Featured Application
This work gives an overview of the present status of anticancer drugs in the environmental water of the Yodo River basin, a specific populated area in Japan. The results indicate importance of application of high-tech treatment(s) including advanced oxidation processes at sewage treatment plant for efficient removal of the discharged drugs.
Abstract
This article reviews the pollution status of anticancer drugs present in the Yodo River basin located in the Kansai district of Japan, covering both the soluble and insoluble (adsorbed on the river sediments and suspended solids) levels. Procedures ranging from sampling in the field and instrumental analytical methods to the data processing for mass balance estimation of the target basin are also described. All anticancer drugs concerned with this article were detected in sewage and river waters, where the presence of bicalutamide (BLT) was identified at considerably high concentrations (maximum 254 ng/L in the main stream, 151 ng/L in tributaries, and 1032 ng/L in sewage treatment plant (STP) effluents). In addition, sorption distribution coefficient (logKd) values showed a tendency to become higher in the silty sediments at Suita Bridge than in the sandy sediments at Hirakata Bridge; these trends were supported by the results of the laboratory-scale sorption experiment. STPs were concluded to be the main sources of the anticancer drug load in the river, and a mass flux evaluation revealed that the effect of attenuation in the river environment was small. The effectiveness of ozonation in the sewage treatment process for removal of these anticancer drugs was further confirmed. The present article should be of value for facilitating the environmental risk assessment of a wide range of drugs in a broader geographical area.
1. Introduction
The emerging problem of the pollution of river environments by pharmaceuticals and personal care products (PPCPs) has received a large amount of attention [1,2,3]. Pharmaceuticals are designed to have specific physiological effects on target areas of the body. Concern is therefore rising about their toxic effects on ecosystems when discharged into environmental water, even when they are present at low concentrations. Furthermore, their impacts on human health via residues contaminating drinking water should be taken into consideration [4,5,6,7]. Generally, the concentrations of these compounds are low (roughly in the range from ng/L to μg/L) worldwide [7,8,9]. Even in this low concentration range, however, there have been reports of the endocrine-disrupting chemicals that have serious environmental impacts, such as the feminization of male fishes [10,11].
PPCPs include different groups of compounds typified by their chemical characteristics, structure, mechanism of action, mode of action, and their therapeutic use to treat specific diseases. Particularly, anticancer drugs are emerging as an area of growing interest because of their promotion of a long lifespan via the suppression of human death through their use as a chemotherapy [12,13].
Human life is changing in response to recent developments in science and technology. In Japan, cancer has been the country’s top cause of death since 1981, accounting for 29% of all deaths in 2015. This was nearly double the rate of the second-highest cause of death, heart disease (15%) [14]. In addition, because of the aging of the Japanese population [14], the number of cancer patients who need treatment is likely to increase in future. Increasing numbers of new pharmaceuticals for chemotherapy [15], which, along with surgery and radiotherapy, is an important treatment for cancer, have been developed in recent years [16]. The use of anticancer agents in clinical situations is also increasing, whereas the rates of adoption of surgical therapy and chemotherapy in Japan have stayed about the same [17].
According to their mechanisms of action, anticancer drugs are mainly classified into alkylating agents, antimetabolites, hormone antagonists, cytotoxic antibiotics, antimitotics, cytotoxic quinolones, and topoisomerase inhibitors. Because all of these anticancer drugs have physiological activity and are highly cytostatic, there are many concerns about their cytostatic effects on aquatic ecosystems and on organisms living in the aquatic environments [12,18,19,20]. In fact, an anticancer drug, tamoxifen (TAM), inhibited the reproduction and growth of Daphnia at 120 ng/L [21] and showed a predicted no-effect concentration of 81 ng/L for fish, plankton, and algae [22]. In addition, anticancer drugs are present in the sediments in river environments. Therefore, serious pollution problems sometimes arise from contaminated river water because of the exposure of benthonic organisms and bioaccumulation in predators such as fish along the ecological chain through predation [23,24]. The sedimented substances affect not only river environments, but also marine environments, and humans are at risk when they eat fish polluted with them through bioaccumulation [25,26,27].
At present, the pollution aspect of anticancer drugs in Japan has been analyzed only in the very limited area surrounding the Yodo River since 2009 [13,28,29,30,31]. Their occurrence in the river water and sediments, including from hospital and sewage treatment effluents [13,28,32], their fate after discharge [31], and their attenuation properties [29,30], were reported in a separate fashion. The time has come to make an overview of the status of anticancer drugs in the environmental water of the Yodo River basin.
The aim of this article is summarize the state of anticancer drugs in the regional river water and sediments in the subcatchment of the Yodo River basin in Japan, providing an invaluable fundamental basis for further spreading of the related studies into a wide range of PPCPs across a wide area to achieve the final goal for conducting effective environmental risk assessments of discharged PPCPs in the future.
2. Materials and Methods
2.1. Sampling of Environmental Waters and Sediments
The question of primary importance is of how to select the sampling field to determine the distribution of pharmaceuticals in the environmental waters. For this purpose, considering the spreading out of the geographical region to core cities throughout Japan, the sampling areas in the Kansai district of Japan were selected; the locations were on the right bank of the middle-to-downstream region of the Yodo River [32] and on two other rivers, the Kanzaki and the Ai, and their tributaries. This area, known as the Kanzaki–Ai River basin, covers an important commercial and urban area of about 790 km2 in Osaka and Hyogo prefectures and is home to 2 million people [33,34].
To collect three different types of waters (river, tributary, and sewage treatment plant (STP)), 13 sampling sites consisting of six river sites (R1 to R6), four tributary sites (T1 to T4), and three STP sites (S1 to S3) (Figure 1) were set in the Kanzaki–Ai River basin. The sampling site at the Suita Bridge (R5) was set as the farthest downstream boundary. One sample was basically taken per site, but two effluents, S3(1) and S3(2), were sampled at S3, compiling 14 samples per each sampling time. Names of the sampling sites were used for identification (ID) of the samples. A conventional activated sludge (CAS) process followed by chlorination for disinfection was used in all STPs except S3 where ozonation (8.6 mg/L ozone) was used after partial CAS. The properties of the STPs including treatment process are shown in Table 1 [35]. Annual flow rates and BOD (biological oxygen demand, mg/L) at the sampling sites in the Kanzaki–Ai River basin are listed in Table 2 [30,36]. Stainless-steel pails were used to collect water samples, while a stainless-steel bottom sampler was used for river sediment samples collected at R5 (Suita Bridge) and R6 (Hirakata Bridge). All samples were transferred in separate glass bottles, transported to the laboratory within 2 h and kept at 4 °C under dark. Analysis of the concentration of the target anticancer drugs in each sample was started within 24 h after collection by filtration through a GF/B glass fiber filter (pore size, 1-μm) for separation of liquid from the suspended solids [37].
