Comparison of the Characteristic Mass Fragmentations of Phenethylamines and Tryptamines by Electron Ionization Gas Chromatography Mass Spectrometry, Electrospray and Matrix-Assisted Laser Desorption Ionization Mass Spectrometry
Abstract
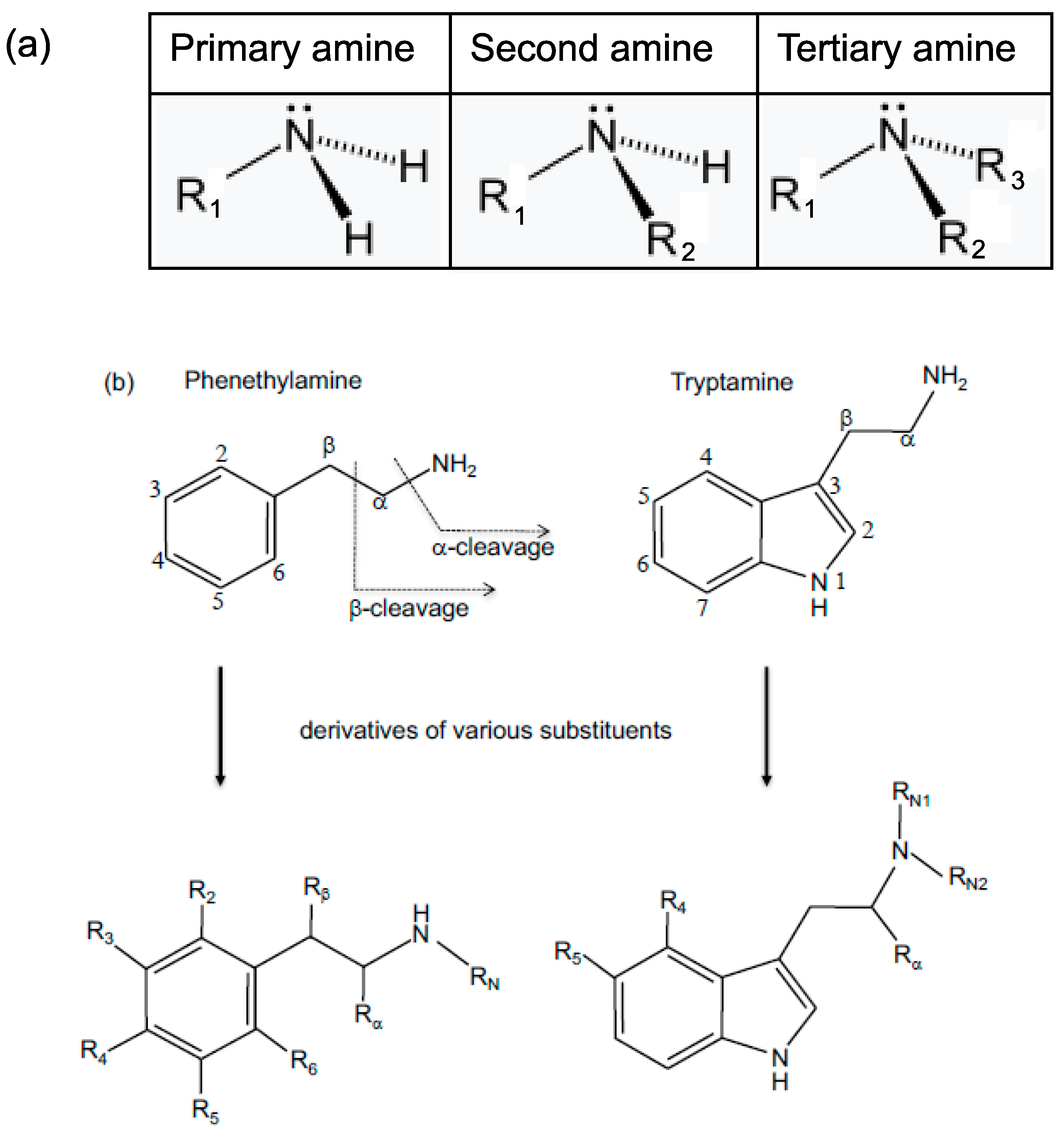
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Reagents
2.3. Sample Preparation
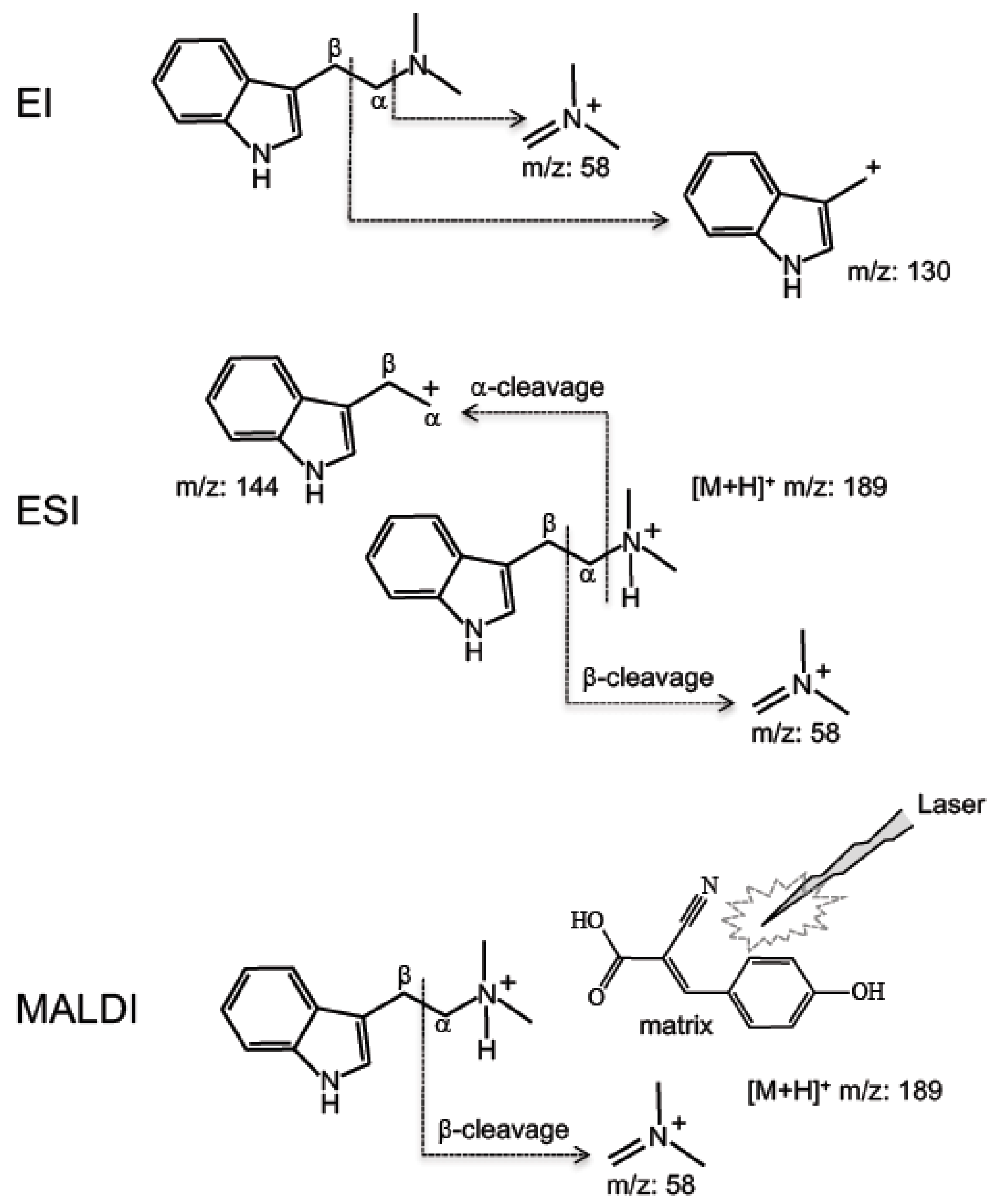
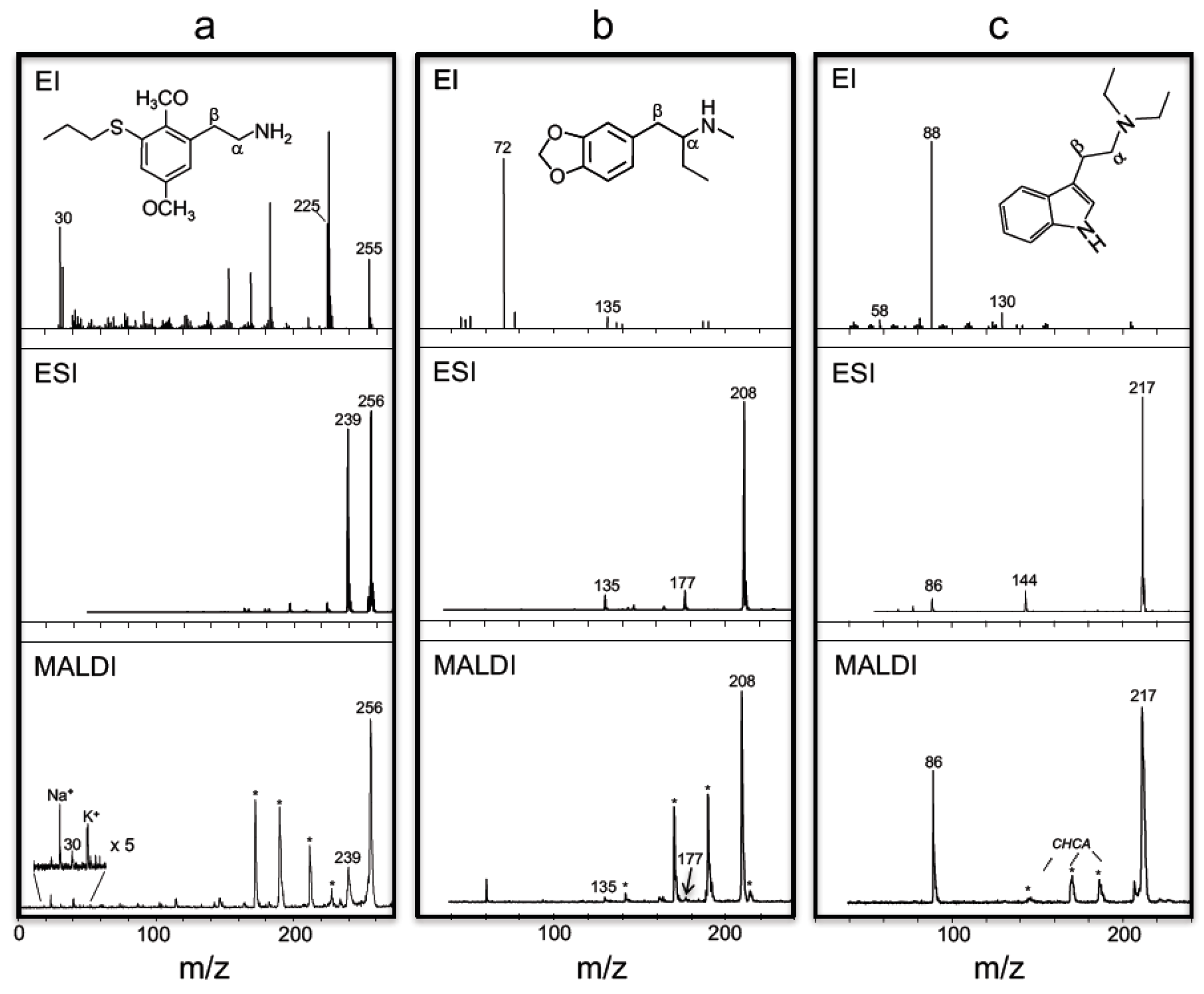
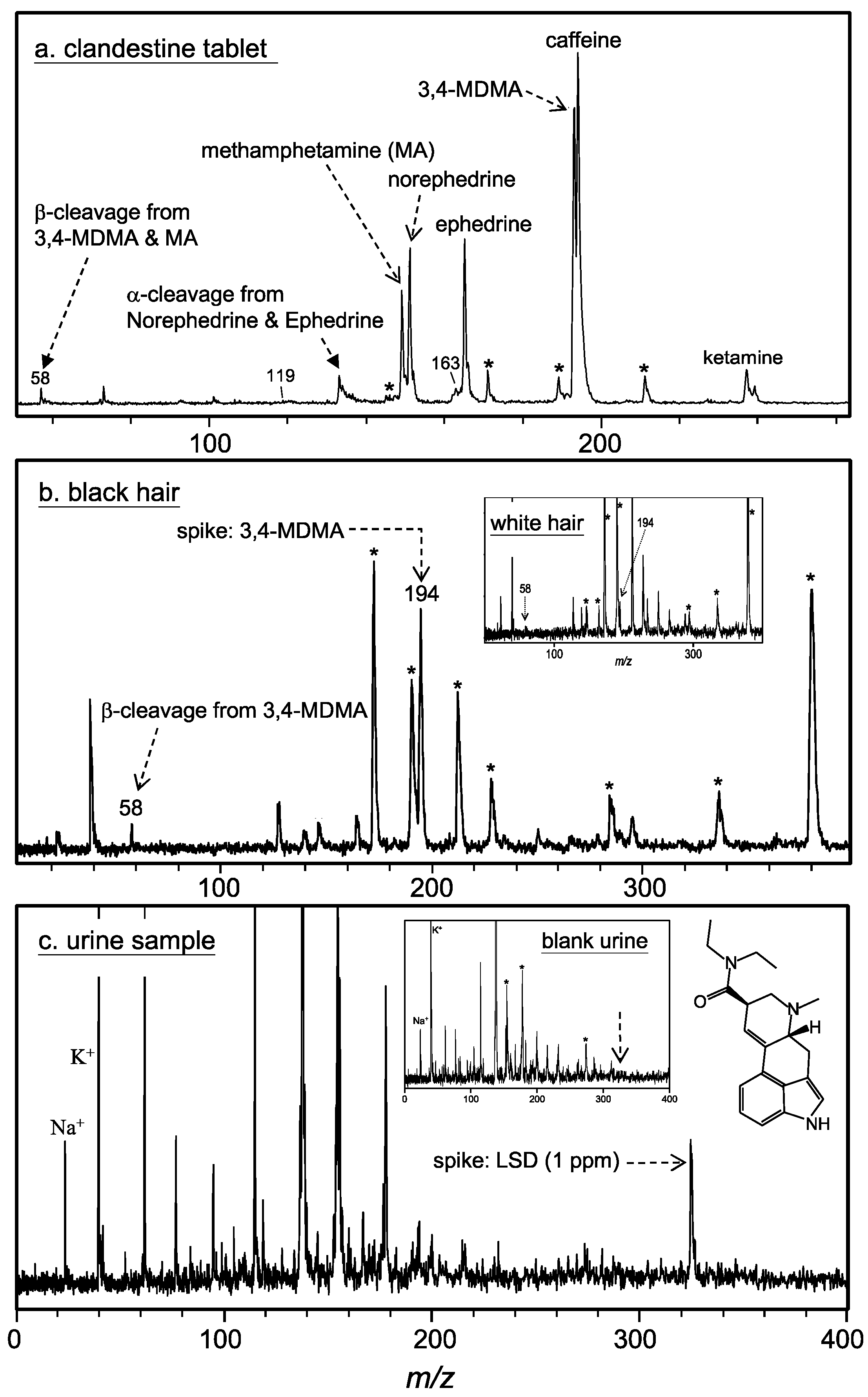
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, L.-C.; Liu, J.-T.; Lin, C.-H. Identification of 2,5-dimethoxy-4-ethylthiophenethylamine and its metabolites in the urine of rats by gas chromatography–mass spectrometry. J. Chromatogr. B 2003, 798, 241–247. [Google Scholar] [CrossRef]
- Valentine, J.L.; Middleton, R. GC-MS identification of sympathomimetic amine drugs in urine: Rapid methodology applicable for emergency clinical toxicology. J. Anal. Toxicol. 2000, 24, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Jurado, C.; Gimenez, M.P.; Soriano, T.; Menendez, M.; Repetto, M. Rapid analysis of amphetamine, methamphetamine, MDA, and MDMA in urine using solid-phase microextraction, direct on-fiber derivatization, and analysis by GC-MS. J. Anal. Toxicol. 