Characterization of Human Dermal Papilla Cells in Alginate Spheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Human Hair Follicles
2.2. Culture of Dermal Papilla Cells
2.3. Fabrication of Alginate Microencapsulated Cells Using Electrospinning
2.4. Direct Cell Count
2.5. RNA Isolation and Real Time PCR
2.6. CMDil Labeling of Cells
2.7. Cultivation of Explants
2.8. Statistical Analysis and Normalization
3. Results
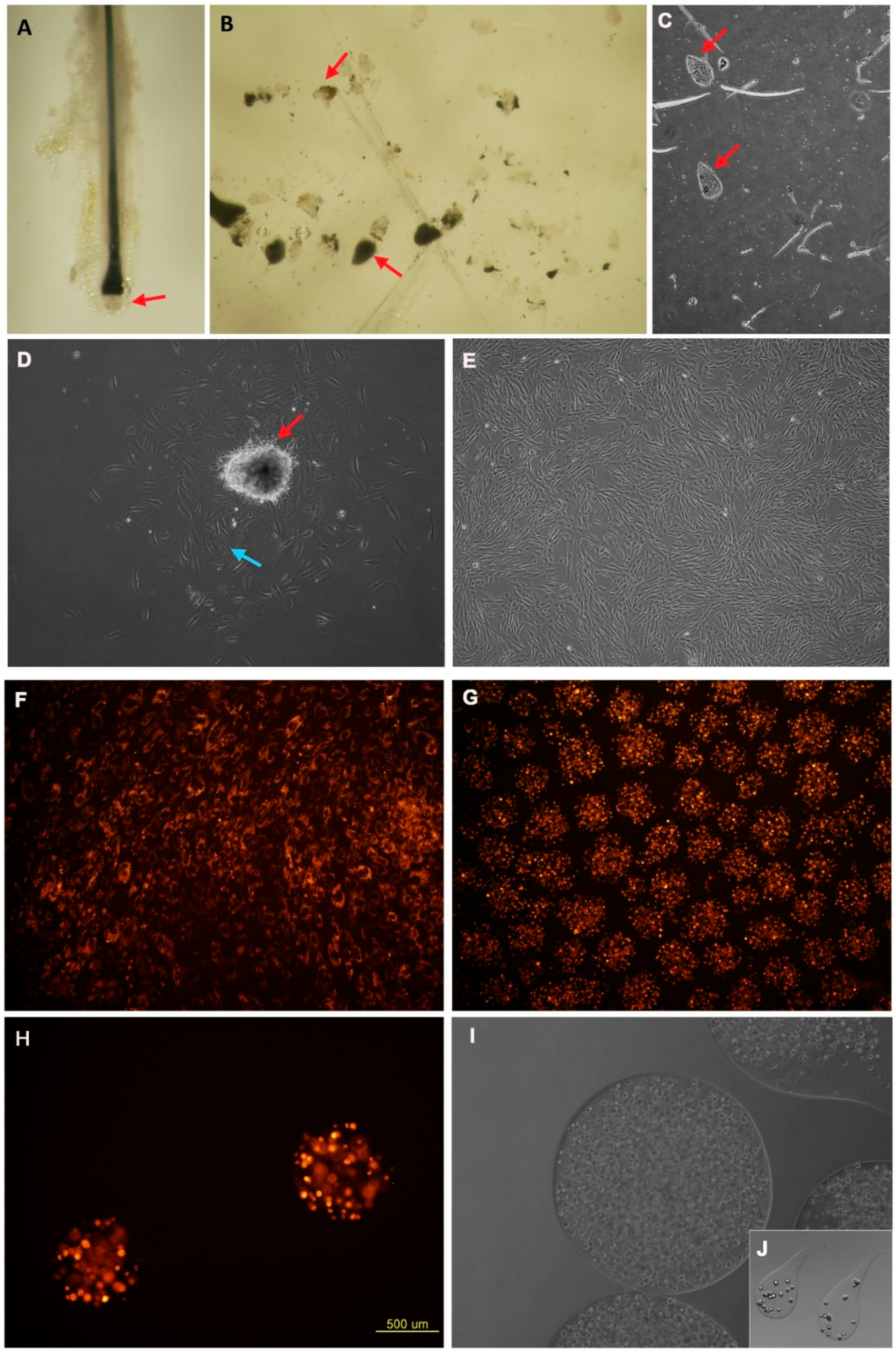
3.1. Characteristics and Cultivation of Human Dermal Papilla Cells in In Vitro and in Alginate Spheres
3.2. Effect of Different Size of Alginate Spheres on DPCs Proliferation
3.3. Effect of Cellular Density of Alginate Spheres on DPCs Proliferation
3.4. Behavior of DPCs from the Alginate Sphere
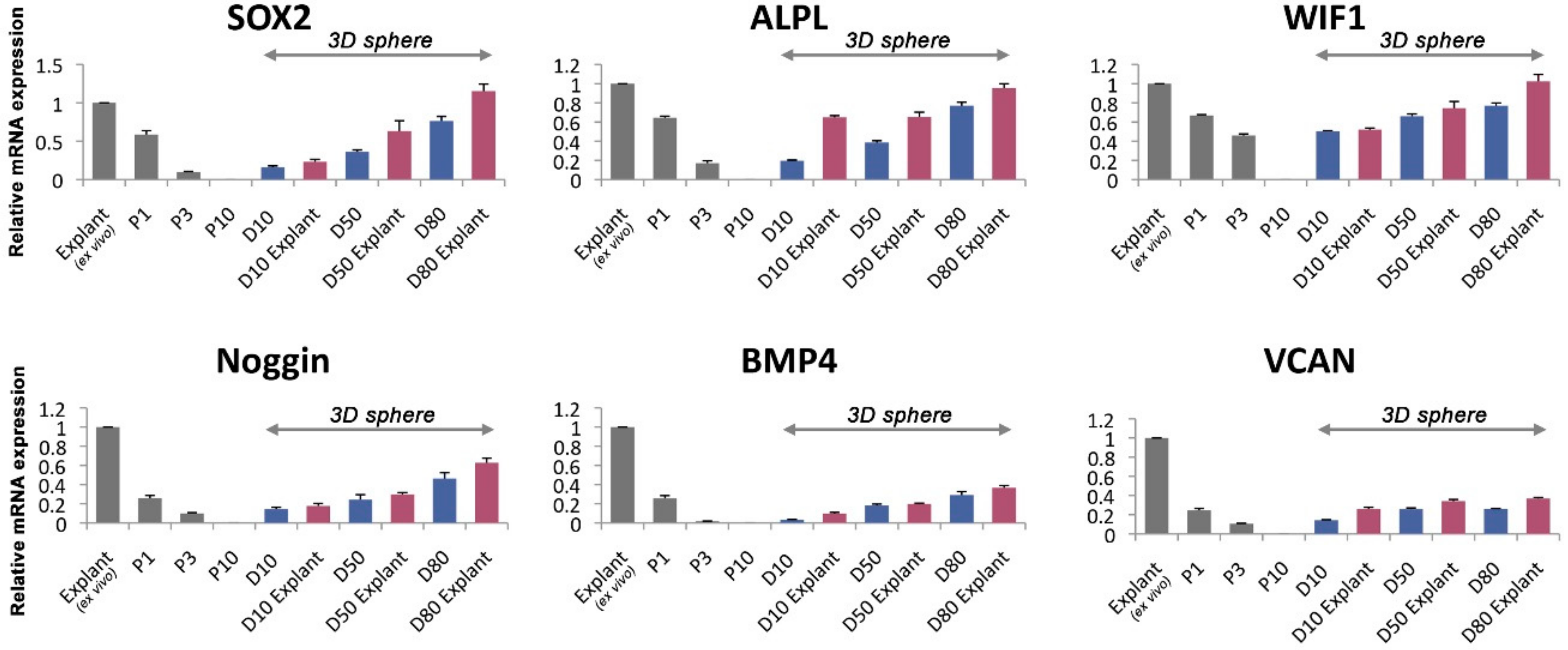
3.5. The Gene Expression Level of the Human DPCs in the Alginate Sphere
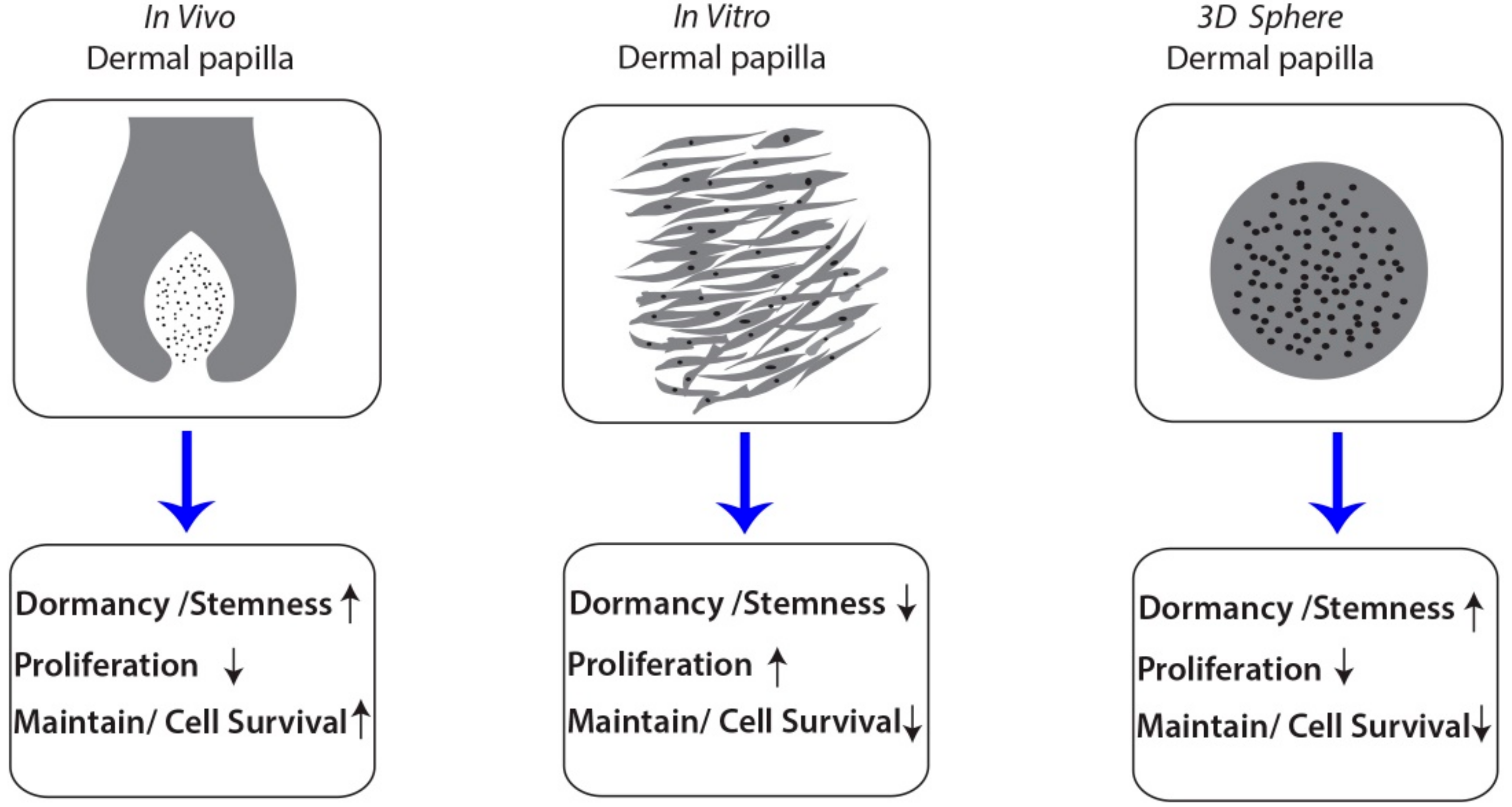
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflict of interest
References
- Mueller-Klieser, W. Three-dimensional cell cultures: From molecular mechanisms to clinical applications. Am. J. Physiol.-Cell Physiol. 1997, 273, C1109–C1123. [Google Scholar] [CrossRef]
- Fennema, E.; Rivron, N.; Rouwkema, J.; Van Blitterswijk, C.; De Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.W.; Hsi, T.C.; Guerrero-Juarez, C.F.; Ramos, R.; Plikus, M.V. Organotypic skin culture. J. Investig. Dermatol. 2013, 133, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Itaka, K.; Ohba, S.; Nishiyama, N.; Chung, U.I.; Yamasaki, Y.; Kataoka, K. 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials 2009, 30, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Suyama, T.; Hatta, M.; Hata, S.; Ishikawa, H.; Yamazaki, J. Differentiation of rat dermal mesenchymal cells and calcification in three-dimensional cultures. Tissue Eng. Regener. Med. 2016, 13, 527–537. [Google Scholar] [CrossRef]