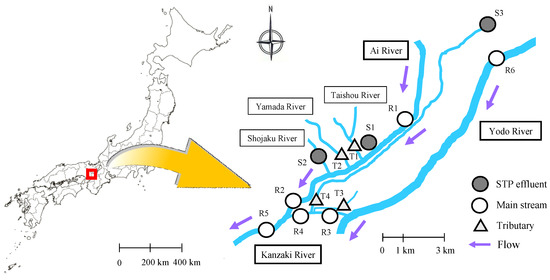

Table 1.
Information of sewage treatment plants (STPs) located in the Kanzaki–Ai River basin (Figure 1) (reproduced from the data in [30]).

Table 2.
Information of flow rate and biological oxygen demand (BOD) values at the sampling sites in the Kanzaki–Ai River basin (Figure 1) (reproduced from the data in [30]).
The surveying anticancer drugs was conducted once in the four seasons in 2013 and 2014; on 4 April (spring), 29 July (summer), 18 December (late autumn), and 4 February (winter). Sampling days were selected on rain-less days accompanied by 2 more ahead fine days (rainfall ≤ 1 mm) [38].
2.2. Selection of Anticancer Drugs and Their Quantification
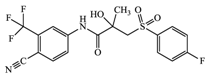
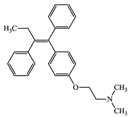
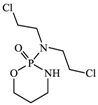
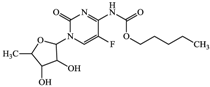
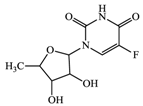
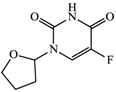
With consideration of their highly frequent therapeutic use in Japan and high excretion rates in unchanged form [39,40], this article focused on the following six anticancer drugs listed in Table 3. Bicalutamide (BLT) is an active antiandrogen medication used to treat prostate cancer. Capecitabine (CAP) is a chemotherapy medication used to treat breast cancer, gastric cancer, and colorectal cancer. Cyclophosphamide (CP) is a chemotherapy medication used to treat ovarian cancer, breast cancer, and small cell lung cancer. Doxifluridine (DFUR) is a chemotherapy medication used to treat gastric cancer, intestinal cancer, and breast cancer. TAM is a medication used to treat breast cancer. Tegafur (TGF) is a chemotherapeutic prodrug of 5-fluorouracil and is used to treat various types of cancer. Once these anticancer drugs are discharged in the environment, their distribution will be affected by their individual physicochemical properties; of these, surface charge due to differences in pKa, pH, and hydrophobic properties estimable from logP were of importance to establish their solubility and degree of adsorption onto organic matters and sedimented materials. The properties of the target anticancer drugs are summarized in Table 3.

Table 3.
Details of the target anticancer drugs typified by their action mechanisms (reconstructed based on the data listed in [41]).
For quantitative analysis of CP and TAM, gas chromatography (GC) combined with mass spectrometry (MS) was used initially after purification and derivatization [42,43,44]. Recent in-depth quantification of these drugs together with the other anticancer drugs was achieved by the simultaneous combination of procedures known as solid-phase extraction (SPE) and liquid chromatography–mass spectrometry (LC-MS/MS) [16,45,46,47], with special care taken for differences in the protocols applied for the liquid and the solid samples. The concentration and purification steps of the anticancer drugs in the liquid samples were performed by SPE [13,31]. In the case of the river sediments and the suspended solids (solid state samples), solvent extraction of the adsorbed materials was achieved by sonication and filtration [31,48,49] prior to analysis.
The final step of measurement was to separate and quantify the target pharmaceuticals by the LC-MS/MS system equipped with an appropriate PC for regulation and calculation. The optimized MS/MS parameters are summarized in Table 4. The cone voltages for detection of the hormone antagonists (BLT and TAM) were somewhat higher than the other anticancer drugs, while lower cone voltages were adequate for the analysis of the antimetabolites (CAP, DFUR, and TGF), which have similar pyrimidine-analogous structures.

Table 4.
LC-MS/MS parameters for the target anticancer drugs (reproduced from [30]).
Quantification of the anticancer drugs was done by subtracting the blank data from the data given by the spiked sample solutions for accounting matrix effects and loss during sample extraction [30,50]. Similarly, recovery rates were calculated from the deviations between the spiked data and the standard data to perform calibration. Recovery rates varied in the range of 63–124% for river water, 52–116% for STP effluent, 23–112% for river sediment, and 33–122% for suspended solids. These values were mostly similar to the values previously reported for pharmaceuticals in river and sewage samples [48,51]. The vales of limit of detection (LOD) and limit of quantification (LOQ), which are important for estimating the sensitivity of the methods, were calculated based on the concentrations at signal-to-noise ratios of 3 and 10 [52,53]. The estimated LOD and LOQ values for liquid samples were in ranges of 0.1–0.4 and 0.3–1.4 ng/L, respectively, but the corresponding values for solid samples appeared in larger ranges of 2.4–13 and 8.2–42 ng/kg, respectively.
2.3. Estimation of Sorption Distribution Coefficient (LogKd) and Mass Balance
The sorption distribution coefficients (logKd) between the liquid samples and the solid samples were determined as log(Cs/Cw) in accordance with the previous reports [54,55], where Cs (ng/kg) is the concentration of pharmaceuticals in the solid samples and Cw (ng/L) is the concentration of pharmaceuticals in the liquid samples.
Experimental sorption of the target anticancer drugs onto the river sediments was done separately by following the procedure as described previously [56,57,58] as well as OECD guideline No. 106 [59]. Sediment samples were suspended in ammonium acetate buffer (pH 7) containing a known amount of the anticancer drugs (0–200 μg/L), and the mixtures were shaken reciprocally for 24 h at 20 °C in the dark to prevent photolysis [56,57]. After incubation, each solution was recovered by filtration through a GD/X glass fiber filter and the concentrations of the anticancer drugs were measured by LC-MS/MS. In the cases for the experimental sorption, the logKd values for the river sediment were determined from the slope of the linear regression lines produced by the initial and equilibrated concentrations in the dissolved phase and the dry weight of the adsorbent [24,60,61].
For estimation of the mass balance of the target anticancer drugs discharged into the Kanzaki–Ai River basin, the mass flux (g/day) of each anticancer drug at each site was calculated by multiplying the detected concentration by the mean river flow rate or the mean STP discharge rate in terms of m3/day. The flow rates at the sampling sites are listed in Table 1 and Table 2. Total mass flux for each drug was calculated by numerical summation of the mass flux values from the upstream region to the farthest downstream boundary site at the Suita Bridge (R5). The mass balance of each drug was then estimated as a percentage of the mass flux for that drug at this boundary site.