2000, 24, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Pujadas, M.; Pichini, S.; Poudevida, S.; Menoyo, E.; Zuccaro, P.; Farre, M.; Torre, R. Development and validation of a gas chromatography-mass spectrometry assay for hair analysis of amphetamine, methamphetamine and methylenedioxy derivatives. J. Chromatogr. B 2003, 798, 249–255. [Google Scholar] [CrossRef]
- Peters, F.T.; Schaefer, S.; Staack, R.F.; Kraemer, T.; Maurer, H.H. Screening for and validated quantification of amphetamines and of amphetamine- and piperazine-derived designer drugs in human blood plasma by gas chromatography/mass spectrometry. J. Mass Spectrom. 2003, 38, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Cody, J.T.; Valtier, S. Differentiation of the 2,3-methylenedioxy regioisomer of 3,4-MDMA (ecstasy) by gas chromatography-mass spectrometry. J. Anal. Toxicol. 2002, 26, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Stout, P.R.; Horn, C.K.; Klette, K.L. Rapid simultaneous determination of amphetamine, methamphetamine, 3,4-methylenedioxyamphetamine, 3,4-methylenedioxymethamphetamine, and 3,4-methylenedioxyethylamphetamine in urine by solid-phase extraction and GC-MS: A method optimized for high-volume laboratories. J. Anal. Toxicol. 2002, 26, 253–261. [Google Scholar] [PubMed]
