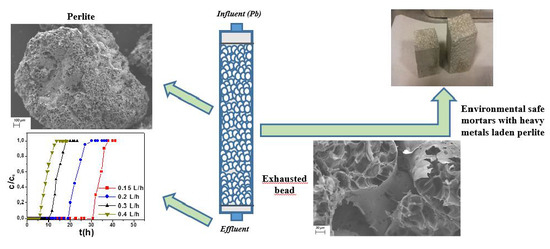
Lead Ion Sorption by Perlite and Reuse of the Exhausted Material in the Construction Field
Abstract
1. Introduction
2. Materials and Methods
2.1. The Sorbent
2.2. Lead Ion Sorption Tests
2.3. Reuse of Perlite Particles in the Construction Field after Lead Exhaustion
3. Results and Discussion
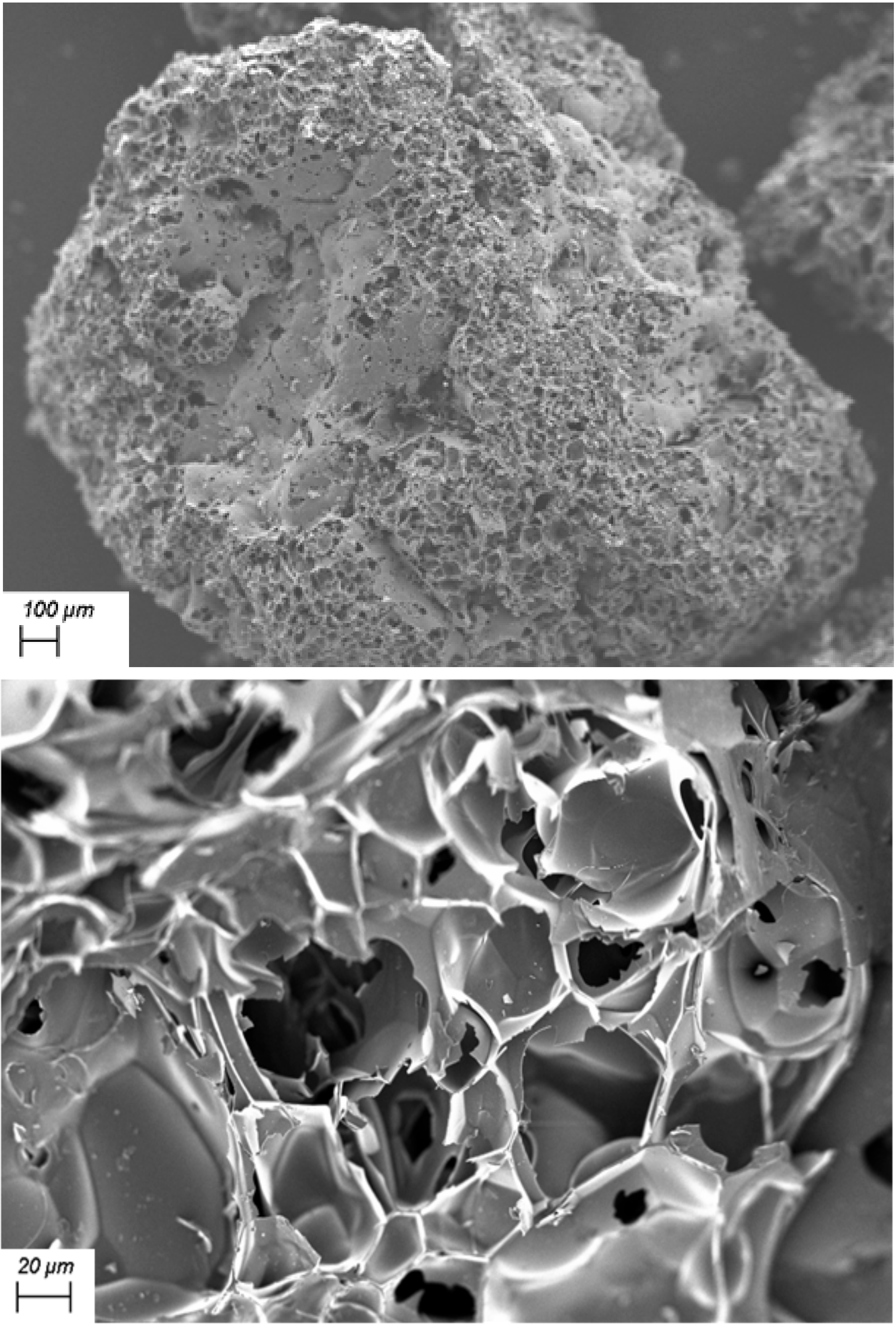
3.1. Perlite Characterization and Perlite as a Lead Ion Sorbent
3.2. Reuse of the Metal Laden Perlite in the Construction Field
4. Conclusions
- -
- From microscopic and BET measurements, metal retention was ascribed to surface area and to the large sorbent pores exposed to the liquid-phase, which allowed for free migration of hydrated lead ions to the functional groups of the silico-aluminate matrix.