3. Results and Discussion
3.1. Distribution of Anticancer Drugs in River Waters and STP Effluents
Table 5 summarized the detected concentrations of the target anticancer drugs. All anticancer drugs were present in both the river waters and the STP effluents [30]. The median concentrations of BLT, TAM, CP, CAP, DFUR, and TGF were 32 ng/L, N.D., 2 ng/L, 2 ng/L, N.D., and N.D., respectively, in the main stream samples, 30 ng/L, N.D., 3 ng/L, 1 ng/L, N.D., and N.D., respectively, in the tributaries, and 245 ng/L, N.D., 10 ng/L, 6 ng/L, N.D., and 23 ng/L, respectively, in the STP effluents (excluding the ozonation STP). The concentrations of BLT, CP, CAP, and TGF in the STP effluents were thus several times higher than those in the river waters. In the previous studies, CP and TAM were detected in the range of N.D. to several tens of ng/L in river water [12,45,62,63] and from N.D. to 100 ng/L in the STP effluent [12,19,47,63,64,65]. On the other hand, in the case of BLT, which is frequently used to treat prostate cancer [66], the maximum concentrations were about 2–10-fold higher than the concentrations of the other drugs: 254 ng/L in main stream samples, 151 ng/L in tributaries, and 1032 ng/L in STP effluents (excluding ozonation). Detection frequencies in the main stream and tributary samples were quite high (83–100%) for BLT and CAP, but low—in the range of 6-44%—for DFUR, TAM, and TGF. The corresponding values for CP were moderate (56–63%). In the cases of TAM and DFUR, their detected concentrations appreciably decreased in the STP effluents. This indicates easiness of degradation of these anticancer drugs by the usual treatment at STPs. Although concentration differences were detected among the seasons, the orders were about the same throughout the year. In addition, the quantities of water to be treated at these STPs and the river flow rates in the target basin were fairly stable throughout the year [36]. Consequently, the data shown in this article suggest that the target six anticancer drugs were being used all year round.

Table 5.
Detection of the target anticancer drugs in river waters and STP effluents (n = 24 (main stream), n = 16 (tributary), n = 10 (STP effluent), n = 10 (STP effluent—ozonation)) (reproduced from [30]).
The concentrations of the anticancer drugs detected in the STP effluents (BLT, CP, CAP, and TGF) tended to be several times higher than those in the river waters. Clarification of the levels and mass balances of all discharged anticancer drugs in the urban river environment, together with the optimization of clean-up treatments at STPs, will be helpful in maintaining the health of residents in the sampling area. In the effluent samples from the STP that used ozonation, the mean concentrations of all anticancer drugs ranged as low as from N.D. to several ng/L, which were roughly one-tenth to one-hundredth of the concentrations detected in the effluents from STPs with chlorination after biological treatment (Table 5). This kind of observation was in accord with those of the previous studies [67,68,69], indicating the effectiveness of ozonation treatment for removing a wide range of pharmaceutical compounds, including anticancer drugs from water samples.
3.2. Allocation of Anticancer Drugs in the Sediment and Suspended Solid Samples
The occurrences of anticancer drugs in the solid state samples (river sediments and suspended solids) at Suita Bridge (R5) and Hirakata Bridge (R6) are summarized in Figure 2 [31]. Three anticancer drugs (BLT, TAM, and CAP) were detected at appreciably higher concentrations in the suspended solids at 628, 13–658, and 231 μg/kg, respectively, with higher levels at the Hirakata Bridge than at the Suita Bridge. In the river sediments, however, only lower levels of the same two anticancer drugs (BLT and TAM) and DFUR were detected at 391, 42–250, and 392 ng/kg, respectively, with no clear difference between both of the sites. These profiles indicate an abundance of particles which have high affinity to the anticancer drugs in the river water. However, the concentration of the suspended solids at the Hirakata Bridge (2.6 mg) was about 30% of that of the Suita Bridge (9.0 mg). Based on these results, the pollution load of the anticancer drugs originating from the suspended solids was concluded to be not very large. Localization of DFUR and CAP in the different solid samples is attributable to the difference in the affinity of these drugs to the particles present in the samples.
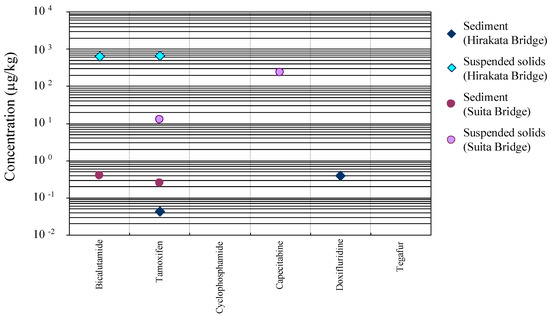
Figure 2.
Distribution of the target anticancer drugs in the river sediments and suspended solids (abbreviation of each anticancer drug is shown in Table 1) (reproduced from [31]).
The sorption property of the present anticancer drugs on the river sediments was further characterized by a sorption experiment [31]. The measured logKd values varied in a range from −0.4 to 2.1 (Table 6). The actual logKd values of the individual anticancer drugs in the sediments at the Hirakata Bridge and the Suita Bridge were 0.4 and 1.4 (BLT), 2.1 and 1.6 (TAM), 0.8 and 0.6 (CP), −0.4 and 0.1 (CAP), 0.9 and 1.5 (DFUR), and 1.3 and 0.4 (TGF), respectively. The concentrations of the anticancer drugs at the Suita Bridge showed a tendency to become higher than those at the Hirakata Bridge. This result is attributable to differences in the particle types in the sediment: sandy sediment at the Hirakata Bridge (moisture content of 9% with 79% sand, 3% silt, and 0% clay) and silty sediment at the Suita Bridge (moisture content of 49%, but no available data for its composition). Similar observation has also been reported in previous studies dealt with different pharmaceuticals [60,67].

Table 6.
Sorption distribution coefficients (logKd) of anticancer drugs for river sediments in sorption experiments (reproduced from [31]).
These observations suggest that the anticancer drugs present in the river water environment were mainly distributed in the liquid phase. The major reason of this biased view could be ascribed to the difference in their octanol–water partition coefficients, logKOW (logP) values [68], which ranged from −0.7 to 5.1 (Table 1). However, the overall results suggest that not only the logP values but also the electric charge and its density (associated with the functional groups in the anticancer drugs) participate as factors which determine the degree of adsorption onto the particles [37,69].