- Weinmann, W.; Renz, M.; Vogt, S.; Pollak, S. Automated solid-phase extraction and two-step derivatisation for simultaneous analysis of basic illicit drugs in serum by GC/MS. Int. J. Legal Med. 2000, 113, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Marquet, P.; Lacassie, E.; Battu, C.; Faubert, H.; Lachâtre, G. Simultaneous determination of amphetamine and its analogs in human whole blood by gas chromatography-mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1997, 700, 77–82. [Google Scholar] [CrossRef]
- Su, A.-K.; Liu, J.-T.; Lin, C.-H. Rapid drug-screening of clandestine tablets by MALDI-TOF mass spectrometry. Talanta 2005, 67, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Su, A.-K.; Liu, J.-T.; Lin, C.-H. Rapid drug-screening and quantitation of 3,4-methylenedioxymethamphetamine in urine by MALDI-TOF mass spectrometr. Anal. Chim. Acta. 2005, 546, 193–198. [Google Scholar] [CrossRef]
- Chen, B.-H.; Liu, J.-T.; Chen, W.-X.; Chen, H.-M.; Lin, C.-H. A general approach to the screening and confirmation of tryptamines and phenethylamines by mass spectral fragmentation. Talanta 2008, 74, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Shulgin, A.; Shulgin, A. PHINKAL (Phenethylamines I Have Known and Loved): The Continuation; Transform Press: Berkeley, CA, USA, 1991. [Google Scholar]
- Shulgin, A.; Shulgin, A. THINKAL (Tryptamines I Have Known and Loved): The Continuation; Transform Press: Berkeley, CA, USA, 1997. [Google Scholar]
