Modulation of Metamorphic and Regenerative Events by Cold Atmospheric Pressure Plasma Exposure in Tadpoles, Xenopus laevis
Abstract
:1. Introduction
1.1. Plasma Properties
1.2. Plasma and Reactive Oxygen Species and Reactive Nitrogen Species
1.3. Plasma and Wound Healing in Mammals and Amphibians
1.4. Cell Organelles, Oxidative Stress, and Apoptosis
1.5. Developmental Plasticity in Tadpoles
1.6. Organ Regeneration in Amphibians
2. Materials and Methods
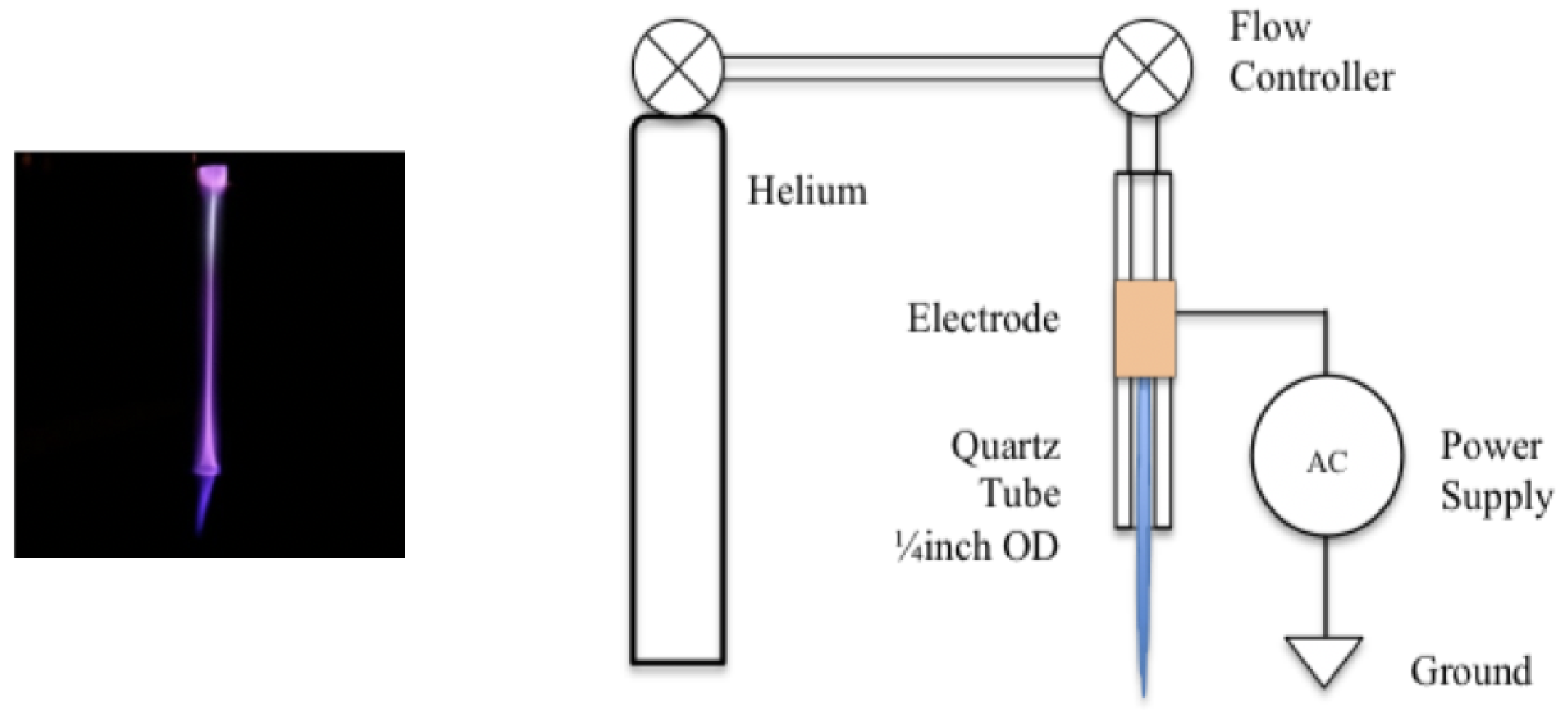
2.1. Plasma Discharge Source
2.2. Maintenance of Tadpoles
2.3. Study of Metamorphosis
- Group 1: Amputated and treated with plasma and this group will be referred as experimental in the text.