When the logKd values were estimated for the anticancer drugs in the solid state samples, positive values were detected only in the case of BLT; 4.3 for the suspended solids at the Hirakata Bridge, while 0.8 for the river sediments at the Suita Bridge. The value at the Suita Bridge was similar to that obtained by the laboratory experiment for the river sediments at this bridge, suggesting the possibility that the capacity for sorption onto the river sediments could be predicted by estimation. However, the logKd values for the other anticancer drugs whose concentrations were below LOD remained undetermined. This lack of logKd values or abundance of concentrations below LOD was the consequence of attenuation due to photodegradation by sunlight and biodegradation through water flow over time [28,70]. The higher positive value for the suspended solids compared to the sediments was in association with the relatively low abundance of the suspended solids.
3.3. Source Distribution of Anticancer Drugs
Based on the data compiled in this article, the major load source distribution of anticancer drugs in the Kanzaki–Ai River basin could be estimated. The amount of the individual anticancer drugs flowing from the upstream region (S3, R6) to the farthest downstream sampling site (R5) was determined. The contribution of each load source to the total mass flux was then calculated as mass flux (%) (Figure 3) [30].
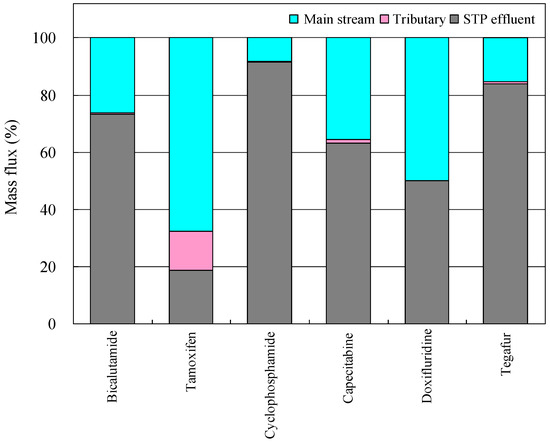
Figure 3.
Source distribution of anticancer drugs in the Kanzaki–Ai River basin (abbreviation of each anticancer drug is shown in Table 1) (reproduced from [30]).
For all anticancer drugs except TAM, the contribution of STP effluents as pollutant loading sources was large, amounting about 50–92% of the total load, while the contribution of tributaries was as low as in the level of 0.3–2.0%. A typical example of these mass flow transitions by using the case of BLT as a representative anticancer drug is shown in Figure 4 [30]. Consequently, STP effluent was strengthened to be a major contributor to the pollution of river waters by the target anticancer compounds in the surveyed area.
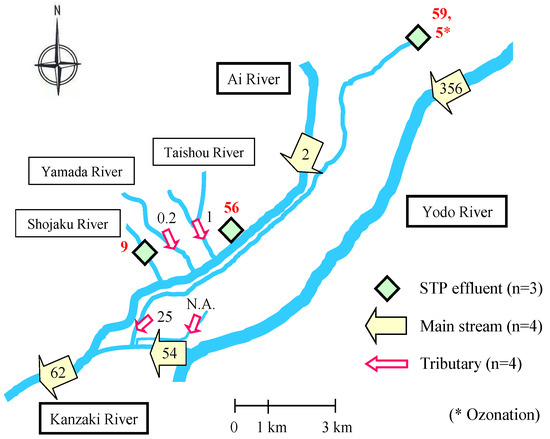
Figure 4.
Transition of mass flows (g/day) from upstream to downstream regions in the Kanzaki–Ai River basin for bicalutamide (BLT). N.A.: not available (reproduced from [30]).
These results also showed that the contribution of STP effluents to the total load of TAM was low (19%; Figure 3). In the case of this anticancer drug, the contributions of the main stream and tributaries were higher than the other anticancer drugs. Recently, a screening assessment of TAM has been published [71], along with a review of its ecotoxicological effects [21,22,72]. However, because of a lack of enough information about the fate of this drug after its discharge into environmental waters, more extended investigations are needed to explain the findings mentioned above. In addition, the bulk of the contributions remained as unassigned in the total mass flux of the target anticancer drugs came from the upstream points surveyed in the main stream [73]. In the present article, pollution loads might have come from areas beyond the sample collection points, because the Kanzaki River originates from a branch on the right bank of the Yodo River [74]. For this reason, advanced water-processing techniques should be introduced not only at STPs located in the middle and downstream reaches, as well as the upstream reaches, to decrease entire pollution loads and improve the water quality of the Kanzaki–Ai River system.
3.4. Mass Balance of Anticancer Drugs
The mass balance for each target anticancer drug in the Kanzaki–Ai River basin could further be estimated by calculation of percent contribution of total efflux load in the influent load through the farthest downstream boundary site (R5). In the present article, the total efflux load for each anticancer drug was obtained by summation of the mass fluxes from two data sets from Kanzaki River (R3 and T4) and Ai River (R1, S1, T1, T2, and S2). The influx load was estimated as the load passing through the boundary site (R5). The resulting mass balance data are shown in Figure 5 [30].
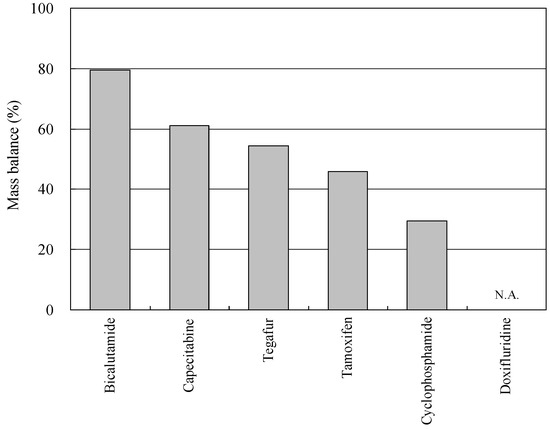
Figure 5.
Mass balances of anticancer drugs in the Kanzaki–Ai River basin. N.A.: not available. The abbreviation of each anticancer drug is shown in Table 1 (reproduced from [30]).
The load of CP was reduced by 70% as it was flowed downstream indicating occurrence of attenuation, but the attenuation rates for the other anticancer drugs were smaller, roughly in the range of 15–50%. BLT has a high recalcitrant property and detected at high concentrations in the river waters [75,76]. Therefore, some of the anticancer drugs are attenuated while being flowed to the lower reaches, but the rate of the attenuation is slow and such anticancer drugs become the matter of environmental pollution.