- Fang, C.; Chung, Y.-L.; Liu, J.-T.; Lin, C.-H. Rapid analysis of 3,4-methylenedioxymethamphetamine: A comparison of nonaqueous capillary electrophoresis/fluorescence detection with GC/MS. Forensic Sci. Int. 2000, 125, 142–148. [Google Scholar] [CrossRef]
| A. Phenethylamines | |||||||||||||||
| Abbrev. | R | R2 | R3 | Ra | R4 | R5 | R6 | RN1 | Full Name | ||||||
| MA | CH3 | H | H | H | H | H | CH3 | methamphetamine | |||||||
| 2,4-DOB | CH3 | OCH3 | H | OCH3 | Br | H | H | 2,4-dimethoxy-5-bromoamphetamine | |||||||
| 3,4-MDA | H | H | -OCH2O- | H | H | H | 3,4-methylenedioxyamphetamine | ||||||||
| 3,4-BDB | CH2CH3 | H | -OCH2O- | H | H | H | 3,4-benzodioxol-5-yl-2-butylamine | ||||||||
| 3,4-MDMA | CH3 | H | -OCH2O- | H | H | CH3 | 3,4-methylenedioxymethamphetamine | ||||||||
| MBDB | CH2CH3 | H | -OCH2O- | H | H | CH3 | N-methyl-1-(1,3-benzodioxol-5-yl)-2-butylamine | ||||||||
| 2C-T-7 | H | H | OCH3 | SCH2CH2CH3 | H | OCH3 | H | 4-propylthio-2,5-dimethoxy-β-phenethylamine | |||||||
| 2C-T-2 | H | H | OCH3 | SCH2CH3 | H | OCH3 | H | 4-ethylthio-2,5-dimethoxy-β-phenethylamine | |||||||
| 2C-C | H | H | OCH3 | Cl | H | OCH3 | H | 4-chloro-2,5-dimethoxy-β-phenethylamine | |||||||
| 2C-B | H | H | OCH3 | Br | H | OCH3 | H | 4-bromo-2,5-dimethoxy-β-phenethylamine | |||||||
