Facile Synthetic Route of a Solution-Processable, Thieno[3,4-c]pyrrolo-4,6-dione-Based Conjugated Small Molecule and Control of the Optoelectronic Properties via Processing Additives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization
2.2.1. 5-(2.-Ethylhexyl)-thieno[3,4-c]pyrrole-4,6-dione
2.2.2. 1,3-Dibromo-5-(2-ethylhexyl)-thieno[3,4-c]pyrrole-4,6-dione (6)
2.2.3. 1-Bromo-5-(2-ethylhexyl)-thieno[3,4-c]pyrrole-4,6-dione (7)
2.2.4. 5,5’-Bis(5-(2-ethylhexyl)-thieno[3,4-c]pyrrole-4,6-dione)-2,2’-bithiophene (8)
2.2.5. 5,5’-Bis{3-(5’-hexyl-2,2’-bithiophene-5-yl)-5-(2-ethylhexyl)-thieno[3,4-c]pyrrole-4,6-dione}-2,2’-bithiophene (M1)
2.3. Solar Cell Fabrication and Characterization
3. Results and Discussion
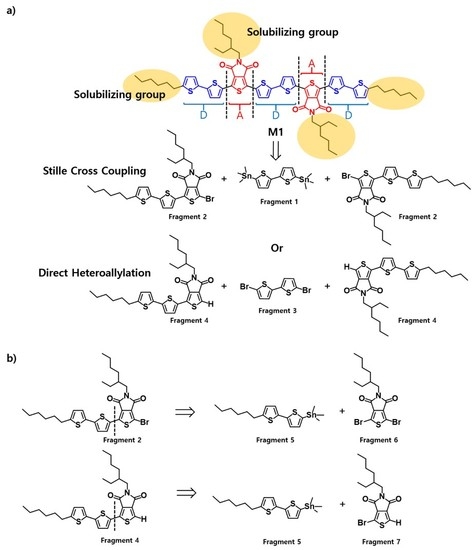
3.1. Molecular Design and Synthesis
3.2. Optical, Electrochemical and Thermal Properties
3.3. Photovoltaic Properties and Surface Morphologies of Blend Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thompson, B.C.; Fréchet, J.M.J. Polymer-fullerene composite solar cells. Angew. Chem. Int. Ed. 2008, 47, 58–77. [Google Scholar] [CrossRef] [PubMed]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef] [Green Version]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.C.; MacKenzie, J.D.; McCulloch, I.; Rivany, J.; Salleo, A. Materials and Applications for Large Area Electronics: Solution-Based Approaches. Chem. Rev. 2010, 110, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, N.; Hosel, M.; Angmo, D.; Krebs, F. Solar cells with one-day energy payback for the factories of the future. Energy Environ. Sci. 2012, 5, 5117–5132. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; You, W. Rational Design of High Performance Conjugated Polymers for Organic Solar Cells. Macromolecules 2012, 45, 607–632. [Google Scholar] [CrossRef]
- Facchetti, A. π-Conjugated Polymers for Organic Electronics and Photovoltaic Cell Applications. Chem. Mater. 2011, 23, 733–758. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Fréchet, J.M.J. Molecular Design and Ordering Effects in π-Functional Materials for Transistor and Solar Cell Applications. J. Am. Chem. Soc. 2011, 133, 20009–20029. [Google Scholar] [CrossRef]
- Amb, C.M.; Chen, S.; Graham, K.R.; Subbiah, J.; Small, C.E.; So, F.; Reynolds, J.R. Dithienogermole as a fused electron donor in bulk heterojunction solar cells. J. Am. Chem. Soc. 2011, 133, 10062–10065. [Google Scholar] [CrossRef]
- Gendron, D.; Leclerc, M. New conjugated polymers for plastic solar cells. Energy Environ. Sci. 2011, 4, 1225–1237. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Stuart, A.C.; Price, S.C.; Liu, S.; You, W. Development of fluorinated benzothiadiazole as a structural unit for a polymer solar cell of 7 % efficiency. Angew. Chem. Int. Ed. 2011, 50, 2995–2998. [Google Scholar] [CrossRef] [PubMed]