Today, STPs play an important role for maintaining the quality of river water environment. In Japan, the contributions of pharmaceutical components, including endocrine-disrupting chemicals (EDCs) such as estrogen, to the pollutant loads in the river waters range from 50% to nearly 100% [73,77], because of the high coverage of sewerage systems by STPs (more than 90% of urban areas) [35]. As a result, STPs become indispensable facilities responsible for reducing the levels of pharmaceuticals included in sewage effluent [16]. The conventional activated sludge (CAS) process, which is often used as the convenient physicochemical and biological treatment at STPs, covers 35% of biological water treatment in Japan [35]. However, its insufficient ability to eliminate anticancer drugs at STPs, as described above, necessitates the introduction of additional post-treatment technologies. Importance of such an advanced water treatment systems at STPs is becoming widely recognized [78,79,80], because of high power for removing not only PPCPs but also other chemicals such as EDCs [81], persistent organic pollutants (POPs) [82], bacterial pathogens, and viruses [83].
3.5. Advanced Technologies for Removal of Anticancer Drugs
Advanced oxidation treatment [84], membrane treatment [85], and hybrid treatment of adsorption with ozonation and/or UV [86] are representative treatments, which can be effectively used to solve the water pollution problems and ensure the safety of the water environment. Examples of these treatments applied to CP, one of the target anticancer drugs discussed in this article, are listed in Table 7.

Table 7.
Removal efficiency of cyclophosphamide (CP) from wastewater [86,87,88,89,90,91,92,93].
Ozonation is one of the techniques known as advanced oxidation processes (AOPs), which use HO· generated from ozone (O3). HO· is the second strongest oxidant, after fluorine. Combined techniques with UV (O3/UV), H2O2 (O3/H2O2), and both (O3/UV/H2O2) were also developed and applied to remove CP. UV and H2O2 are known to increase the rate of HO· formation [89]. Application of these treatments to remove the anticancer drugs has an outstanding benefit of higher degradability with lower toxicity of the reaction byproducts, but is accompanied by the shortcomings of the undesirable consumption of the oxidizing agents by the associated scavengers in the samples and the possible formation of less biodegradable byproducts and/or conversion into still more highly toxic byproducts. Additional treatments are sometimes still needed to achieve complete mineralization. These processes are thought to be cost-effective and their practical utilization has already started at some model STPs, as described in this article.
Removal of the target compounds by adsorption is the simplest, most cost-effective, and versatile way. Various kinds of materials are usable as adsorbents, such as activated carbon, mesoporous silica, zeolite, biochar, carbon nanotubes, clays, graphene oxide, chitosan, biomass wastes, and functionalized resin [90]. In addition, two types of operation systems, batch-wise and continuous, are also available practically. By using the affinity of the special adsorbent for a specific drug, selective removal of recalcitrant drugs could be achieved.
This article is concentrated to a specific area in Japan where the population is high. Evidently, there are similar areas in many other countries with a high population and a rather limited flow of streams, where concentrations of anticancer drugs may thus be more or less similar to those presented in this article. Finally, the present article should have value for conducting future ecotoxicity assessments of anticancer drugs and the risks they pose to human health via drinking water.
4. Conclusions
The present article contributed summative clarification of the distribution of six anticancer drugs (BLT, TAM, CP, CAP, DFUR, and TGF) in the river waters and sediments of the Yodo River basin in Japan, including effluents from sewage treatment plants (STPs). In the year-round survey, all anticancer drugs were detected at medium concentrations in the range of N.D.–32 ng/L in the river water and N.D.–245 ng/L in the STP effluents, with the highest levels for BLT (254 ng/L in river water and 1032 ng/L in the STP effluents). STPs were the primary sources of anticancer drugs in the river water, and the attenuation effect of the river environment was small. Ozonation was effective in removing these drugs. BLT, DFUR, and TAM were also detected in the river sediments at maximum concentrations of 391, 392, and 250 ng/kg, respectively. In addition, the sorption distribution coefficient (logKd) in river sediments appeared to be higher in the silty sediments at Suita Bridge than in the sandy sediments at Hirakata Bridge, in accord with the results of the laboratory-scale sorption experiment. The present article provides fundamental data as an initiative for conducting risk assessments of environmental pharmaceuticals in a wider geographic area.
Author Contributions
This manuscript was entirely conceptualized, developed, and performed by T.A.
Funding
This research was funded by the River Foundation (25-1263-024, 26-1263-017), the Kurita Water and Environment Foundation (14E007), and the Ministry of Education, Culture, Sports, Science, and Technology of Japan (16K16218) for funding in the form of research grants and scholarships.
Acknowledgments
We thank the staff of the STPs for sampling the water.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A review-Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A review-Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Tiedeken, E.J.; Tahar, A.; McHugh, B.; Rowan, N.J. Monitoring, sources, receptors, and control measures for three European Union watch list substances of emerging concern in receiving waters—A 20 year systematic review. Sci. Total Environ. 2017, 574, 1140–1163. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, M.I.; Lambrianides, A.; Schneider, M.; Kümmerer, K.; Fatta-Kassinos, D. Environmental side effects of pharmaceutical cocktails: What we know and what we should know. J. Hazard. Mater. 2014, 279, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Evgenidou, E.N.; Konstantinou, I.K.; Lambropoulou, D.A. Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: A review. Sci. Total Environ. 2015, 505, 905–926. [Google Scholar] [CrossRef] [PubMed]
- Bound, J.P.; Voulvoulis, N. Pharmaceuticals in the aquatic environment-a comparison of risk assessment strategies. Chemosphere 2004, 56, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Jobling, S.; Williams, R.; Johnson, A.; Taylor, A.; Gross-Sorokin, M.; Nolan, M.; Tyler, C.R.; van Aerle, R.; Santos, E.; Brighty, G. Predicted exposures to steroid estrogens in U.K. rivers correlate with widespread sexual disruption in wild fish populations. Environ. Health Perspect. 2005, 114, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Besse, J.-P.; Latour, J.-F.; Garric, J. Anticancer drugs in surface waters: What can we say about the occurrence and environmental significance of cytotoxic, cytostatic and endocrine therapy drugs? Environ. Int. 2012, 39, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Arima, N.; Tsukada, A.; Hirami, S.; Matsuoka, R.; Moriwake, R.; Ishiuchi, H.; Inoyama, T.; Teranishi, Y.; Yamaoka, M.; et al. Detection of pharmaceuticals and phytochemicals together with their metabolites in hospital effluents in Japan, and their contribution to sewage treatment plant influents. Sci. Total Environ. 2016, 548–549, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Labour and Welfare (Japan). Vital Statistics in Japan (In Japanese); Ministry of Health Labour and Welfare: Tokyo, Japan, 2017; pp. 1–55.
- Nussbaumer, S.; Bonnabry, P.; Veuthey, J.-L.; Fleury-Souverain, S. Analysis of anticancer drugs: A review. Talanta 2011, 85, 2265–2289. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barceló, D. Incidence of anticancer drugs in an aquatic urban system: From hospital effluents through urban wastewater to natural environment. Environ. Pollut. 2014, 193, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Social Welfare and Public Health in Tokyo (Japan). Summary of Medical Practices Survey in Tokyo, Japan; Social Welfare and Public Health: Tokyo, Japan, 2012; pp. 1–16.
