1. Introduction
Articular cartilage tissue consists of chondrocytes growing in an extracellular matrix (ECM), which is responsible for the adhesion and differentiation of cells and cell-to-cell communication. The principal components of ECM are certain fibrous proteins; e.g., type II collagen, proteoglycans, hyaluronic acid, and chondroitin sulfate [
1]. Articular cartilage protects the bone ends from friction and compressive loads. The injuries and articular diseases are frequent and may cause pain, arthrodesis, and joint arthoplasty. Due to the limited regenerative capacity of cartilage, tissue repair methods have been sought for. Traditionally, autografts and allografts are used to treat these lesions. However, the donor cartilage tissue is not only available in insufficient amounts but also may induce an immune response, leading to the implant’s failure. Tissue engineering (TE) is the promising alternative of cartilage defects treatment. However, its success depends on four key factors such as cells, mechanical environment, growth factors, and scaffolds. An ideal scaffold must fulfill a wide range of criteria, such as biocompatibility, porosity and suitable 3D structure, high water absorption capacity, and high mechanical strength. Furthermore, it has to promote the proliferation of chondrocytes and ensure the diffusion of nutrients, active substances and growth factors. Over the last years, various materials have been studied as potential matrices for TE, being able to mimic the ECM. They are classified as either synthetic (polylactic acid, polyethylene glycol) or natural that are based on proteins (collagen, fibrin, silk) and polysaccharides (hyaluronic acid, chitosan, alginate, cellulose) [
2,
3].
Like plant cellulose, bacterial bionanocellulose (BNC) is a linear polymer composed of β-1,4-linked
d-glucopyranose residues. It is produced as an exopolysaccharide having a form of the three-dimensional network of nano-sized fibers by various species of bacteria, such as those of the genera
Komagataeibacter,
Agrobacterium,
Rhizobium, and
Sarcina [
4]. BNC exhibits many desirable and unique properties such as biocompatibility [
5], high mechanical strength, and water holding capacity up to 100 times its dry mass [
6] as well as high crystallinity and porosity. Biocompatibility of BNC-based products makes them suitable for numerous biomedical applications, such as wound dressings [
7,
8], artificial vessels [
9,
10], cornea replacements [
11], drug delivery systems [
12,
13] and scaffolds for tissue engineering [
14].
The interactions of the biomaterial with surrounding cells depend on the proper distribution of functional groups in three-dimensional space [
15]. The native BNC-based scaffolds promote neither the excellent cell adhesion nor growth because cellulose is biologically inactive, mainly due to its neutral charge [
16,
17]. Therefore, there is a need to modulate the intrinsic properties that may be particularly beneficial in the biomedical application by introducing for instance a negative charge promoting interactions with proteins in the extracellular matrix [
18]. For this purpose, we created a composite of bacterial nanocellulose with sulfate groups-rich κ-carrageenan (κ-Car). κ-Car, widely used in the food industry due to its excellent gelling capacity, belongs to the family of linear polysaccharides extracted from red seaweeds,
Rodophyta. Carrageenans, built from 3-linked β-
d-galactopyranose and 4-linked α-
d-galactopyranose or 4-linked 3,6-anhydro-α-
d-galactopyranose units [
19], are divided into the iota (ι-), kappa (κ-) and lambda (λ-) forms, and their mu (μ-) and nu (ν-) precursors [
20,
21]. The classification is based on a number and position of sulfate groups on the repeating disaccharide units. κ-Car, potentially used as a pharmaceutical material due to its good biocompatibility and low toxicity, contains one sulfate group on the C4 of β-
d-galactopyranose residue [
22]. Upon cooling it forms gels in the presence of K
+, Na
+ or Ca
2+ ions via aggregation of double helices [
23]. Due to its gelation ability, high water absorption capacity, and an ester sulfate content of 25–30%, κ-Car has a great applicability in drug delivery systems [
24,
25], for cells encapsulation [
26] and as a wound dressing [
27]. The composition imitating glycosaminoglycans makes it a potential scaffold for tissue engineering of cartilage. Nevertheless, there are same inconveniences when using κ-Car as an independent scaffold, such as limited swelling properties, unsatisfactory mechanical strength, or the fragility of created gels [
28].
The proposed BNC/κ-Car composites in this work enable to overcome the limitations of the two polymers when used separately. To the best of our knowledge, this is the first report on the production of bacterial cellulose/κ-carrageenan composites using an in situ method.
In this paper, we report the preparation of new BNC/κ-carrageenan composites and the evaluation of their properties. The morphology of fibers, chemical composition, crystallinity, tensile, and compression strength, water holding capacity, water retention ratio and swelling properties were examined and compared with those of pure BNC. Furthermore, we examined the influence of BNC/κ-carrageenan composites on chondrogenic differentiation as a goal of cartilage tissue engineering. To evaluate the utility of the BNC/κ-Car composites as potential scaffolds for TE, we applied murine chondrogenic ATDC5 cells that represent a chondroprogenitor clone and assessed the impact of the composites on their viability as well as the specific expression patterns of
Col1α1,
Col2α1,
Runx2, and
Sox9, which are indicative of ATDC5 chondrogenic differentiation [
29].