- Price, S.C.; Stuart, A.C.; Yang, L.; Zhou, H.; You, W. Fluorine substituted conjugated polymer of medium band gap yields 7% efficiency in polymer-fullerene solar cells. J. Am. Chem. Soc. 2011, 133, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.-Y.; Lu, J.; Beaupre, S.; Zhang, Y.; Pouliot, J.-R.M.; Wakim, S.; Zhou, J.; Leclerc, M.; Li, Z.; Ding, J.; et al. Bulk Heterojunction Solar Cells Using Thieno[3,4-c]pyrrole-4,6-dione and Dithieno[3,2-b:2′,3′-d]silole Copolymer with a Power Conversion Efficiency of 7.3%. J. Am. Chem. Soc. 2011, 133, 4250–4253. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhong, C.; Huang, X.; Wong, W.-Y.; Wu, H.; Chen, L.; Su, S.; Cao, Y. Simultaneous enhancement of open-circuit voltage, short-circuit current density, and fill factor in polymer solar cells. Adv. Mater. 2011, 23, 4636–4643. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Choy, W.C.; Huo, L.; Xie, F.; Sha, W.E.; Ding, B.; Guo, X.; Li, Y.; Hou, J.; You, J.; et al. Dual plasmonic nanostructures for high performance inverted organic solar cells. Adv. Mater. 2012, 24, 3046–3052. [Google Scholar] [CrossRef] [PubMed]
- Service, R.F. Solar energy. Outlook brightens for plastic solar cells. Science 2011, 332, 6027. [Google Scholar] [CrossRef] [PubMed]
- Small, C.E.; Chen, S.; Graham, K.R.; Subbiah, J.; Amb, C.M.; Tseng, S.W.; Lai, T.H.; Reynolds, J.R.; So, F. High-efficiency inverted dithienogermole-thienopyrrolodione-based polymer solar cells. Nat. Photonics 2012, 6, 115–120. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Yang, S.-H.; Hsu, C.-S. Synthesis of Conjugated Polymers for Organic Solar Cell Applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef]
- Zhang, W.; Smith, J.; Watkins, S.E.; Gysel, R.; McGehee, M.; Salleo, A.; Kirkpatrick, J.; Ashraf, S.; Anthopoulos, T.; Heeney, M.; et al. Indacenodithiophene Semiconducting Polymers for High-Performance, Air-Stable Transistors. J. Am. Chem. Soc. 2010, 132, 11437–11439. [Google Scholar] [CrossRef]
- Zhang, M.; Tsao, H.N.; Pisula, W.; Yang, C.; Mishra, A.K.; Müllen, K. Field-Effect Transistors Based on a Benzothiadiazole−Cyclopentadithiophene Copolymer. J. Am. Chem. Soc. 2007, 129, 3472–3473. [Google Scholar] [CrossRef]
- Tsao, H.N.; Cho, D.M.; Park, I.; Hansen, M.R.; Mavrinskiy, A.; Yoon, D.Y.; Graf, R.; Pisula, W.; Spiess, H.W.; Müllen, K. Ultrahigh mobility in polymer field-effect transistors by design. J. Am. Chem. Soc. 2011, 133, 2605–2612. [Google Scholar] [CrossRef]
- Bijleveld, J.C.; Shahid, M.; Gilot, J.; Wienk, M.M.; Janssen, R.A.J. Copolymers of Cyclopentadithiophene and Electron-Deficient Aromatic Units Designed for Photovoltaic Applications. Adv. Funct. Mater. 2009, 19, 3262–3270. [Google Scholar] [CrossRef]
- Bijleveld, J.C.; Zoombelt, A.P.; Mathijssen, S.G.J.; Wienk, M.M.; Turbiez, M.; de Leeuw, D.M.; Janssen, R.A.J. Poly(diketopyrrolopyrrole−terthiophene) for Ambipolar Logic and Photovoltaics. J. Am. Chem. Soc. 2009, 131, 16616–16617. [Google Scholar] [CrossRef] [PubMed]
- Bijleveld, J.C.; Gevaerts, V.S.; Nuzzo, D.D.; Turbiez, M.; Mathijssen, S.G.J.; de Leeuw, D.M.; Wienk, M.M.; Janssen, R.A.J. Efficient Solar Cells Based on an Easily Accessible Diketopyrrolopyrrole Polymer. Adv. Mater. 2010, 22, E242–E246. [Google Scholar] [CrossRef] [PubMed]