- -
- Metal removal efficiencies increased with increasing amounts of perlite, together with breakpoints being reached at increasing bed volumes.
- -
- Increase in retention capacities was observed with an increase of initial metal concentration, together with anticipation of the breakthrough point.
- -
- A more complete saturation of the column was detected at low flow-rates, associated with long liquid/solid contact times, and with corresponding higher values of the breakthrough points.
- -
- The best lead ion retention was obtained at 0.3 L·h−1, with 4 g of perlite and 10 mg·L−1 of Pb2+ (3.5 mg of Pb2+gsorbent−1).
- -
- Film diffusion control was the kinetic step of the process.
- -
- After column breakthrough, the sorbent was considered a special waste and incorporated into cement conglomerates as a lightweight, eco-friendly aggregate.
- -
- With the increase of perlite volume, thermal insulating properties of the samples were improved, together with a decrease in mechanical strength.
- -
- The cement conglomerates could be used in the construction industry in plaster or panels, without adverse environmental effects, as the metals would be incorporated into the matrix, with negligible leaching.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, N.; Agarwal, A.K.; Eastwood, P.; Gupta, T.; Singh, A.P. Introduction to air pollution and its control. In Air Pollution and Control. Energy, Environment, and Sustainability; Sharma, N., Agarwal, A., Eastwood, P., Gupta, T., Singh, A., Eds.; Springer: Singapore, 2018; pp. 3–7. ISBN 978-981-10-7184-3. [Google Scholar]
- Basile, T.; Petrella, A.; Petrella, M.; Boghetich, G.; Petruzzelli, V.; Colasuonno, S.; Petruzzelli, D. Review of endocrine-disrupting-compound removal technologies in water and wastewater treatment plants: An EU perspective. Ind. Eng. Chem. Res. 2011, 50, 8389–8401. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, G.; Yu, R. Fates and impacts of nanomaterial contaminants in biological wastewater treatment system: A review. Water Air Soil Pollut. 2018, 229, 9. [Google Scholar] [CrossRef]
- Cachada, A.; Rocha-Santos, T.; Duarte, A.C. Soil and pollution: An introduction to the main issues. In Soil Pollution, 1st ed.; Duarte, A., Cachada, A., Rocha-Santos, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–28. [Google Scholar]
- Spasiano, D.; Luongo, V.; Petrella, A.; Alfè, M.; Pirozzi, F.; Fratino, U.; Piccinni, A.F. Preliminary study on the adoption of dark fermentation as pretreatment for a sustainable hydrothermal denaturation of cement-asbestos composites. J. Clean. Prod. 2017, 166, 172–180. [Google Scholar] [CrossRef]
- Dmitrienko, M.A.; Strizhak, P.A. Coal-water slurries containing petrochemicals to solve problems of air pollution by coal thermal power stations and boiler plants: An introductory review. Sci. Total Environ. 2018, 613, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.L.; DeSutter, T.M.; Casey, F.X.; Khan, E.; Wick, A.F. Thermal remediation alters soil properties—A review. J. Environ. Manag. 2018, 206, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Petrella, A.; Boghetich, G.; Petrella, M.; Mastrorilli, P.; Petruzzelli, V.; Petruzzelli, D. Photocatalytic degradation of azo dyes. Pilot plant investigation. Ind. Eng. Chem. Res. 2014, 53, 2566–2571. [Google Scholar] [CrossRef]
- Petrella, A.; Mascolo, G.; Murgolo, S.; Petruzzelli, V.; Ranieri, E.; Spasiano, D.; Petruzzelli, D. Photocatalytic oxidation of organic micro-pollutants: Pilot plant investigation and mechanistic aspects of the degradation reaction. Chem. Eng. Commun. 2016, 203, 1298–1307. [Google Scholar] [CrossRef]
- Zhang, K.; Chai, F.; Zheng, Z.; Yang, Q.; Zhong, X.; Fomba, K.W.; Zhou, G. Size distribution and source of heavy metals in particulate matter on the lead and zinc smelting affected area. J. Environ. Sci. 2018, 71, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, E.; Fratino, U.; Petrella, A.; Torretta, V.; Rada, E.C. Ailanthus Altissima and Phragmites Australis for chromium removal from a contaminated soil. Environ. Sci. Pollut. R. 2016, 23, 15983–15989. [Google Scholar] [CrossRef] [PubMed]