2. Materials and Methods
2.1. Microbial Strains
Komagataeibacter xylinus E25 strain used in this study was derived from the Institute of Technical Biochemistry (Lodz University of Technology) collection. The strain belongs to BOWIL Biotech Ltd., Poland.
2.2. Culture Media
K. xylinus E25 strain was cultivated under stationary conditions in a Schramm-Hestrin (SH) medium containing glucose (20 g/L), yeast extract (5.0 g/L), peptone (5.0 g/L), MgSO4·7H2O (0.5 g/L), anhydrous Na2HPO4 (2.7 g/L) and citric acid (1.15 g/L), dissolved in distilled water. The pH of the culture medium was adjusted to 5.7 using 0.1 M acetic acid before autoclaving at 121 °C for 20 min.
2.3. Preparation of BNC, κ-Car and BNC/κ-Car Composites
K. xylinus E25 strain was activated by cultivation in the SH medium at 30 °C for 3 days and used as an inoculum. The medium was supplemented with ethanol (1% (v/v)) directly before the inoculation with bacterial pre-culture (5% (v/v)). BNC membranes and BNC/κ-Car composites were obtained in stationary conditions at the temperature of 30 °C after 7-day culture. The composites were prepared using two in situ methods. The first method was based on supplementation of the SH medium with κ-Car (final concentrations of 2, 5 and 8 g/L) before the medium sterilization that yielded the composites BNC/κ-Car0.2, BNC/κ-Car0.5, and BNC/κ-Car0.8, respectively. The composites with higher content of κ-Car (BNC/κ-Car2.0, BNC/κ-Car4.0, and BNC/κ-Car6.0) were obtained using the two-step method. In the first step, the SH medium was supplemented with κ-Car (2 g/L) and sterilized (121 °C for 20 min). After cooling, additional portions of sterile κ-Car were added to obtain the final concentrations of 20, 40 and 60 g/L (the composites obtained are referred to as BNC/κ-Car2.0, BNC/κ-Car4.0 and BNC/κ-Car6.0, respectively).
The purification steps of the obtained membranes included rinsing with tap water until flushing out of the remaining medium, 24 h washing with 1% (w/v) sodium hydroxide for bacterial cells removal, and subsequent immersing in 1% (v/v) acetic acid and then in distilled water for pH neutralization. Purified cellulose membranes were packed and sterilized by autoclaving at 121 °C and stored at 4 °C before further analytical steps.
κ-Car gels were prepared for Fourier-transform infrared spectroscopy (FTIR) analysis and cell viability study. κ-Car was dissolved in 0.1 M CaCl2 by autoclaving (121 °C for 20 min) to obtain solution with concentrations of 2 and 4 g/L, which were immediately transferred into a 96-well plate (1 mL per well), under sterile conditions. Upon cooling κ-Car formed round-shaped pieces of gel (κ-Car0.2 and κ-Car0.4, respectively), which were freeze-dried (an Alpha 1–2 LD plus lyophilizer, Christ, Germany) and stored in desiccators before using.
2.4. Characterization of BNC/κ-Car Composites
2.4.1. Scanning Electron Microscopy (SEM)
SEM was applied for visual inspection of BNC/κ-Car composites. Freeze-dried samples of the composites were sputter coated with gold layer and observed using a FEI QUANTA 250 FEG microscope (Thermo Fischer Scientific, Waltham, MA, USA) (HV 2 kV, magnification 40,000×). Fibers thickness was evaluated on the basis on SEM images using the Makroaufmassprogramm software. Histograms were drown using the R (v. 3.4.1) software.
2.4.2. Fourier-Transform Infrared Spectroscopy
The incorporation of κ-Car into BNC membranes was evaluated with the FT-IR in an attenuated total reflectance mode (ATR) using a Nicolet 6700 FT-IR (Thermo Fischer Scientific, Waltham, MA, USA). The spectra were recorded over the range from 4000 to 650 cm−1 by the accumulation of 200 scans, with a spectral resolution of 4 cm−1.
2.4.3. X-ray Diffractometry
Room temperature powder X-ray diffraction patterns were collected using a PANalytical X’Pert Pro MPD diffractometer (Malvern Panalytical Ltd., Royston, UK) with the Bragg–Brentano reflection geometry and the graphite monochromated Cu-Kα radiation. All data were collected in the 2θ range 5–60° with a step of 0.0167° and an exposure per step of 30 s. The samples were spun during data collection to minimize preferred orientation effects. A PANalytical X’Celerator detector based on the Real Time Multiple Strip technology and simultaneously measuring intensities in the 2θ range of 2.122° was used.
The crystallinity index was calculated as the ratio of the integral intensity under all crystalline peaks to the sum of integral intensity under the crystalline peaks and amorphous halo [
30] using the WAXFIT program [
31]. Each diffraction curve was analyzed by the creation of a theoretical function best fitted to the experimental curve, according to the hybrid optimization procedure, which combines a genetic algorithm with a modified Rosenbrock optimization method. A linear combination of the Gauss and Lorentz profiles was used to construct the theoretical function approximating crystalline peaks and an amorphous halo. As a result of fitting, all the parameters of the component functions were determined.