- Higgins, C.A.; Chen, J.C.; Cerise, J.E.; Jahoda, C.A.; Christiano, A.M. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc. Natl. Acad. Sci. USA 2013, 110, 19679–19688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, C.A.; Richardson, G.D.; Ferdinando, D.; Westgate, G.E.; Jahoda, C.A. Modelling the hair follicle dermal papilla using spheroid cell cultures. Exp. Dermatol. 2010, 19, 546–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenn, K.; Paus, R. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.W.; Kloepper, J.; Langan, E.A.; Kim, Y.; Yeo, J.; Kim, M.J.; Hsi, T.-C.; Rose, C.; Yoon, G.S.; Lee, S.-J. A guide to studying human hair follicle cycling in vivo. J. Investig. Dermatol. 2016, 136, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; Dela Cruz-Racelis, J.; Fuchs, E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009, 4, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.; Messenger, A.G.; Stephenson, T.J. Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: Implications for the control of hair follicle size and androgen responses. J. Investig. Dermatol. 1999, 113, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Enshell-Seijffers, D.; Morgan, B.A. De novo production of dermal papilla cells during the anagen phase of the hair cycle. J. Investig. Dermatol. 2010, 130, 2664–2666. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J.; Gunin, A.; Magerl, M.; Paus, R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme during the hair growth cycle: Implications for growth control and hair follicle transformations. J. Investig. Dermatol. Symp. Proc. 2003, 8, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Lichti, U.; Weinberg, W.C.; Goodman, L.; Ledbetter, S.; Dooley, T.; Morgan, D.; Yuspa, S.H. In vivo regulation of murine hair growth: Insights from grafting defined cell populations onto nude mice. J. Investig. Dermatol. 1993, 101, S124–S129. [Google Scholar] [CrossRef]
- Rahmani, W.; Abbasi, S.; Hagner, A.; Raharjo, E.; Kumar, R.; Hotta, A.; Magness, S.; Metzger, D.; Biernaskie, J. Hair follicle dermal stem cells regenerate the dermal sheath, repopulate the dermal papilla and modulate hair type. Dev. Cell 2014, 31, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M. The human hair cycle. J. Investig. Dermatol. 1959, 33, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Sung, Y.K.; Chung, E.J.; Im, S.U.; Ahn, J.S.; Kim, M.K.; Kim, J.C. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Investig. Dermatol. 