- Booker, V.; Halsall, C.; Llewellyn, N.; Johnson, A.; Williams, R. Prioritising anticancer drugs for environmental monitoring and risk assessment purposes. Sci. Total Environ. 2014, 473–474, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Negreira, N.; de Alda, M.L.; Barceló, D. Cytostatic drugs and metabolites in municipal and hospital wastewaters in Spain: Filtration, occurrence, and environmental risk. Sci. Total Environ. 2014, 497–498, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Toolaram, A.P.; Kümmerer, K.; Schneider, M. Environmental risk assessment of anti-cancer drugs and their transformation products: A focus on their genotoxicity characterization-state of knowledge and short comings. Mutat. Res. Rev. Mutat. Res. 2014, 760, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Borgatta, M.; Waridel, P.; Decosterd, L.-A.; Buclin, T.; Chèvre, N. Multigenerational effects of the anticancer drug tamoxifen and its metabolite 4-hydroxy-tamoxifen on Daphnia pulex. Sci. Total Environ. 2016, 545–546, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Orias, F.; Bony, S.; Devaux, A.; Durrieu, C.; Aubrat, M.; Hombert, T.; Wigh, A.; Perrodin, Y. Tamoxifen ecotoxicity and resulting risks for aquatic ecosystems. Chemosphere 2015, 128, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Heimann, K.; Ridd, M.; Negri, A.P. Pesticide contamination and phytotoxicity of sediment interstitial water to tropical benthic microalgae. Water Res. 2013, 47, 5211–5221. [Google Scholar] [CrossRef] [PubMed]
- Radović, T.T.; Grujić, S.D.; Kovačević, S.R.; Laušević, M.D.; Dimkić, M.A. Sorption of selected pharmaceuticals and pesticides on different river sediments. Environ. Sci. Pollut. Res. 2016, 23, 25232–25244. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Wang, Z.; Zhang, K.; Yu, G.; Duan, X. Sediment pollution and its effect on fish through food chain in the Yangtze River. Int. J. Sediment Res. 2008, 23, 338–347. [Google Scholar] [CrossRef]
- Lopes, C.; Persat, H.; Babut, M. Transfer of PCBs from bottom sediment to freshwater river fish: A food-web modelling approach in the Rhône River (France) in support of sediment management. Ecotoxicol. Environ. Saf. 2012, 81, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Yoon, J. Chronological trends of emission, environmental level and human exposure of POPs over the last 10 years (1999–2010) in Korea: Implication to science and policy. Sci. Total Environ. 2014, 470–471, 1346–1361. [Google Scholar] [CrossRef] [PubMed]
- Hanamoto, S.; Nakada, N.; Yamashita, N.; Tanaka, H. Modeling the photochemical attenuation of down-the-drain chemicals during river transport by stochastic methods and field measurements of pharmaceuticals and personal care products. Environ. Sci. Technol. 2013, 47, 13571–13577. [Google Scholar] [CrossRef] [PubMed]
- Hanamoto, S.; Kawakami, T.; Nakada, N.; Yamashita, N.; Tanaka, H. Evaluation of the photolysis of pharmaceuticals within a river by 2 year field observations and toxicity changes by sunlight. Environ. Sci. Process. Impacts 2014, 16, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Ishiuchi, H.; Inoyama, T.; Teranishi, Y.; Yamaoka, M.; Sato, T.; Mino, Y. Occurrence and fate of selected anticancer, antimicrobial, and psychotropic pharmaceuticals in an urban river in a subcatchment of the Yodo River basin, Japan. Environ. Sci. Pollut. Res. 2015, 22, 18676–18686. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Arima, N.; Tsukada, A.; Hirami, S.; Matsuoka, R.; Moriwake, R.; Ishiuchi, H.; Inoyama, T.; Teranishi, Y.; Yamaoka, M.; et al. Distribution of six anticancer drugs and a variety of other pharmaceuticals, and their sorption onto sediments, in an urban Japanese river. Environ. Sci. Pollut. Res. 2017, 24, 19021–19030. [Google Scholar] [CrossRef] [PubMed]
- Lake Biwa-Yodo River Water Quality Preservation Organization (Japan) 2016. Lake Biwa-Yodo River Water Quality Preservation Organization (BYQ) Report on water environment in Biwa Lake-Yodo River System 2014 (In Japanese); Lake Biwa-Yodo River Water Quality Preservation Organization: Japan, Osaka, 2016; pp. 1–94. [Google Scholar]
- Ministry of the Environment (Japan). Type of Environmental Standard for Water Quality for Conservation of Aquatic Organism; 3rd Report; Ministry of the Environment: Tokyo, Japan, 2009. Available online: http://www.env.go.jp/press/press.php?serial=10970 (accessed on 11 October 2018).
- Osaka Prefecture (Japan). Yodo River System, River Construction Plan for Kanzaki River (In Japanese); Osaka Prefecture: Osaka, Japan, 2012; pp. 1–52.
- Japan Sewage Works Association. Statistics of Sewerage (In Japanese); Japan Sewage Works Association: Tokyo, Japan, 2016.
- Osaka Prefectural Government (Japan). Survey Results based on Water Quality Measurement (In Japanese). Available online: http://www.pref.osaka.lg.jp/kankyohozen/osaka-wan/kokyo-status.html (accessed on 11 October 2018).
- Narumiya, M.; Nakada, N.; Yamashita, N.; Tanaka, H. Phase distribution and removal of pharmaceuticals and personal care products during anaerobic sludge digestion. J. Hazard. Mater. 2013, 260, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Japan Meteorological Agency. Weather Statistics. 2014. Available online: http://www.jma.go.jp/jma/index.html (accessed on 11 October 2018).
- Jiho. Yakuji Handbook (In Japanese); Jiho: Tokyo, Japan, 2014; ISBN 978-4840745666. [Google Scholar]
- Ministry of Health Labour and Welfare (Japan). Annual Report on Statistics of Production by Pharmaceutical Industry in 2015 (In Japanese). Available online: http://www.mhlw.go.jp/topics/yakuji/2014/nenpo/index.html (accessed on 11 October 2018).