2.4.4. Mechanical Strength
BNC/κ-Car composites were examined for tensile and compression strength. The tests were performed using a universal testing machine (Zwick/Roell Z1.0, Zwick GmbH & Co. KG, Ulm, Germany) equipped with either 1 kN cell (tensile) or 0.5 kN cell (compression). Before the tensile tests, membranes were cut into rectangular samples (20 mm × 45 mm) and pressed into flat pieces (thickness of 1 mm). Then, the samples were mounted between two clamps (the gauge length was 15 mm for all samples) and subjected to deformation at a rate of 50 mm/min, while the pre-load was 1 N. The maximum stress and elongation at break were estimated using the TestXpert@II software. Young’s modulus values were calculated from the stress–strain relationship. For the compressive test, membranes were cut into 2 cm × 2 cm rectangular pieces and placed between two metal plates. The direction of compression (at a rate of 50 mm/min) was perpendicular to the surface of the sample (the pre-load was 5 N). Values of the compressive modulus were calculated from the slope of linear stress–strain curves. Both the measurements were performed in 12 replicates.
2.4.5. Water Holding Capacity (WHC)
The WHC measurements were conducted according to [
32]. Briefly, rectangular pieces (2 cm × 2 cm) of never-dried BNC/κ-Car membranes were quickly shaken twice and weighted to determine the wet mass. Then, they were dried in a vacuum gel dryer (Model 543, Bio-Rad, Hercules, CA, USA) at 80 °C for 4 h until the constant weight. Water holding capacity was calculated from the following equation:
where
Wd is the weight of dry membrane and
WW is the weight of wet membrane. The experiment was carried out in triplicate for each BNC/κ-Car composite.
2.4.6. Swelling Properties and Water Retention Ratio (WRR)
The swelling ratio was determined by placing lyophilized, round-shaped BNC/κ-Car membranes with diameter of 38 mm, in distilled water. The samples were removed from water after certain period of time, initially after 10 min, and an excess water was gently drained with filter paper. Afterwards, samples were weighted. The content of water in swollen membranes was calculated using the following equation:
where
Wd is the initial weight of dry sample and
WW is the weight of the wet membrane after incubation in distilled water. All the measurements were performed in triplicate.
To estimate the WRR values, the round-shaped, wet BNC/κ-Car membranes with a diameter of 38 mm, were weighted at different time intervals according to [
33] until the complete water evaporation. Samples were stored at ambient temperature between measurements. Then, the percentage of water remained in BNC/κ-Car membranes was plotted against the time. Tests were done in triplicate for all the BNC/κ-Car composites.
2.5. ATDC5 Cell Viability Study
2.5.1. ATDC5 Cell Culture
The chondrogenic ATDC5 cell line originating from mouse teratocarcinoma AT805 was purchased from The Health Protection Agency (supplied by Sigma Aldrich, St. Louis, MO, USA). The cells were cultured in Dulbecco’s Modified Eagle Medium: Nutrient mixture F12 (1:1; DMEM:F12) supplemented with 2 mM glutamine, 5% Fetal Bovine Serum (FBS), 100 U/mL penicillin, 100 mg/mL neomycin, and 2.5 µg/mL amphotericin B. All the culture medium components were obtained from the Life Technologies (Carlsbad, CA, USA). Cells were incubated at 37 °C in a humidified atmosphere supplemented with 5% CO2.
2.5.2. Seeding of ATDC5 Cells on Scaffolds
Round-shaped BNC, κ-Car and BNC/κ-Car membranes were placed in 24–well polystyrene plates and soaked in complete the DMEM:F12 medium for 24 h. Then an excess of the culture medium was removed and ATDC5 cells were seeded into the prepared scaffolds at a density of 1.5 × 10
5 per well in the complete medium. Cells were incubated at 37 °C and 5% CO
2 for the next 21 days with medium exchange every 2–3 days. During ATDC5 cells culture, the morphology was observed and documented with a Leica M205 microscope (Leica Microsystems GmbH, Wetzlar, Germany) equipped with a Leica MC170 HD camera (
Figure A2).
2.5.3. Cell Viability Assay
After 21 days of growth, the BNC, κ-Car and BNC/κ-Car scaffolds with ATDC5 cells were transferred into new 24-well plates (to reduce the false positive response from cells adhered to the plastic surface of wells). Subsequently, 500 µL of DMEM:F12 medium was added to each well and the number of viable cells was quantified by PrestoBlue viability assay. 40 μL of PrestoBlue cell viability reagent (Life Technologies, CA, USA), a resazurin-based solution, was added into each well and incubated further for 90 min at 37 °C and 5% CO
2. The number of viable cells was determined based on measurements of the fluorescent signal F
530/590 using a Synergy 2 Microplate Reader (Bio-Rad, CA, USA). Values of fluorescence magnitudes were used to calculate cell viability expressed as a percentage of the viability of the cells growing on the surface of BNC/κ-Car or κ-Car in comparison to pure BNC
where
RFUsample is the fluorescence of cells growing on the BNC/κ-Car composites,
RFUmedium is the fluorescence of medium without cells and
RFUcontrol is the fluorescence of cells, which grew on the BNC surface.