- Roncali, J. Molecular Bulk Heterojunctions: An Emerging Approach to Organic Solar Cells. Acc. Chem. Res. 2009, 42, 1719–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, B.; Kim, C.; Nguyen, T.-Q. Small Molecule Solution-Processed Bulk Heterojunction Solar Cells. Chem. Mater. 2011, 23, 470–482. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Q.; Li, Z.; Pei, J.; Tian, W. Solution processable D–A small molecules for bulk-heterojunction solar cells. Energy Environ. Sci. 2010, 3, 1427–1436. [Google Scholar] [CrossRef]
- Riede, M.; Mueller, T.; Tress, W.; Schueppel, R.; Leo, K. Small-molecule solar cells-status and perspectives. Nanotechnology 2008, 19, 424001–424013. [Google Scholar] [CrossRef]
- Hains, A.W.; Liang, Z.; Woodhouse, M.A.; Gregg, B.A. Molecular semiconductors in organic photovoltaic cells. Chem. Rev. 2010, 110, 6689–6735. [Google Scholar] [CrossRef]
- Broggi, A.; Tomasi, I.; Bianchi, L.; Marrochi, A.; Vaccaro, L. Small molecular aryl acetylenes: Chemically thailoring high-efficiency organic semiconductors for solar cells and field-effect transistors. ChemPlusChem 2014, 79, 486–508. [Google Scholar] [CrossRef]
- Burckstummer, H.; Kronenberg, N.M.; Gsanger, M.; Stolte, M.; Meerholz, K.; Wurthner, F. Tailored merocyanine dyes for solution-processed BHJ solar cells. J. Mater. Chem. 2010, 20, 240–243. [Google Scholar] [CrossRef]
- Bagnis, D.; Beverine, L.; Huang, H.; Silvestri, F.; Yao, Y.; Yan, H.; Pagani, G.A.; Marks, T.J.; Facchetti, A. Marked Alkyl- vs. Alkenyl-Substitutent Effects on Squaraine Dye Solid-State Structure, Carrier Mobility, and Bulk-Heterojunction Solar Cell Efficiency. J. Am. Chem. Soc. 2010, 132, 4074–4075. [Google Scholar] [CrossRef] [PubMed]
- Mayerhoffer, U.; Deing, K.; Gruss, K.; Braunschweig, H.; Meerholz, K.; Wurthner, F. Outstanding short-circuit currents in BHJ solar cells based on NIR-absorbing acceptor-substituted squaraines. Angew. Chem. Int. Ed. 2009, 48, 8776–8779. [Google Scholar] [CrossRef]
- Wei, G.D.; Lunt, R.R.; Sun, K.; Wang, S.Y.; Thompson, M.E.; Forrest, S.R. Efficient, ordered bulk heterojunction nanocrystalline solar cells by annealing of ultrathin squaraine thin films. Nano Lett. 2010, 10, 3555–3559. [Google Scholar] [CrossRef] [PubMed]
- Ilmi, R.; Haque, A.; Khan, M.S. High efficiency small molecule-based donor materials for organic solar cells. Org. Electron. 2018, 58, 53–62. [Google Scholar] [CrossRef]
- Bin, H.; Yao, J.; Yang, Y.; Angunawela, I.; Sun, C.; Gao, L.; Ye, L.; Qiu, B.; Xue, L.; Zhu, C.; et al. High-efficiency all-small-molecule organic solar cells based on an organic molecule donor with alkylsily-thienyl conjugated side chains. Adv. Mater. 2018, 30, 1706361–1706368. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, M.T.; Anthony, J.E.; Malliaras, G.G. Photovoltaics from soluble small molecules. Mater. Today 2007, 10, 34–41. [Google Scholar] [CrossRef]