| 2C-I | H | H | OCH3 | I | H | OCH3 | H | 4-iodo-2,5-dimethoxy-β-phenethylamine | |||||||
| B. Tryptamines | |||||||||||||||
| Abbrev. | R5 | R | RN1 | RN2 | Full Name | ||||||||||
| AMT | H | CH3 | H | H | α-methyltryptamine | ||||||||||
| DMT | H | H | CH3 | CH3 | N,N-dimethyltryptamine | ||||||||||
| 5-MeO-AMT | OCH3 | CH3 | H | H | 5-methoxy-α-methyltryptamine | ||||||||||
| DET | H | H | CH2CH3 | CH2CH3 | N,N-diethyltryptamine | ||||||||||
| DPT | H | H | CH2CH2CH3 | CH2CH2CH3 | N,N-dipropyltryptamine | ||||||||||
| DBT | H | H | CH2CH2CH2CH3 | CH2CH2CH2CH3 | N,N-dibutyltryptamine | ||||||||||
| 5-MeO-DMT | OCH3 | H | CH3 | CH3 | 5-methoxy-N,N-dimethyltryptamine | ||||||||||
| DIPT | H | H | CH(CH3)2 | CH(CH3)2 | N,N-diisopropyltryptamine | ||||||||||
| 5-MeO-DIPT | OCH3 | H | CH(CH3)2 | CH(CH3)2 | 5-methoxy-N,N-diisopropyltryptamine | ||||||||||
| Abbrev. | EI/MS | ESI/MS | MALDI/TOFMS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cβ··Cα | Cα··N | M+ | β-Cleavage | α-Cleavage | [M+H]+ | β-Cleavage | α-Cleavage | [M+H]+ | ||
| 1° | 2C-T-7 | 30, 225 | -- | 255 | -- | 239 | 256 | 30 | 239 | 256 |
| 1° | 2C-T-2 | 30, 211 | -- | 241 | -- | 225 | 242 | 30 | 225 | 242 |
| 1° | 2C-C | 30, 185 | -- | 215 | -- | 199 | 216 | 30 | 199 | 216 |
| 1° | 2C-B | 30, 229 | -- | 259 | -- | 243 | 260 | 30 | 243 | 260 |