- Fabbricino, M.; Ferraro, A.; Luongo, V.; Pontoni, L.; Race, M. Soil washing optimization, recycling of the solution, and ecotoxicity assessment for the remediation of Pb-contaminated sites using EDDS. Sustainability 2018, 10, 636. [Google Scholar] [CrossRef]
- Petruzzelli, D.; Petruzzelli, V.; Basile, T.; Petruzzelli, M.; Petrella, A.; Maggiore, M. Chemical and geochemical characterisation of a disused red brick factory area of central Italy. Chem. Ecol. 2011, 27, 143–152. [Google Scholar] [CrossRef]
- Bairq, Z.A.S.; Li, R.; Li, Y.; Gao, H.; Sema, T.; Teng, W.; Kumar, S.; Liang, Z. New advancement perspectives of chloride additives on enhanced heavy metals removal and phosphorus fixation during thermal processing of sewage sludge. J. Clean. Prod. 2018, 188, 185–194. [Google Scholar] [CrossRef]
- Shahat, A.; Hassan, H.M.; Azzazy, H.M.; El-Sharkawy, E.A.; Abdou, H.M.; Awual, M.R. Novel hierarchical composite adsorbent for selective lead (II) ions capturing from wastewater samples. Chem. Eng. J. 2018, 332, 377–386. [Google Scholar] [CrossRef]
- Petrella, A.; Petrella, M.; Boghetich, G.; Basile, T.; Petruzzelli, V.; Petruzzelli, D. Heavy metals retention on recycled waste glass from solid wastes sorting operations: A comparative study among different metal species. Ind. Eng. Chem. Res. 2012, 51, 119–125. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, Y.; Li, L.; Xu, H.; Sun, Y.; Wang, Y. Novel synthesis of cyano-functionalized mesoporous silica nanospheres (MSN) from coal fly ash for removal of toxic metals from wastewater. J. Hazard. Mater. 2018, 345, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.C.; Loganathan, P.; Nguyen, T.V.; Kandasamy, J.; Naidu, R.; Vigneswaran, S. Adsorptive removal of five heavy metals from water using blast furnace slag and fly ash. Environ. Sci. Pollut. Res. 2018, 25, 20430–20438. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Kucinska, A.; Cyganiuk, A.; Lukaszewicz, J.P. A microporous and high surface area active carbon obtained by the heat-treatment of chitosan. Carbon 2012, 50, 3098–3101. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Yang, L.; Liu, H. Removal of chromium(VI) from wastewater using weakly and strongly basic magnetic adsorbents: Adsorption/desorption property and mechanism comparative studies. RSC Adv. 2016, 6, 18471–18482. [Google Scholar] [CrossRef]
- Petrella, A.; Petruzzelli, V.; Ranieri, E.; Catalucci, V.; Petruzzelli, D. Sorption of Pb(II), Cd(II) and Ni(II) from single- and multimetal solutions by recycled waste porous glass. Chem. Eng. Commun. 2016, 203, 940–947. [Google Scholar] [CrossRef]
- Petrella, A.; Spasiano, D.; Acquafredda, P.; De Vietro, N.; Ranieri, E.; Cosma, P.; Rizzi, V.; Petruzzelli, V.; Petruzzelli, D. Heavy metals retention (Pb (II), Cd (II), Ni (II)) from single and multimetal solutions by natural biosorbents from the olive oil milling operations. Process Saf. Environ. 2018, 114, 79–90. [Google Scholar] [CrossRef]
- Coppola, L.; Bellezze, T.; Belli, A.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Cabrini, M.; Candamano, S.; Cappai, M.; Caputo, D.; et al. Binders alternative to Portland cement and waste management for sustainable construction–Part 2. J. Appl. Biomater. Funct. Mater. 2018, 16, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Coppola, L.; Bellezze, T.; Belli, A.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Cabrini, M.; Candamano, S.; Cappai, M.; Caputo, D.; et al. Binders alternative to Portland cement and waste management for sustainable construction-part 1. J. Appl. Biomater. Funct. Mater. 2018, 16, 186–202. [Google Scholar] [PubMed]
- Turan, M.; Mart, U.; Yüksel, B.; Celik, M.S. Lead removal in fixed-bed columns by zeolite and sepiolite. Chemosphere 2005, 60, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, R.; Vijayaraghavan, K.; Thilakavathi, M.; Iyer, P.V.R.; Velan, M. Application of seaweeds for the removal of lead from aqueous solution. Biochem. Eng. J. 2007, 33, 211–216. [Google Scholar] [CrossRef]