- Shang, H.; Fan, H.; Liu, Y.; Hu, W.; Li, Y.; Zhan, X. A solution-processable star-shaped molecule for high-performance organic solar cells. Adv. Mater. 2011, 23, 1554–1557. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, D.; Xu, Y.; Feng, X. Thiophene-based conjugated oligomers for organic solar cells. J. Mater. Chem. 2011, 21, 17590–17600. [Google Scholar] [CrossRef]
- Feng, G.; Xu, Y.; Zhang, J.; Wang, Z.; Zhou, Y.; Li, Y.; Wei, Z.; Li, C.; Li, W. All-small-molecule organic solar cells based on electron donor incorporating binary electron deficient units. J. Mater. Chem. 2016, 4, 6056–6063. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, X.; Long, G. High performance photovoltaic applications using solution-processed small molecules. Acc. Chem. Res. 2013, 46, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.P.; Yiu, A.T.; Beaujuge, P.M.; Woo, C.H.; Holcomb, T.W.; Millstone, J.E.; Douglas, J.D.; Chen, M.S.; Fréchet, J.M.J. Efficient small molecule bulk heterojunction solar cells with high fill factors via pyrene-directed molecular self-assembly. Adv. Matter. 2011, 23, 5359–5363. [Google Scholar] [CrossRef] [PubMed]
- Mikroyannidis, J.A.; Stylianakis, M.M.; Dong, Q.; Zhou, Y.; Tian, W. New 4,7-dithienobenzothiadiazole derivatives with cyano-vinylene bonds: synthesis, photophysics and photovoltaics. Syn. Met. 2009, 159, 1471–1477. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Lu, C.-W.; Huang, W.-C.; Chen, Y.-H.; Lin, H.-W.; Wong, K.-T. New A-A-D-A-A-type electron donors for small molecule organic solar cells. Org. Lett. 2011, 13, 4962–4965. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Cheng, P.; Li, Y.; Zhan, X. A Solution Processable D-A-D Molecule based on Thiazolothiazole for High Performance Organic Solar Cells. Adv. Energy Mater. 2012, 2, 63–67. [Google Scholar] [CrossRef]
- McDowell, C.; Abdelsamie, M.; Toney, M.F.; Bazan, G.C. Solvent Additives: Key morphology-directing agents for solution-processed organic solar cells. Adv. Mater. 2018, 30, 1707114–1707143. [Google Scholar] [CrossRef]
- Liu, X.; Huettner, S.; Rong, Z.; Sommer, M.; Friend, R.H. Solvent additive control of morphology and crystallization in semiconducting polymer blends. Adv. Mater. 2014, 24, 669–674. [Google Scholar] [CrossRef]
- Liao, H.-C.; Ho, C.-C.; Chan, C.-Y.; Jao, M.-H.; Darling, S.B.; Su, W.-F. Additives for morphology control in high-efficiency organic solar cells. Mater. Today 2013, 16, 326–336. [Google Scholar] [CrossRef]
- Henson, Z.B.; Welch, G.C.; van der Poll, T.; Bazan, G.C. Pyridalthiadiazole-Based Narrow Band Gap Chromophores. J. Am. Chem. Soc. 2012, 134, 3766–3779. [Google Scholar] [CrossRef]
- Takacs, C.J.; Sun, Y.; Welch, G.C.; Perez, L.A.; Liu, X.; Wen, W.; Bazan, G.C.; Heeger, A.J. Solar cell efficiency, self-assembly, and dipole-dipole interactions of isomorphic narrow-band-gap molecules. J. Am. Chem. Soc. 2012, 134, 16597–16606. [Google Scholar] [CrossRef]