2008, 128, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.M.; Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Sphere formation increases the ability of cultured human dermal papilla cells to induce hair follicles from mouse epidermal cells in a reconstitution assay. J. Investig. Dermatol. 2012, 132, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Oliver, R.F. The induction of hair follicle formation in the adult hooded rat by vibrissa dermal papillae. Development 1970, 23, 219–236. [Google Scholar]
- Kishimoto, J.; Burgeson, R.E.; Morgan, B.A. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000, 14, 1181–1185. [Google Scholar] [PubMed]
- Rendl, M.; Polak, L.; Fuchs, E. Bmp signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008, 22, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Mun, C.H.; Hwang, J.Y.; Lee, S.H. Microfluidic spinning of the fibrous alginate scaffolds for modulation of the degradation profile. Tissue Eng. Regener. Med. 2016, 13, 140–148. [Google Scholar] [CrossRef]
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun collagen nanofibers and their applications in skin tissue engineering. Tissue Eng. Regener. Med. 2017, 14, 699–718. [Google Scholar] [CrossRef]
- Oh, J.W.; Choi, J.Y.; Kim, M.; Abdi, S.I.H.; Lau, H.C.; Kim, M.; Lim, J.O. Fabrication and characterization of epithelial scaffolds for hair follicle regeneration. Tissue Eng. Regener. Med. 2012, 9, 147–156. [Google Scholar] [CrossRef]
- Shin, H.; Kwack, M.H.; Shin, S.H.; Oh, J.W.; Kang, B.M.; Kim, A.A.; Kim, J.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Identification of transcriptional targets of wnt/beta-catenin signaling in dermal papilla cells of human scalp hair follicles: Ep2 is a novel transcriptional target of wnt3a. J. Dermatol. Sci. 2010, 58, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.M.; Shin, S.H.; Kwack, M.H.; Shin, H.; Oh, J.W.; Kim, J.; Moon, C.; Moon, C.; Kim, J.C.; Kim, M.K.; et al. Erythropoietin promotes hair shaft growth in cultured human hair follicles and modulates hair growth in mice. J. Dermatol. Sci. 2010, 59, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, M.; Kobayashi, T.; Sasaki, T.; Shimizu, A.; Amagai, M. Restoration of the intrinsic properties of human dermal papilla in vitro. J. Cell Sci. 2012, 125, 4114–4125. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Cotsarelis, G. Review of hair follicle dermal cells. J. Dermatol. Sci. 2010, 57, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahoda, C.A.; Horne, K.A.; Oliver, R.F. Induction of hair growth by implantation of cultured dermal papilla cells. Nature 1984, 311, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Schell, J.; Krall, A.S.; Jelinek, D.; Miranda, M.; Grigorian, M.; Braas, D.; White, A.C.; Zhou, J.L.; Graham, N.A.; et al. Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat. Cell Biol. 2017, 19, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Trumpp, A.; Essers, M.; Wilson, A. Awakening dormant haematopoietic stem cells. Nat. Rev. Immunol. 2010, 10, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.M.; He, X.C.; Sugimura, R.; Li, L. Stem cell dormancy: Maintaining a reserved population. Rev. Cell Biol. Mol. Med. 2006. [Google Scholar] [CrossRef]
- Wang, Q.; Oh, J.W.; Lee, H.L.; Dhar, A.; Peng, T.; Ramos, R.; Guerrero-Juarez, C.F.; Wang, X.; Zhao, R.; Cao, X.; et al. A multi-scale model for hair follicles reveals heterogeneous domains driving rapid spatiotemporal hair growth patterning. eLife 2017, 6, e22772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutberg, S.E.; Kolpak, M.L.; Gourley, J.A.; Tan, G.; Henry, J.P.; Shander, D. Differences in expression of specific biomarkers distinguish human beard from scalp dermal papilla cells. J Investig. Dermatol. 2006, 126, 2583–2595. [Google Scholar] [CrossRef] [PubMed]
- Inamatsu, M.; Matsuzaki, T.; Iwanari, H.; Yoshizato, K. Establishment of rat dermal papilla cell lines that sustain the potency to induce hair follicles from afollicular skin. J Investig. Dermatol. 1998, 111, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Botchkarev, V.A.; Kishimoto, J. Molecular control of epithelial–mesenchymal interactions during hair follicle cycling. J. Investig. Dermatol. Symp. Proc. 2003, 8, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Oh, J.W.; Spandau, D.F.; Tholpady, S.; Diaz, J., 3rd; Schroeder, L.J.; Offutt, C.D.; Glick, A.B.; Plikus, M.V.; Koyama, S.; et al. Estrogen modulates mesenchyme-epidermis interactions in the adult nipple. Development 2017, 144, 1498–1509. Available online: http://dev.biologists.org/content/develop/144/8.toc.pdf (accessed on 19 July 2018). [CrossRef] [PubMed] [Green Version]