- Group 2: Amputated and not treated which will be referred as control in the text.
- Group 3: This group of tadpoles were not amputated or treated with plasma to see the progress of metamorphosis which served as the control for group 2.
2.4. Tail Regeneration Study
- Group 1: Tadpoles amputated and exposed to plasma as mentioned above (experimental).
- Group 2: Tadpoles amputated but not exposed to plasma (control).
2.5. Ca Quantification Assay
2.6. Confocal Microscopy
2.7. Transmission Electron Microscopy
3. Results
3.1. Metamorphic Studies
3.2. Ca
3.3. mPTP
3.4. Peroxisomes
3.5. Light and Electron Microscopy
4. Discussion
4.1. Adaptive Plasticity
4.2. Tail Regeneration
4.3. Ca, Endoplasmic Reticulum (ER), Mitochondria and Peroxisomes
4.4. Ultrastructure of Tadpole Epidermis (Epidermal Morphology and Junctional Complex)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APP | Atmospheric Pressure Plasma |
| BL | Blastema |
| ER | Endoplasmic Reticulum |
| M | Mitochondria |
| mPTP | mitochondrial Permeability Transition Pore |
| MV | Microvilli |
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitrogen Species |
| RER | Rough Endoplasmic Reticulum |
| sccm | standard cubic centimeters per minute |
| WE | Wound Epithelium |
Appendix A

References
- von Woedtke, T.; Metelmann, H.R.; Weltmann, K.D. Clinical plasma medicine: State and perspectives of in vivo application of cold atmospheric plasma. Contrib. Plasma Phys. 2014, 54, 104–117. [Google Scholar] [CrossRef]
- Langmuir, I. Oscillations in ionized gases. Proc. Natl. Acad. Sci. USA 1928, 14, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Schütze, A.; Jeong, J.Y.; Babayan, S.E.; Park, J.; Selwyn, G.S.; Hicks, R.F. The atmospheric-pressure plasma jet: A review and comparison to other plasma sources. IEEE Trans. Plasma Sci. 2006, 26, 1685–1694. [Google Scholar] [CrossRef]
- Winter, J.; Brandenburg, R.; Weltmann, K.D. Atmospheric pressure plasma jets: An overview of devices and new directions. Plasma Sources Sci. Technol. 2015, 24, 064001. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Iza, F.; Brandenburg, R. Foundations of atmospheric pressure non-equilibrium plasmas. Plasma Sources Sci. Technol. 2017, 26, 123002. [Google Scholar] [CrossRef] [Green Version]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B At. Spectrosc. 2006, 21, 2–30. [Google Scholar] [CrossRef]
- Park, G.Y.; Park, S.J.; Choi, M.Y.; Koo, I.G.; Byun, J.H.; Hong, J.W.; Sim, J.Y.; Collins, G.J.; Lee, J.K. Atmospheric-pressure plasma sources for biomedical applications. Plasma Sources Sci. Technol. 2012, 21, 043001. [Google Scholar] [CrossRef]
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold atmospheric plasma: Methods of production and application in dentistry and oncology. Med. Gas Res. 2013, 3, 21. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.; Vasilets, V.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Laroussi, M. Plasma medicine: A brief introduction. Plasma 2018, 1, 47–60. [Google Scholar] [CrossRef]
- Morfill, G.; Kong, M.; Zimmermann, J. Focus on plasma medicine. New J. Phys. 2009, 11, 115011. [Google Scholar] [CrossRef]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; Van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Tanaka, H.; Hori, M. Medical applications of non-thermal atmospheric pressure plasma. J. Clin. Biochem. Nutr. 2017, 60, 29–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.W.; Kang, S.U.; Kim, Y.E.; Park, J.K.; Yang, S.S.; Kim, Y.S.; Lee, Y.S.; Lee, Y.; Kim, C.-H. Novel therapeutic effects of non-thermal atmospheric pressure plasma for muscle regeneration and differentiation. Sci. Rep. 2016, 6, 28829. [Google Scholar] [CrossRef] [PubMed]