- Abdel-Halim, S.H.; Shehata, A.M.A.; El-Shahat, M.F. Removal of lead ions from industrial waste water by different types of natural materials. Water Res. 2003, 37, 1678–1683. [Google Scholar] [CrossRef]
- Bahadir, T.; Bakan, G.; Altas, L.; Buyukgungor, H. The investigation of lead removal by biosorption: An application at storage battery industry wastewaters. Enzym. Microb. Technol. 2007, 41, 98–102. [Google Scholar] [CrossRef]
- Chakir, A.; Bessiere, J.; Kacemi, K.E.; Marouf, B. A comparative study of the removal of trivalent chromium from aqueous solutions by bentonite and expanded perlite. J. Hazard. Mater. 2002, 95, 29–46. [Google Scholar] [CrossRef]
- Doğan, M.; Alkan, M. Adsorption kinetics of methyl violet onto perlite. Chemosphere 2003, 50, 517–528. [Google Scholar] [CrossRef]
- Sowmeyan, R.; Swaminathan, G. Evaluation of inverse anaerobic fluidized bed reactor for treating high strength organic wastewater. Bioresour. Technol. 2008, 99, 3877–3880. [Google Scholar] [CrossRef] [PubMed]
- Shavisi, Y.; Sharifnia, S.; Hosseini, S.N.; Khadivi, M.A. Application of TiO2/perlite photocatalysis for degradation of ammonia in wastewater. J. Ind. Eng. Chem. 2014, 20, 278–283. [Google Scholar] [CrossRef]
- Saja, S.; Bouazizi, A.; Achiou, B.; Ouammou, M.; Albizane, A.; Bennazha, J.; Younssi, S.A. Elaboration and characterization of low-cost ceramic membrane made from natural Moroccan perlite for treatment of industrial wastewater. J. Environ. Chem. Eng. 2018, 6, 451–458. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Tukker, A. Product services for a resource-efficient and circular economy—A review. J. Clean. Prod. 2015, 97, 76–91. [Google Scholar] [CrossRef]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Yuan, Q.; Tang, H.; Yu, F.; Lv, X. A green adsorbent derived from banana peel for highly effective removal of heavy metal ions from water. RSC Adv. 2016, 2, 45041–45048. [Google Scholar] [CrossRef]
- Ranieri, E.; Gorgoglione, A.; Petrella, A.; Petruzzelli, V.; Gikas, P. Benzene removal in horizontal subsurface flow constructed wetlands treatment. Int. J. Appl. Eng. Res. 2015, 10, 14603–14614. [Google Scholar]
- Petrella, A.; Petruzzelli, V.; Basile, T.; Petrella, M.; Boghetich, G.; Petruzzelli, D. Recycled porous glass from municipal/industrial solid wastes sorting operations as a lead ion sorbent from wastewaters. React. Funct. Polym. 2010, 70, 203–209. [Google Scholar] [CrossRef]
- Italian Organization for Standardization (UNI). Cement Composition, Specifications and Conformity Criteria for Common Cements. EN 197-1. Available online: http://store.uni.com/magento-1.4.0.1/index.php/en-197-1-2011.html (accessed on 14 September 2011).
- Italian Organization for Standardization (UNI). Methods of Testing Cement-Part 1: Determination of Strength. EN 196-1. Available online: http://store.uni.com/magento-1.4.0.1/index.php/en-196-1-2016.html (accessed on 27 April 2016).
- International Organization for Standardization (ISO). Cement, Test Methods, Determination of Strength. ISO 679. Available online: http://store.uni.com/magento-1.4.0.1/index.php/iso-679-2009.html (accessed on 24 April 2009).
- Petrella, A.; Cosma, P.; Rizzi, V.; De Vietro, N. Porous alumosilicate aggregate as lead ion sorbent in wastewater treatments. Separations 2017, 4, 25. [Google Scholar] [CrossRef]
- Paul, S.C.; Šavija, B.; Babafemi, A.J. A comprehensive review on mechanical and durability properties of cement based materials containing waste recycled glass. J. Clean. Prod. 2018, 198, 891–906. [Google Scholar] [CrossRef]
- Italian Organization for Standardization (UNI). Characterization of Waste-Compliance Test for Leaching of Granular Waste Materials and Sludges. EN 12457-2. Available online: http://store.uni.com/magento-1.4.0.1/index.php/en-12457-2-2002.html (accessed on 18 September 2002).