- Lee, M.H.; Im, S.; Shin, S.H.; Kwack, M.H.; Jun, S.E.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Conditioned media obtained from human outer root sheath follicular keratinocyte culture activates signalling pathways that contribute to maintenance of hair-inducing capacity and increases trichogenicity of cultured dermal cells. Exp. Dermatol. 2012, 21, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Oh, J.W.; Kim, S.J.; Lim, J.O. The effect of bsm-alginate sponge on the enhanced early proliferation of epithelial cells. Biomater. Res. 2013, 17, 26–30. [Google Scholar]
- Matsuzaki, T.; Inamatsu, M.; Yoshizato, K. The upper dermal sheath has a potential to regenerate the hair in the rat follicular epidermis. Differentiation 1996, 60, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Yamao, M.; Toyoshima, K.-E.; Yoshizato, K. Comparison of hair-inducing ability between the upper and the lower dermal papilla: P4.44. J. Dtsch. Dermatol. Ges. 2004, 2, 526. [Google Scholar]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mali, N.M.; Kim, Y.-H.; Park, J.M.; Kim, D.; Heo, W.; Dao, B.L.; Lim, J.O.; Oh, J.W. Characterization of Human Dermal Papilla Cells in Alginate Spheres. Appl. Sci. 2018, 8, 1993. https://doi.org/10.3390/app8101993
Mali NM, Kim Y-H, Park JM, Kim D, Heo W, Dao BL, Lim JO, Oh JW. Characterization of Human Dermal Papilla Cells in Alginate Spheres. Applied Sciences. 2018; 8(10):1993. https://doi.org/10.3390/app8101993
Chicago/Turabian StyleMali, Nanda Maya, Yong-Hee Kim, Jung Min Park, Donghyun Kim, Wook Heo, Buu Le Dao, Jeong Ok Lim, and Ji Won Oh. 2018. "Characterization of Human Dermal Papilla Cells in Alginate Spheres" Applied Sciences 8, no. 10: 1993. https://doi.org/10.3390/app8101993
APA StyleMali, N. M., Kim, Y.-H., Park, J. M., Kim, D., Heo, W., Dao, B. L., Lim, J. O., & Oh, J. W. (2018). Characterization of Human Dermal Papilla Cells in Alginate Spheres. Applied Sciences, 8(10), 1993. https://doi.org/10.3390/app8101993