| 1° | 2C-I | 30, 277 | -- | 307 | -- | 291 | 308 | 30 | 291 | 308 |
| 1° | AMT | 44, 130 | -- | 174 | -- | 158 | 175 | 44 | 158 | 175 |
| 1° | 5-MeO-AMT | 44, 160 | -- | -- | -- | 188 | 205 | 44 | 188 | 205 |
| 1° | 2,4-DOB | 44, 230 | -- | -- | -- | 257 | 274 | 44 | 257 | 274 |
| 1° | 3,4-MDA | 44, 135 | -- | -- | -- | 163 | 180 | 44 | 163 | 180 |
| 1° | 3,4-BDB | 58, 135 | -- | -- | -- | 177 | 194 | 58 | 177 | 194 |
| 2° | 3,4-MDMA | 58, 135 | -- | -- | -- | 163 | 194 | 58 | 163 | 194 |
| 2° | 3,4-MBDB | 72, 135 | -- | -- | -- | 177 | 208 | 72 | 177 | 208 |
| 2° | MA | 58, 91 | -- | -- | -- | 119 | 150 | 58 | 119 | 150 |
| 3° | DMT | 58, 130 | -- | 188 | 58 | 144 | 189 | 58 | -- | 189 |
| 3° | DET | 86, 130 | -- | -- | 86 | 144 | 217 | 86 | -- | 217 |
| 3° | DPT | 114, 130 | -- | -- | 114 | 144 | 245 | 114 | -- | 245 |
| 3° | DBT | 130, 142 | -- | -- | 142 | 144 | 273 | 142 | -- | 273 |
| 3° | 5-MeO-DMT | 58, 160 | -- | 218 | 58 | 174 | 219 | 58 | -- | 219 |
| 3° | DIPT | 114, 130 | -- | -- | 114 | 144 | 245 | 114 | -- | 245 |
| 3° | 5-MeO-DIPT | 114, 160 | -- | -- | 114 | 174 | 275 | 114 | -- | 275 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.-H.; Liu, J.-T.; Chen, H.-M.; Chen, W.-X.; Lin, C.-H. Comparison of the Characteristic Mass Fragmentations of Phenethylamines and Tryptamines by Electron Ionization Gas Chromatography Mass Spectrometry, Electrospray and Matrix-Assisted Laser Desorption Ionization Mass Spectrometry. Appl. Sci. 2018, 8, 1022. https://doi.org/10.3390/app8071022