- Welch, G.C.; Bakus, R.C., II; Teat, S.J.; Bazan, G.C. Impact of regiochemistry and isoelectronic bridgehead substitution on the molecular shape and bulk organization of narrow bandgap chromophores. J. Am. Chem. Soc. 2013, 135, 2298–2305. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Najari, A.; Berrouard, P.; Beaupré, S.; Aïch, B.R.; Tao, Y.; Leclerc, M. A thieno[3,4-c]pyrrole-4,6-dione-based copolymer for efficient solar cells. J. Am. Chem. Soc. 2010, 132, 5330–5331. [Google Scholar] [CrossRef] [PubMed]
- Piliego, C.; Holcombe, T.W.; Douglas, J.D.; Woo, C.H.; Beaujuge, P.M.; Fréchet, J.M.J. Synthetic control of structural order in N-alkylthieno[3,4-c]pyrrole-4,6-dione-based polymers for efficient solar cells. J. Am. Chem. Soc. 2010, 132, 7595–7597. [Google Scholar] [CrossRef] [PubMed]
- Su, M.-S.; Kuo, C.-Y.; Yuan, M.-C.; Jeng, U.-S.; Su, C.-J.; Wei, K.-H. Improving device efficiency of polymer/fullerene bulk heterojunction solar cells through enhanced crystallinity and reduced grain boundaries induced by solvent additives. Adv. Mater. 2011, 23, 3315. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Pron, A.; Berrouard, P.; Leong, W.L.; Yuen, J.D.; Moon, J.S.; Leclerc, M.; Heeger, A.J. A New Terthiophene-Thienopyrrolodione Copolymer-Based Bulk Heterojunction Solar Cell with High Open-Circuit Voltage. Adv. Energy Mater. 2012, 2, 1397–1403. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Cheng, Y.-H.; Chou, Y.-J.; Su, M.-S.; Chen, C.-M.; Wei, K.-H. Crystalline conjugated polymer containing fused 2,5-di(thiophen-2-yl)thieno[2,3-b]thiophene and thieno[3,4-c]pyrrole-4,6-dione units for bulk heterojunction solar cells. Chem. Commun. 2011, 47, 5064–5066. [Google Scholar] [CrossRef] [PubMed]
- Pron, A.; Berrouard, P.; Leclerc, M. Thieno[3,4-c]pyrrole-4,6-dione-Based Polymers for Optoelectronic Applications. Macromol. Chem. Phys. 2013, 214, 7–16. [Google Scholar] [CrossRef]
- Berrouard, P.; Dufresne, S.; Pron, A.; Veilleux, J.; Leclerc, M. Low-Cost Synthesis and Physical Characterization of Thieno[3,4-c]pyrrole-4,6-dione-Based Polymers. J. Org. Chem. 2012, 77, 8167–8173. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.-C.; Chiu, M.-Y.; Liu, S.-P.; Chen, C.-M.; Wei, K.-H. A Thieno[3,4-c]pyrrole-4,6-dione-Based Donor−Acceptor Polymer Exhibiting High Crystallinity for Photovoltaic Applications. Macromolecules 2010, 43, 6936–6938. [Google Scholar] [CrossRef]
- Lin, Y.; Cheng, P.; Liu, Y.; Zhao, X.; Li, D.; Tan, J.; Hu, W.; Li, Y.; Zhan, X. Solution-processable small molecules based on thieno[3,4-c]pyrrole-4,6-dione for high-performance solar cells. Sol. Energy Mater. Sol. Cells 2012, 99, 301–307. [Google Scholar] [CrossRef]
- Berrouard, P.; Najari, A.; Pron, A.; Gendron, D.; Morin, P.-O.; Pouliot, J.-R.; Veilleux, J.; Leclerc, M. Synthesis of 5-alkyl[3,4-c]thienopyrrole-4,6-dione-based polymers by direct heteroarylation. Angew. Chem. Int. Ed. 2011, 51, 2068–2071. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Kim, C.; Walker, B.; Nguyen, T.-Q.; Seo, J.H. Morphology control of solution processable small molecule bulk heterojunction solar cellsviasolvent additives. RSC Adv. 2012, 2, 2232–2234. [Google Scholar] [CrossRef]