2.5.4. Gene Expression Analysis
After 21 days of ATDC5 cells growth, the BNC and BNC/κ-Car scaffolds were transferred to Falcon test tubes and washed twice with 2 mL of phosphate buffer saline. Subsequently, 1.5 mL aliquots of 0.25% trypsin/EDTA solution were added to detach ATDC5 cells. The suspensions of cells were collected and the density of cells was determined. Templates for one step real-time RT-PCR, without RNA extraction process, were prepared from 1.0 × 10
4 cells using a CellAmp Direct RNA Prep Kit (Takara Bio Inc., Kusatsu, Japan) according to the manufacturer’s instructions. Primers (
Table 1) were designed using the NCBI’s Primer-BLAST software (National Center for Biotechnology Information) based on sequences from the GenBank database and purchased from Genomed, Poland.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed using a One Step PrimeScript™ RT-PCR Kit (Takara Bio Inc., Kusatsu, Japan) and a CFX96™ detection system (Bio-Rad, Hercules, CA, USA). The process started with reverse transcription (5 min; 42 °C) and cDNA melting (10 s; 95 °C). 40 cycles of real time-PCR consisted of denaturation (5 s; 95 °C), annealing (30 s; 60 °C), and elongation (30 s; 72 °C). Nonspecific products and primer dimers were excluded by melting curve analysis performed by maintaining the temperature at 50 °C for 2 s, followed by a gradual increase in temperature to 95 °C. The final products were separated electrophoretically on a 2.7% agarose gel in TAE buffer (TRIS/acetate/EDTA) containing a nucleic acid stain (Midori Green Advance DNA stain, NIPPON Genetics, Tokyo, Japan). Constitutively expressed GAPDH gene was selected as an endogenous control. Expression of examined genes were normalized to GAPDH expression and control samples genes expression. The target gene expression levels were calculated using the Livak and Schmittgen equations [
34]:
2.6. Statistical Analysis
Statistical significance of data was evaluated by analysis of variance (ANOVA; p < 0.05) using the MiniTab18 software. For significant effects the Tukey method of multiple comparisons was performed.
3. Results and Discussion
3.1. Biosynthesis of BNC and BNC/κ-Car Composites
BNC/κ-Car composites were biosynthesized in situ using two methods, which exploited the viscoelastic behavior of κ-Car chains in
aqueous solutions. In the first method, κ-Car (0.2, 0.5 and 0.8%) was added to the SH culture medium and sterilized. Above the temperature of 70 °C, κ-Car forms a colloidal solution and upon cooling, the double helices of biopolymer form a three-dimensional network. Afterwards, the growing BNC penetrated κ-Car matrix and the compact structure of the composite was formed. The viscosity of culture medium grew with the increasing concentration of κ-Car. This in turn decreased the oxygen transfer rate and reduced BNC formation. The similar observation was reported in case of aloe vera juice addition to a culture medium [
35].
In the second method, sterile κ-Car was added to the cooled SH culture medium previously supplemented with 2.0 g/L κ-Car. Under such conditions, the supplementary κ-Car did not form the gel spontaneously. Instead, it formed large agglomerates whose appearance was ascribed to formation of water layer around each κ-Car coil. Strong stirring allowed to obtain stable homogenous medium containing 2, 4 and 6 of κ-Car on the basis of the matrix created by 0.2% κ-Car. During cultivation, cellulose fibers surrounded the κ-Car agglomerates and formed the loose 3D network. The increase in κ-Car concentration resulted in a decrease in the BNC composite mass. In the case of BNC/κ-Car6.0 very thin and soft pellicle was obtained and therefore it was excluded from further characterization.
Both the in situ methods allowed to obtain new and unique structures of membranes. The presence of κ-Car in the BNC-based membranes strongly affected their properties, such as fibers morphology, chemical composition, crystallinity, tensile and compression strength, water holding capacity, water retention ratio, and swelling properties.