- Mills, A.F. Basic Heat and Mass Transfer, 2nd ed.; Prentice Hall: Upper Saddler River, NJ, USA, 1999; Volume 2, pp. 745–833. ISBN 978-0130962478. [Google Scholar]
- Ahmadi, P.F.; Ardeshir, A.; Ramezanianpour, A.M.; Bayat, H. Characteristics of heat insulating clay bricks made from zeolite, waste steel slag and expanded perlite. Ceram. Int. 2018, 44, 7588–7598. [Google Scholar] [CrossRef]
- Di Mundo, R.; Petrella, A.; Notarnicola, M. Surface and bulk hydrophobic cement composites by tyre rubber addition. Constr. Build. Mater. 2018, 172, 176–184. [Google Scholar] [CrossRef]
- Liuzzi, S.; Rubino, C.; Stefanizzi, P.; Petrella, A.; Boghetich, A.; Casavola, C.; Pappalettera, G. Hygrothermal properties of clayey plasters with olive fibers. Constr. Build. Mater. 2018, 158, 24–32. [Google Scholar] [CrossRef]






| Test No. | Flow Rate (L·h−1) | Influent Conc. (mg·L−1) | Bead Size (mm) | Bed Volume (cm3) | Bed Amount (g) | Qexp (mg·g−1) | BV |
|---|---|---|---|---|---|---|---|
| 1 | 0.3 | 2 | 1–2 | 16 | 2 | 1.10 ± 0.05 | 55 ± 3 |
| 2 | 0.3 | 2 | 1–2 | 20 | 2.6 | 1.45 ± 0.07 | 80 ± 4 |
| 3 | 0.3 | 2 | 1–2 | 24 | 3.2 | 1.7 ± 0.08 | 91 ± 5 |
| 4 | 0.3 | 2 | 1–2 | 30 | 4 | 2.0 ± 0.1 | 100 ± 5 |
| 5 | 0.3 | 4 | 1–2 | 30 | 4 | 2.5 ± 0.12 | 53 ± 3 |
| 6 | 0.3 | 7 | 1–2 | 30 | 4 | 3.1 ± 0.15 | 40 ± 2 |
| 7 | 0.3 | 10 | 1–2 | 30 | 4 | 3.5 ± 0.17 | 27 ± 1 |
| 8 | 0.15 | 2 | 1–2 | 30 | 4 | 2.5 ± 0.12 | 153 ± 8 |
| 9 | 0.2 | 2 | 1–2 | 30 | 4 | 2.2 ± 0.11 | 130 ± 7 |
| 10 | 0.4 | 2 | 1–2 | 30 | 4 | 1.6 ± 0.08 | 73 ± 4 |
| Sample | ρ (Kg m−3) | λ (W m−1 K−1) | Rc (Mpa) |
|---|---|---|---|
| Normalized mortar | 1960 ± 100 | 2.02 ± 0.10 | 49 ± 3 |
| Sand (100%) | 1920 ± 100 | 1.59 ± 0.08 | 48 ± 3 |
| Sand (75%)/Perlite (25%) | 1750 ± 88 | 1.2 ± 0.06 | 34.5 ± 2 |
| Sand (50%)/Perlite (50%) | 1600 ± 80 | 0.90 ± 0.05 | 26 ± 1 |
| Sand (25%)/Perlite (75%) | 1350 ± 68 | 0.60 ± 0.03 | 21 ± 1 |
| Perlite (100%) | 1050 ± 53 | 0.39 ± 0.02 | 16.4 ± 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrella, A.; Spasiano, D.; Rizzi, V.; Cosma, P.; Race, M.; De Vietro, N. Lead Ion Sorption by Perlite and Reuse of the Exhausted Material in the Construction Field. Appl. Sci. 2018, 8, 1882. https://doi.org/10.3390/app8101882
Petrella A, Spasiano D, Rizzi V, Cosma P, Race M, De Vietro N. Lead Ion Sorption by Perlite and Reuse of the Exhausted Material in the Construction Field. Applied Sciences. 2018; 8(10):1882. https://doi.org/10.3390/app8101882
Chicago/Turabian StylePetrella, Andrea, Danilo Spasiano, Vito Rizzi, Pinalysa Cosma, Marco Race, and Nicoletta De Vietro. 2018. "Lead Ion Sorption by Perlite and Reuse of the Exhausted Material in the Construction Field" Applied Sciences 8, no. 10: 1882. https://doi.org/10.3390/app8101882
APA StylePetrella, A., Spasiano, D., Rizzi, V., Cosma, P., Race, M., & De Vietro, N. (2018). Lead Ion Sorption by Perlite and Reuse of the Exhausted Material in the Construction Field. Applied Sciences, 8(10), 1882. https://doi.org/10.3390/app8101882