Chen B-H, Liu J-T, Chen H-M, Chen W-X, Lin C-H. Comparison of the Characteristic Mass Fragmentations of Phenethylamines and Tryptamines by Electron Ionization Gas Chromatography Mass Spectrometry, Electrospray and Matrix-Assisted Laser Desorption Ionization Mass Spectrometry. Applied Sciences. 2018; 8(7):1022. https://doi.org/10.3390/app8071022
Chicago/Turabian StyleChen, Bo-Hong, Ju-Tsung Liu, Hung-Ming Chen, Wen-Xiong Chen, and Cheng-Huang Lin. 2018. "Comparison of the Characteristic Mass Fragmentations of Phenethylamines and Tryptamines by Electron Ionization Gas Chromatography Mass Spectrometry, Electrospray and Matrix-Assisted Laser Desorption Ionization Mass Spectrometry" Applied Sciences 8, no. 7: 1022. https://doi.org/10.3390/app8071022
APA StyleChen, B.-H., Liu, J.-T., Chen, H.-M., Chen, W.-X., & Lin, C.-H. (2018). Comparison of the Characteristic Mass Fragmentations of Phenethylamines and Tryptamines by Electron Ionization Gas Chromatography Mass Spectrometry, Electrospray and Matrix-Assisted Laser Desorption Ionization Mass Spectrometry. Applied Sciences, 8(7), 1022. https://doi.org/10.3390/app8071022