3.2. Characterization of BNC/κ-Car Composites
3.2.1. Morphology and Fibers’ Architecture of BNC/κ-Car Composites
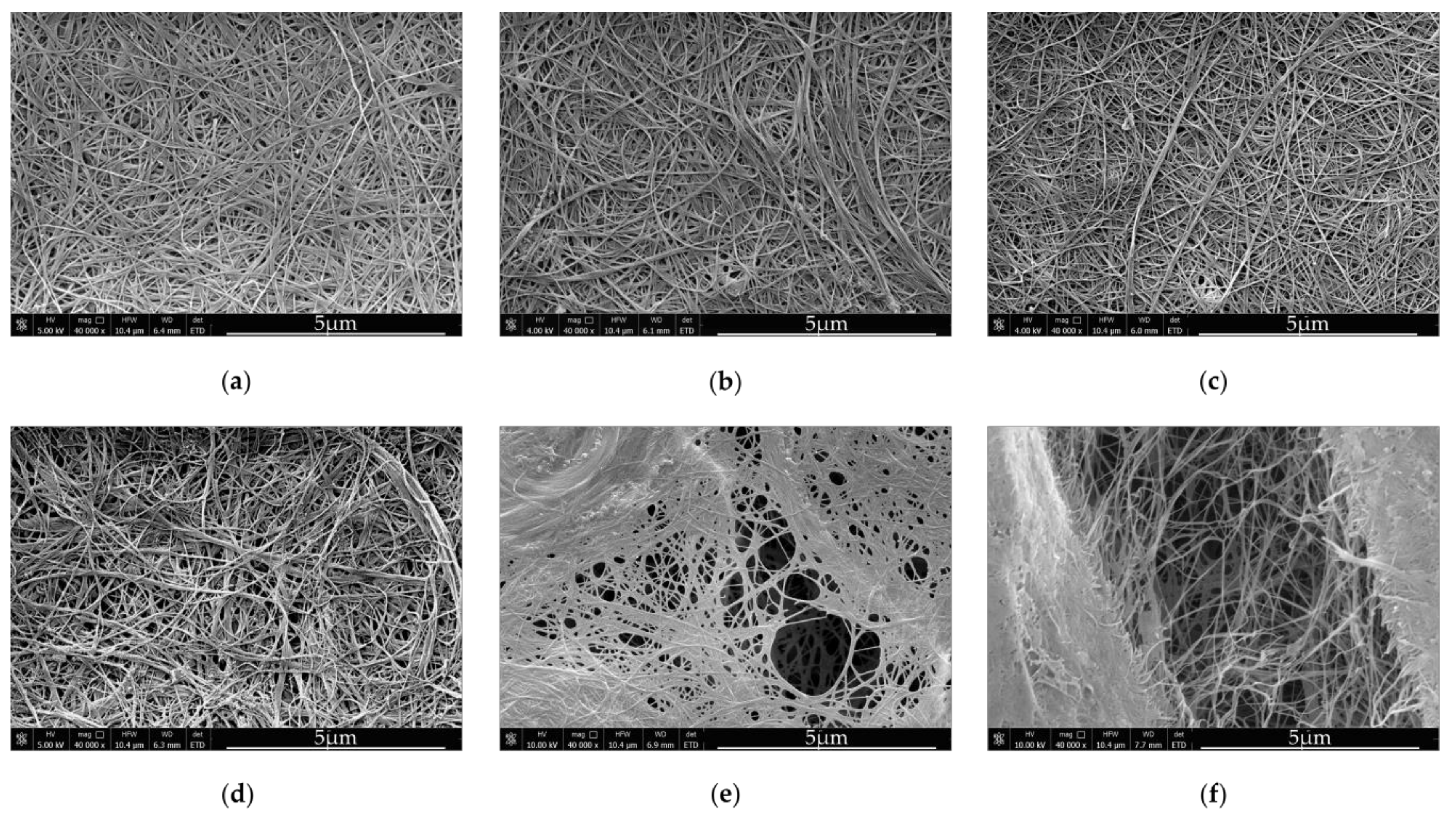
Bionanocellulose sub-elementary fibrils, which are 3–6 nm in diameter, and are extruded by bacterial cells during cultivation, immediately form microfibrils and crystallize, yielding type I cellulose [
36]. The network of fibers is compact and diameters of single fibers are around 40–60 nm (
Figure 1a). Medium viscosity has a great impact on the bacterial cellulose structure. According to [
37] BNC synthesized in viscous culture media consist of bundles, which either represent type II cellulose or contain a mixture of type I and type II cellulose.
The structure of composites produced by the first method, was slightly different from the structure of BNC derived from the unmodified SH medium (
Figure 1b–d). The spaces between cellulose fibers, visible in the
Figure 1, were enlarged when the concentration of κ-Car in the culture medium was increased. Only in case of the BNC
0.2 composite, which was obtained at a relatively low κ-Car concentration, the empty spaces between BNC fibers were not visible. We assume that the medium viscosity as well as adhesion of κ-Car to cellulose ribbons significantly influenced the diameter of fibers in case of BNC κ-Car
0.5 and BNC κ-Car
0.8. The average diameters of strands were 83 nm and 119 nm, respectively (
Figure 2c,d). The latter value was 2.4-fold greater compared to the unmodified BNC fibers.
The second method resulted in formation of highly porous structures of both BNC/κ-Car
2.0 and BNC/κ-Car
4.0 composites (
Figure 1e,f). Winding of cellulose ribbons around κ-Car agglomerates gave rise to the very loose 3D network. Furthermore, most of κ-Car was incorporated into the surface layers of membranes that was visible in SEM images. Therefore, despite the increased porosity, the latter composites were relatively rigid, as the compression modulus indicated (
Section 3.2.5). The diameters of fibers were reduced to 48 and 35 nm for BNC/κ-Car
2.0 and BNC/κ-Car
4.0, respectively (
Figure 2e,f).
3.2.2. FTIR-ATR Analysis
The FTIR spectra of BNC, κ-Car and BNC/κ-Car composites are presented in
Figure 3. Samples were scanned over the range of 4000–650 cm
−1 to observe the effect of κ-Car presence in BNC membranes, caused by the in situ modification.