- American Chemical Society SciFinder. American Chemical Society. 2018; SciFinder online database (subscription database). [Google Scholar]
- Steger-Hartmann, T.; Kümmerer, K.; Schecker, J. Trace analysis of the antineoplastics ifosfamide and cyclophosphamide in sewage water by twostep solid-phase extraction and gas chromatography-mass spectrometry. J. Chromatogr. A 1996, 726, 179–184. [Google Scholar] [CrossRef]
- Tauxe-Wuersch, A.; De Alencastro, L.F.; Grandjean, D.; Tarradellas, J. Trace determination of tamoxifen and 5-fluorouracil in hospital and urban wastewaters. Int. J. Environ. Anal. Chem. 2006, 86, 473–485. [Google Scholar] [CrossRef]
- Mullot, J.-U.; Karolak, S.; Fontova, A.; Huart, B.; Levi, Y. Development and validation of a sensitive and selective method using GC/MS-MS for quantification of 5-fluorouracil in hospital wastewater. Anal. Bioanal. Chem. 2009, 394, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Kosjek, T.; Heath, E. Occurrence, fate and determination of cytostatic pharmaceuticals in the environment. TrAC Trends Anal. Chem. 2011, 30, 1065–1087. [Google Scholar] [CrossRef]
- Negreira, N.; Mastroianni, N.; López de Alda, M.; Barceló, D. Multianalyte determination of 24 cytostatics and metabolites by liquid chromatography–electrospray–tandem mass spectrometry and study of their stability and optimum storage conditions in aqueous solution. Talanta 2013, 116, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Rabii, F.W.; Segura, P.A.; Fayad, P.B.; Sauvé, S. Determination of six chemotherapeutic agents in municipal wastewater using online solid-phase extraction coupled to liquid chromatography-tandem mass spectrometry. Sci. Total Environ. 2014, 487, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Radović, T.; Grujić, S.; Petković, A.; Dimkić, M.; Laušević, M. Determination of pharmaceuticals and pesticides in river sediments and corresponding surface and ground water in the Danube River and tributaries in Serbia. Environ. Monit. Assess. 2015, 187, 4092. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Wu, X.-L.; Jiang, Y.-N.; Yan, Q.-Y.; Li, Y.-W.; Huang, X.-P.; Cai, Q.-Y.; Mo, C.-H. Occurrence and risk assessment of tetracycline antibiotics in soil from organic vegetable farms in a subtropical city, south China. Environ. Sci. Pollut. Res. 2016, 23, 13984–13995. [Google Scholar] [CrossRef] [PubMed]
- Prasse, C.; Schlüsener, M.P.; Schulz, R.; Ternes, T.A. Antiviral drugs in wastewater and surface waters: A new pharmaceutical class of environmental relevance? Environ. Sci. Technol. 2010, 44, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Golovko, O.; Koba, O.; Kodesova, R.; Fedorova, G.; Kumar, V.; Grabic, R. Development of fast and robust multiresidual LC-MS/MS method for determination of pharmaceuticals in soils. Environ. Sci. Pollut. Res. 2016, 23, 14068–14077. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.; Škrbić, B.; Živančev, J.; Ferrando-Climent, L.; Barcelo, D. Determination of 81 pharmaceutical drugs by high performance liquid chromatography coupled to mass spectrometry with hybrid triple quadrupole–linear ion trap in different types of water in Serbia. Sci. Total Environ. 2014, 468–469, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Schlüsener, M.P.; Hardenbicker, P.; Nilson, E.; Schulz, M.; Viergutz, C.; Ternes, T.A. Occurrence of venlafaxine, other antidepressants and selected metabolites in the Rhine catchment in the face of climate change. Environ. Pollut. 2015, 196, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Leal, R.M.P.; Alleoni, L.R.F.; Tornisielo, V.L.; Regitano, J.B. Sorption of fluoroquinolones and sulfonamides in 13 Brazilian soils. Chemosphere 2013, 92, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Lu, J.; Liu, Z.; Tong, Y.; Li, S. Concentration and distribution of antibiotics in water–sediment system of Bosten Lake, Xinjiang. Environ. Sci. Pollut. Res. 2015, 22, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.F.d.; Jelic, A.; López-Serna, R.; Mozeto, A.A.; Petrovic, M.; Barceló, D. Occurrence and distribution of pharmaceuticals in surface water, suspended solids and sediments of the Ebro river basin, Spain. Chemosphere 2011, 85, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Broodbank, N. Sediment-water interactions of pharmaceutical residues in the river environment. Water Res. 2014, 48, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Ishida, M.; Hisamatsu, K.; Yunoki, A.; Otomo, K.; Kunitou, M.; Shimizu, M.; Hosomaru, K.; Mikata, S.; Mino, Y. Fate of new three anti-influenza drugs and one prodrug in the water environment. Chemosphere 2017, 169, 550–557. [Google Scholar] [CrossRef] [PubMed]
- OECD. Adsorption-Desorption Using a Batch Equilibrium Method, OECD Guideline for the Testing of Chemicals No.106; OECD: Paris, France, 2000; pp. 1–45. [Google Scholar]
- Sangster, J.L.; Oke, H.; Zhang, Y.; Bartelt-Hunt, S.L. The effect of particle size on sorption of estrogens, androgens and progestagens in aquatic sediment. J. Hazard. Mater. 2015, 299, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L.M. Adsorption and degradation of five selected antibiotics in agricultural soil. Sci. Total Environ. 2016, 545–546, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Ashton, D.; Hilton, M.; Thomas, K.V. Investigating the environmental transport of human pharmaceuticals to streams in the United Kingdom. Sci. Total Environ. 2004, 333, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Simultaneous determination of a selected group of cytostatic drugs in water using high-performance liquid chromatography–triple-quadrupole mass spectrometry. J. Sep. Sci. 2011, 34, 3166–3177. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Yin, J.; Duan, H.; Wu, Y.; Shao, B. Analysis of hormone antagonists in clinical and municipal wastewater by isotopic dilution liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2010, 396, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barceló, D. Development of a UPLC-MS/MS method for the determination of ten anticancer drugs in hospital and urban wastewaters, and its application for the screening of human metabolites assisted by information-dependent acquisition tool (IDA) in sewage samples. Anal. Bioanal. Chem. 2013, 405, 5937–5952. [Google Scholar] [CrossRef] [PubMed]
- Cockshott, I. Bicalutamide: Clinical pharmacokinetics and metabolism. Clin. Pharmacokinet. 2004, 43, 855–878. [Google Scholar] [CrossRef] [PubMed]
- Borisover, M.; Keren, Y.; Usyskin, A.; Bukhanovsky, N. Effects of γ-irradiation of original and organic matter-amended soils on the sorption of triclosan and diuron from aqueous solutions. Chemosphere 2016, 152, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.R. Sources, behaviour and fate of organic contaminants during sewage treatment and in sewage sludges. Sci. Total Environ. 1996, 185, 3–26. [Google Scholar] [CrossRef]
- De Oliveira, T.; Guégan, R.; Thiebault, T.; Milbeau, C.L.; Muller, F.; Teixeira, V.; Giovanela, M.; Boussafir, M. Adsorption of diclofenac onto organoclays: Effects of surfactant and environmental (pH and temperature) conditions. J. Hazard. Mater. 2017, 323, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Barra Caracciolo, A.; Topp, E.; Grenni, P. Pharmaceuticals in the environment: Biodegradation and effects on natural microbial communities. A review. J. Pharm. Biomed. Anal. 2015, 106, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Environment Canada (Canada). Screening Sssessment, Ethanamine, 2-[4-[(1Z)-1,2-diphenyl-1-butenyl]phenoxy]-N,N-dimethyl-(Tamoxifen); Environment Canada: Vancouver, BC, Canada, 2015; pp. 1–63.