- Kwon, O.; Park, S.; Park, J.K.; Wang, D.H. Controlling the optoelectronic properties of narrow bandgap organic chromophores upon isoelectronic bridgehead substitution. Dyes Pigment. 2018, 158, 233–239. [Google Scholar] [CrossRef]
- Dang, M.T.; Wantz, G.; Bejbouji, H.; Urien, M.; Dautel, O.J.; Vignau, L.; Hirsch, L. Polymeric solar cells based on P3HT:PCBM: role of the casting solvent. Sol. Energy Mater. Sol. Cells 2011, 95, 3408–3418. [Google Scholar] [CrossRef]
- Dhass, A.D.; Natarajan, E.; Lakshmi, P. Influence of shunt resistance on the performance of solar photovoltaic cell. In Proceedings of the 2012 International Conference on Emerging Trends in Electrical Engineering and Energy Management (ICETEEEM), Chennai, India, 13–15 December 2012; pp. 382–386. [Google Scholar]









| M1:PC71BM (w/w ratio in Chloroform) | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) | Rs (Ω·cm2) | Rsh (Ω·cm2) |
|---|---|---|---|---|---|---|
| 70:30 | 1.34 | 0.90 | 30 | 0.36 | 940 | 4.75 × 106 |
| 60:40 | 1.55 | 0.87 | 37 | 0.50 | 430 | 3.23 × 106 |
| 50:50 | 1.53 | 0.87 | 43 | 0.57 | 240 | 1.84 × 106 |
| 40:60 | 1.83 | 0.86 | 57 | 0.89 | 70 | 0.14 × 106 |
| 30:70 | 1.68 | 0.84 | 49 | 0.69 | 160 | 0.89 × 106 |
| M1:PC71BM (40:60, w/w Ratio in Chlorobenzene) | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) | |
|---|---|---|---|---|---|
| No additives | 2.35 | 0.86 | 61 | 1.23 | |
| DIO (%, v/v) | 0.8 | 2.09 | 0.54 | 34 | 0.38 |
| 0.4 | 3.00 | 0.78 | 42 | 0.98 | |
| 0.2 | 3.03 | 0.81 | 56 | 1.36 | |
| 0.1 | 2.73 | 0.72 | 40 | 0.77 | |
| CN (%, v/v) | 1.0 | 3.0 | 0.76 | 52 | 1.17 |
| 0.5 | 3.53 | 0.85 | 62 | 1.86 | |
| 0.3 | 3.39 | 0.90 | 59 | 1.80 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, O.; Lim, J.; Park, J.K.; Wang, D.H. Facile Synthetic Route of a Solution-Processable, Thieno[3,4-c]pyrrolo-4,6-dione-Based Conjugated Small Molecule and Control of the Optoelectronic Properties via Processing Additives. Appl. Sci. 2018, 8, 2644. https://doi.org/10.3390/app8122644
Kwon O, Lim J, Park JK, Wang DH. Facile Synthetic Route of a Solution-Processable, Thieno[3,4-c]pyrrolo-4,6-dione-Based Conjugated Small Molecule and Control of the Optoelectronic Properties via Processing Additives. Applied Sciences. 2018; 8(12):2644. https://doi.org/10.3390/app8122644
Chicago/Turabian StyleKwon, Obum, Jihyun Lim, Jin Kuen Park, and Dong Hwan Wang. 2018. "Facile Synthetic Route of a Solution-Processable, Thieno[3,4-c]pyrrolo-4,6-dione-Based Conjugated Small Molecule and Control of the Optoelectronic Properties via Processing Additives" Applied Sciences 8, no. 12: 2644. https://doi.org/10.3390/app8122644
APA StyleKwon, O., Lim, J., Park, J. K., & Wang, D. H. (2018). Facile Synthetic Route of a Solution-Processable, Thieno[3,4-c]pyrrolo-4,6-dione-Based Conjugated Small Molecule and Control of the Optoelectronic Properties via Processing Additives. Applied Sciences, 8(12), 2644. https://doi.org/10.3390/app8122644