In the case of pure BNC, two main peaks can be observed. A sharp characteristic peak of O-H stretching appears at 3347 cm
−1 and another important peak of 1645 cm
−1 is assigned to the glucose carbonyl stretching [
38,
39]. A series of bands from 1200 to 1000 cm
−1 are related to the stretching of C-O-C of sugar rings and C-O stretching vibrations of the primary (C
6) and secondary hydroxyl (C
2, C
3) groups. CH
2 deformation can be detected at 1428 cm
−1. The absorption band at 2896 cm
−1 corresponds to CH-stretching and CH
2 asymmetric stretching [
40]. The peak at 1316 cm
−1 results from CH
2 wagging symmetric bending [
41].
The FTIR spectrum of κ-Car demonstrates peaks, which are characteristic of this polymer. The absorption bands at 1234, 929, and 844 cm
−1 are assigned to S=O of sulfate esters, C-O of anhydrogalactose and sulfation of C
4 on galactose [
42], respectively. Sulfonic acid groups can be indicated based on the peak at 1368 cm
−1 [
43]. The spectra of BNC/κ-Car composites provided evidence that the main component of these composites is cellulose. However, these spectra contain additional small peaks in the range of 1260–1240, 940–920 and 810–845 cm
−1, which are typical of κ-Car. The band’s shape at 1030 cm
−1 suggests mixing of two peaks from both the polysaccharides. Shifting of absorption band of CO-SO
3 of the β-1,3-linked residue from 845 to 810 cm
−1 can be observed in the case of all the composites. The shift may be ascribed to the interactions of cellulose chains with κ-Car coils. The region of 3600–3000 cm
−1, which corresponds to –OH stretching is broader in the case of BNC/κ-Car, especially in case of BNC/κ-Car
2.0 and BNC/κ-Car
4.0, in comparison to the native BNC. This may suggest the interleaving of cellulose fibers and carrageenan matrix and occurrence of physical interactions between these biopolymers via additional hydrogen bonds. The latter effect was observed also for BNC composites with other compounds such as sodium alginate [
44], agarose [
45], chitosan [
46], and collagen [
47].
3.2.3. X-ray Diffraction (XRD) Analysis
The XRD method was used to determine the crystallinity index (CI) of the BNC/κ-Car composites (
Table 2). The diffraction patterns (
Figure 4) contain three main peaks that are present at ~14.66°, ~17.26° and ~22.90° for the (101), (10–1) and (200) planes, respectively, and they may be identified as the reflection planes of cellulose I [
30]. The peaks in the diffraction curve of all the samples were relatively broad, because BNC contains amorphous regions apart from the crystalline areas. With the increase of κ-Car concentration, the CI of BNC/κ-Car decreased, from 87.6% for pure BNC to 77.4% for BNC/κ-Car
4.0. This may suggest the occurrence of interactions between cellulose ribbons and helices or coils of κ-Car during crystallization process. These results are consistent with the FTIR data.
3.2.4. Water Holding Capacity, Water Retention Rate, and Swelling Properties
Water holding capacity, water retention rate and swelling properties are very important parameters with regard to medical applications of biomaterials. They decide of water diffusion in membrane and thus the ability of transporting the nutrients and active compounds throughout the biomaterial. The high water retention capacity enables to maintain the moist environment that is crucial for the proper cell growth and favorable interactions with the environment in tissue engineering applications.
The results of WHC measurements for the pure BNC and composites with κ-Car are shown in
Figure 5. The highest water content was observed for BNC and BNC/κ-Car
0.2 (it reached 137 and 133 g water/g of their dry mass, respectively). However, we observed a decline in WHC for higher concentrations of κ-Car (0.5 and 0.8%). WHC was reduced to 109 g
water/g
dry mass in case of BNC/κ-Car
0.8. WHC level is a consequence of structural arrangements of fibrils in BNC network and it is affected by the size of pores, crystallinity index, density, and width of fibers. The addition of gelling agents such as carrageenan or agar to the culture medium resulted in the changed cellulose network consisting of ribbon-like wide fibers [
48]. The contact surface was decreased and less water molecules could be hold within the BNC structure [
49]. On the other hand, the presumable reason of the reduced WHC in the case of BNC/κ-Car
2.0 and BNC/κ-Car
4.0 (117 and 123 g
water/g
dry mass, respectively) was the presence of κ-Car aggregates, especially in surface layers, which filled the empty spaces between fibers.
The swelling ability of hydrogels is essential in tissue engineering and drug delivery applications. During the process of swelling, the structure of the porous materials changes due to the growing distance between the fibers. The absorbed water molecules are directed to free spaces, fill them, and form hydrogen bonds with chains of polymers. Both BNC and κ-Car are excellent water absorbents, and therefore they have found numerous medical applications. However, their swelling profiles are different. Pure κ-Car, which can absorb 11.28 g water per 1 g of its dry mass, swells rapidly and the gel reaches the equilibrium state in 60 min (
Figure A1). BNC swells more slowly and binds 87.75 g
water/g
dry mass, what is 7.8 times more compared to κ-Car. Water completely fills pores in BNC membranes within 3 h (
Figure 6). In the case of BNC/κ-Car
0.2, BNC/κ-Car
0.5, and BNC/κ-Car
0.8, the amount of absorbed water decreased when the concentration of κ-Car in the culture medium increased (79.13, 64.26, and 53.27 g
water/g
dry mass, respectively). These results were consistent with the data obtained for BNC and pure κ-Car. The swelling ratio of BNC/κ-Car composites reflected both the water binding capacity of each of the two biopolymers and their percentages in the composites.