- Sun, L.; Chi, J.; Hu, X.; Fu, Z. Tamoxifen concepts and cancer: New paradigms (Cancer etiology, diagnosis and treatments: Pharmacology -research, safety testing and regulation), Ectoxicological effects of tamoxifen in the aquatic environment. In Tamoxifen Concepts and Cancer: New Paradigms (Cancer Etiology, Diagnosis and Treatments: Pharmacology—Research, Safety Testing and Regulation); Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015; pp. 47–60. [Google Scholar]
- Azuma, T.; Nakada, N.; Yamashita, N.; Tanaka, H. Mass balance of anti-influenza drugs discharged into the Yodo River system, Japan, under an influenza outbreak. Chemosphere 2013, 93, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Osaka Prefectural Government (Japan). River Development Project of Kanzaki River in Yodo River System (In Japanese); Osaka Prefectural Government: Osaka, Japan, 2015; pp. 1–24.
- Andreozzi, R.; Raffaele, M.; Nicklas, P. Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere 2003, 50, 1319–1330. [Google Scholar] [CrossRef]
- Patrolecco, L.; Capri, S.; Ademollo, N. Occurrence of selected pharmaceuticals in the principal sewage treatment plants in Rome (Italy) and in the receiving surface waters. Environ. Sci. Pollut. Res. 2015, 22, 5864–5876. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Hanamoto, S.; Johnson, A.C.; Yamashita, N.; Nakada, N.; Tanaka, H. Elevated risk from estrogens in the Yodo River basin (Japan) in winter and ozonation as a management option. Environ. Sci. Process. Impacts 2014, 16, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Prasse, C.; Stalter, D.; Schulte-Oehlmann, U.; Oehlmann, J.; Ternes, T.A. Spoilt for choice: A critical review on the chemical and biological assessment of current wastewater treatment technologies. Water Res. 2015, 87, 237–270. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A.; Prasse, C.; Eversloh, C.L.; Knopp, G.; Cornel, P.; Schulte-Oehlmann, U.; Schwartz, T.; Alexander, J.; Seitz, W.; Coors, A.; et al. Integrated evaluation concept to assess the efficacy of advanced wastewater treatment processes for the elimination of micropollutants and pathogens. Environ. Sci. Technol. 2017, 51, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Otomo, K.; Kunitou, M.; Shimizu, M.; Hosomaru, K.; Mikata, S.; Mino, Y.; Hayashi, T. Performance and efficiency of removal of pharmaceutical compounds from hospital wastewater by lab-scale biological treatment system. Environ. Sci. Pollut. Res. 2018, 25, 14647–14655. [Google Scholar] [CrossRef] [PubMed]
- Gmurek, M.; Olak-Kucharczyk, M.; Ledakowicz, S. Photochemical decomposition of endocrine disrupting compounds—A review. Chem. Eng. J. 2017, 310, 437–456. [Google Scholar] [CrossRef]
- Barceló, D.; Petrovic, M. Emerging Contaminants from Industrial and Municipal Waste; Springer: Berlin/Heidelberg, Germany, 2008; Volume 5S/2. [Google Scholar]
- Verbyla, M.E.; Mihelcic, J.R. A review of virus removal in wastewater treatment pond systems. Water Res. 2015, 71, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Zhang, T.C.; Valero, J.R. Membrane processes for removal of pharmaceutically active compounds (PhACs) from water and wastewaters. Sci. Total Environ. 2016, 547, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Kovalova, L.; Siegrist, H.; von Gunten, U.; Eugster, J.; Hagenbuch, M.; Wittmer, A.; Moser, R.; McArdell, C.S. Elimination of micropollutants during post-treatment of hospital wastewater with powdered activated carbon, ozone, and UV. Environ. Sci. Technol. 2013, 47, 7899–7908. [Google Scholar] [CrossRef] [PubMed]
- Seira, J.; Sablayrolles, C.; Montréjaud-Vignoles, M.; Albasi, C.; Joannis-Cassan, C. Elimination of an anticancer drug (cyclophosphamide) by a membrane bioreactor: Comprehensive study of mechanisms. BioChem. Eng. J. 2016, 114, 155–163. [Google Scholar] [CrossRef]
- Wang, L.; Albasi, C.; Faucet-Marquis, V.; Pfohl-Leszkowicz, A.; Dorandeu, C.; Marion, B.; Causserand, C. Cyclophosphamide removal from water by nanofiltration and reverse osmosis membrane. Water Res. 2009, 43, 4115–4122. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.J.; McConville, M.; Verliefde, A.R.D.; van der Aa, L.T.J.; Heijman, S.G.J.; Verberk, J.Q.J.C.; Rietveld, L.C.; van Dijk, J.C. Development of a predictive model to determine micropollutant removal using granular activated carbon. Drink. Water Eng. Sci. 2009, 2, 57–62. [Google Scholar] [CrossRef]
- Lin, A.-C.; Hsueh, J.-F.; Hong, P.K.A. Removal of antineoplastic drugs cyclophosphamide, ifosfamide, and 5-fluorouracil and a vasodilator drug pentoxifylline from wastewaters by ozonation. Environ. Sci. Pollut. Res. 2015, 22, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Česen, M.; Kosjek, T.; Laimou-Geraniou, M.; Kompare, B.; Širok, B.; Lambropolou, D.; Heath, E. Occurrence of cyclophosphamide and ifosfamide in aqueous environment and their removal by biological and abiotic wastewater treatment processes. Sci. Total Environ. 2015, 527–528, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Nanaboina, V.; Chen, F.; Korshin, G.V. Removal of polycyclic synthetic musks and antineoplastic drugs in ozonated wastewater: Quantitation based on the data of differential spectroscopy. J. Hazard. Mater. 2016, 304, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Ferre-Aracil, J.; Valcárcel, Y.; Negreira, N.; de Alda, M.L.; Barceló, D.; Cardona, S.C.; Navarro-Laboulais, J. Ozonation of hospital raw wastewaters for cytostatic compounds removal. Kinetic modelling and economic assessment of the process. Sci. Total Environ. 2016, 556, 70–79. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).