The swelling profiles for BNC/κ-Car2.0 and BNC/κ-Car4.0 were different compared to the other composites. The plateau was achieved after 36 and 48 h, respectively. After 3 h, when BNC was completely hydrated, the degrees of swelling of BNC/κ-Car2.0 and BNC/κ-Car4.0 were only 55 and 53%, respectively. This difference may be ascribed to the high concentration of κ-Car in surface layers since the latter polymer swells quickly but binds less water. The occurrence of negative charges on its coils causes that water molecules to bind strongly and their diffusion to the interior of the membrane takes more time. BNC/κ-Car2.0 and BNC/κ-Car4.0 absorbed 70.52 and 95.65 gwater/gdry mass, respectively. The difference in the swelling ratio is a consequence of different porosity and contact surface area, which are greater in the case of BNC/κ-Car4.0 and therefore, the latter composite bound more water.
The water retention rate of BNC and its composites is a vital parameter in case of medical applications [
50]. Like WHC, WRR is affected by the thickness and structure of fibers as well as the presence of additives inside the membrane [
33]. Interestingly, the WRR profiles of the BNC/κ-Car composites obtained in this study were different from the WHC profiles. In case of BNC/κ-Car prepared by the first method, the rate of water evaporation decreased with the increase in κ-Car concentration, with an exception of BNC/κ-Car
0.8 (
Figure 7). The similar observations were reported by [
51] who studied the effect of an increasing concentration of a single sugar on the α-linked glucuronic acid-based oligosaccharide. The positive impact of carrageenan on WRR of carrageenan/hydroxymethyl cellulose composites was also reported [
52]. In this study, the differences between the composites were visible after 6 h of drying process. Presumably, the molecules of water, which were physically attached to the surface of membranes, were evaporated within this time. Noteworthy, these molecules accounted for less than 20% of bound water. The diffusion of the remaining water molecules, which were entrapped between the fibers, took either 36 h (BNC) or 48 h (BNC/κ-Car
0.2 and BNC/κ-Car
0.5). The composite BNC/κ-Car
0.8 was characterized by the significantly lower value of WRR that may be ascribed to the high thickness of fibers and enhanced porosity, which were visible in the SEM images. Presumably, these parameters had the greater impact on WRR than the presence of κ-Car. Because of the lower surface area and expanded pores, water molecules that are bound either to the surface or inside the membrane may be removed much easier. WRR values of BNC/κ-Car
2.0 and BNC/κ-Car
4.0 were similar and water evaporation took up to 48 h. The effect of κ-Car incorporation into the surface layers of membranes was noticeable. The diffusion of water was hindered like in case of the swelling behavior of modified BNC and within 24 h, BNC/κ-Car
2.0, BNC/κ-Car
4.0 and BNC lost 55, 60 and 68% of bound water, respectively.
3.2.5. Mechanical Strength
Tissue engineering applications commonly encompass the use of scaffolds to provide a suitable microenvironment for the incorporation of cells to regenerate damaged tissues or organs. Scaffolds should exhibit high mechanical strength to maintain integrity until new tissue regeneration. It is especially important in case of cartilage tissue replacements. As the load bearing material, cartilage functions in a challenging mechanical environment. Bacterial nanocellulose is known as a mechanically stable biomaterial with hydrogel-like characteristic [
53]. Interestingly, incorporation of κ-Car into BNC additionally improved the tensile strength of BNC/κ-Car composites at κ-Car concentrations up to 2% (
Table 3). Also, the elongation at break was affected by the incorporation of κ-Car. In the case of the second method of BNC/κ-Car production, the fracture strain was increased to 163 and 180% compared to the native BNC for BNC/κ-Car
2.0 and BNC/κ-Car
4.0, respectively. The Young’s modulus of the native articular cartilage is about 10 MPa [
54,
55]. All the composites obtained in this study, with an exception of BNC/κ-Car
4.0, were characterized by the higher tensile modulus values ranging from 12.32 to 16.35 MPa, in contrast to pure BNC, whose Young’s modulus was below 9 MPa.
As components of cartilage tissue, the chondrocytes are adapted to function under elevated pressure, and mechanical compression is thought to stimulate the proliferation and differentiation of these cells [
56,
57]. Therefore, the compression modulus is the key parameter, which determines the utility of scaffolds in tissue engineering. The study of Toyoda et al. showed that exposition of chondrocytes seeded in a three-dimensional agarose scaffold on the static or pulsing hydrostatic pressure of 5 MPa for 4 h resulted in the increasing of GAG synthesis and a four-fold higher level of aggrecan mRNA [
56]. In the case of BNC and BNC-based composites, the compression properties depend on the arrangement and thickness of fibers as well as the porosity and crystallinity of membranes. The compression of samples between plates causes the removal of water. In case of the composites produced by the first method, the results of measurements of compression properties were consistent with the results of water holding capacity measurements and correlated with their porosity. Upon compression, water entrapped inside the membrane causes the resistance and immediately flows out.
Interestingly, the composites BNC/κ-Car2.0 and BNC/κ-Car4.0 behaved in an unusual manner. Despite the large free spaces between thin fibers, the lower WHC and decline in crystallinity, in comparison to the native BNC, were observed. The compression modulus was 1.9 and 2.2 times higher for BNC/κ-Car2.0 and BNC/κ-Car4.0, respectively, than for BNC. Presumably, the incorporation of κ-Car into the surface layers of membranes caused that the removal of water from the composites was impeded. BNC modified in that way may have a great potential in TE of cartilage connected with mechanical stimulation of chondrocytes, leading to a higher efficacy of their cultivation.
3.3. The Viability of ATDC5 Cells
To evaluate the usefulness of BNC/κ-Car composites as a scaffold material for cartilage repair, cell viability was assessed with Presto Blue
® test. The effect of the scaffold on the viability of ATDC5 cells is presented in
Figure 8.
As the biocompatibility of pure BNC produced by
K. xylinus E25 is well known [
58] the significant differences in the viability of cells cultivated on the BNC control and nearly all BNC/κ-Car composites were promising. After 21 days of growth on various BNC/κ-Car composites ATDC5 cells exhibited the increased cell viability in comparison to the BNC control sample. The only exception was BNC/κ-Car
2.0, for which the ATDC5 cells viability was reduced to 88.5%. The growth of the cells on pure 2 and 4% κ-Car gels was also monitored (at the lower concentrations, κ-Car does not form stable gels). The observed high toxicity of 4% κ-Car was consistent with data obtained by Sathuyan et al. [
59], who reported the correlation between κ-Car concentration and cell viability, and showed that the growing polysaccharide concentration caused the decrease in cell viability, which depended also on the time of growth.
3.4. Relative Gene Expression
Apart from determination of the ATDC5 cells viability, also the relative expression of the selected genes, which are crucial for the process of chondrogenesis, was under scrutiny (
Figure 9). The long expansion time and multiple passaging often lead to “dedifferentiation” of chondrocytes, which is defined as the gradual loss of differentiation molecular markers. Chondrocytes cultivated in vitro on the plastic surfaces dedifferentiate particularly quickly to less specific cells with enhanced ability to proliferate [
60]. As dedifferentiation progresses, the expression of genes encoding the extracellular matrix components, such as collagen type II (
Col2a1) or SRY (sex determining region Y)-box 9 (
Sox9), transcription factor is changed, whilst at the same time the cells acquire an increased fibroblastic phenotype, characterized by the expression of the genes encoding collagen type I (Col1a) or runt related transcription factor 2 (
Runx2) [
61]. Specifically,
Sox9 transcription factor enhances expression of
Col2a1 while overexpression of
Runx2 is connected with the appearance of hypertrophic chondrocytes and leads to the appearance of osteoblasts followed by cell mineralization [
61,
62].
It was shown that some biophysical stimuli, like three-dimensional space for growth, are sufficient for cells to differentiate to chondrocytes [
63]. The surfaces of BNC scaffolds were uneven with a porous structure, which allowed the cells to penetrate into the biopolymer structure. Therefore, pure BNC scaffolds slightly enhanced the differentiation process [
14,
58]. What is more, some findings proved that the negatively charged surfaces of scaffolds could promote cell dedifferentiation [
64].
The Col1α1 expression was found to be nearly the same in case of all BNC/κ-Car composites as compared to the ATDC5 cells growing on either plastic surface (data not shown) or pure BNC. In case of the majority of BNC/κ-Car composites the expression level of Col2α1 was slightly higher than in the control sample. The only exception, for which the level of expressed Col2α1 was statistically increased was the BNC/κ-Car0.8 composite. Sox9 expression was significantly enhanced in case of all the composites with higher κ-Car concentration, namely BNC/κ-Car0.5, BNC/κ-Car0.8, and BNC/κ-Car2.0. Finally, low expression level of Runx2 shows that none of the composites tested induced the chondrocyte hypertrophy. Thus, the results of this study showed that BNC/κ-Car composites supported the dedifferentiation of ATDC5 cells to more chondrogenic phenotype, as demonstrated by the increase in the expression of chondrogenic marker genes Col2α1, Sox9 and the decrease of Col1α1 and Runx2 expression.
4. Conclusions
In this work, two variants of the innovative in situ method of novel bacterial cellulose/κ-carrageenan composites production, based on the stationary culture of GRAS bacteria K. xylinus E25, were established. It was shown that the method enables the production of BNC/κ-Car composites, which outperform the two polymers when used separately as potential scaffolds for tissue engineering, regarding the morphology of fibers, chemical composition, crystallinity, tensile and compression strength, water holding capacity, water retention ratio and swelling properties. Also, the cultures of murine chondrogenic ATDC5 cells provided evidence of the utility of the BNC/κ-Car composites as scaffolds for TE. The positive effect of the composites on the cells viability and chondrogenic differentiation was measured as the expression patterns of Col1α1, Col2α1, Runx2, and Sox9. All the composites obtained in this study supported the differentiation of ATDC5 cells to more chondrogenic phenotype and none of them caused the chondrocyte hypertrophy.