- Chernets, N.; Zhang, J.; Steinbeck, M.J.; Kurpad, D.S.; Koyama, E.; Friedman, G.; Freeman, T.A. Nonthermal atmospheric pressure plasma enhances mouse limb bud survival, growth, and elongation. Tissue Eng. Part A 2015, 21, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cheng, X.; Zhu, W.; Holmes, B.; Keidar, M.; Zhang, L.G. Design of biomimetic and bioactive cold plasma-modified nanostructured scaffolds for enhanced osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Tissue Eng. Part A 2013, 20, 1060–1071. [Google Scholar] [CrossRef]
- Haertel, B.; Hahnel, M.; Blackert, S.; Wende, K.; von Woedtke, T.; Lindequist, U. Surface molecules on HaCaT keratinocytes after interaction with non-thermal atmospheric pressure plasma. Cell Biol. Int. 2012, 36, 1217–1222. [Google Scholar] [CrossRef]
- Panngom, K.; Baik, K.Y.; Nam, M.K.; Han, J.H.; Rhim, H.; Choi, E.H. Preferential killing of human lung cancer cell lines with mitochondrial dysfuction by nonthermal dielectric barrier discharge plasma. Cell Death Dis. 2013, 4, e642. [Google Scholar] [CrossRef]
- Schmidt, A.; Dietrich, S.; Steuer, A.; Weltmann, K.D.; von Woedtke, T.; Masur, K.; Wende, K. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J. Biol. Chem. 2015, 290, 6731–6750. [Google Scholar] [CrossRef]
- Wende, K.; Straßenburg, S.; Haertel, B.; Harms, M.; Holtz, S.; Barton, A.; Masur, K.; von Woedtke, T.; Lindequist, U. Atmospheric pressure plasma jet treatment evokes transient oxidative stress in HaCaT keratinocytes and influences cell physiology. Cell Biol. Int. 2014, 38, 412–425. [Google Scholar] [CrossRef]
- Georgescu, N.; Lupu, A.R. Tumoral and normal cells treatment with high-voltage pulsed cold atmospheric plasma jets. IEEE Trans. Plasma Sci. 2010, 38, 1949–1955. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 10, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Chung, T.H. Cold atmospheric plasma-jet generated RONS and their selective effects on normal and carcinoma cells. Sci. Rep. 2008, 6, 20332. [Google Scholar] [CrossRef] [PubMed]
- Fridman, G.; Shereshevsky, A.; Jostet, M.M. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chem. Plasma Process. 2007, 27, 163–176. [Google Scholar] [CrossRef]
- Barekzi, N.; Laroussi, M. Dose-dependent killing of leukemia cells by low-temperature plasma. J. Phys. D Appl. Phys. 2012, 45, 422002. [Google Scholar] [CrossRef]
- Kalghatgi, S.U.; Fridman, G.; Cooper, M.; Nagaraj, G.; Peddinghaus, M.; Balasubramanian, M.; Vasilets, V.N.; Gutsol, A.F.; Fridman, A.; Friedman, G. Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasma. IEEE Trans. Plasma Sci. 2007, 35, 1559–1566. [Google Scholar] [CrossRef]
- Ma, R.N.; Feng, H.Q.; Liang, Y.D.; Zhang, Q.; Tian, Y.; Su, B.; Zhang, J.; Fang, J. An atmospheric-pressure cold plasma leads to apoptosis in Saccharomyces cerevisiae by accumulating intracellular reactive oxygen species and calcium. J. Phys. D Appl. Phys. 2013, 46, 285401–285408. [Google Scholar] [CrossRef]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295. [Google Scholar] [CrossRef]
- Lee, H.J.; Shon, C.H.; Kim, Y.S.; Kim, S.; Kim, G.C.; Kong, M.G. Degradation of adhesion molecules of G361 melanoma cells by a non-thermal atmospheric pressure microplasma. New J. Phys. 2009, 11, 115026. [Google Scholar] [CrossRef]
- Kim, G.C.; Kim, G.J.; Park, S.R.; Jeon, S.M.; Seo, H.J.; Iza, F.; Lee, J.K. Air plasma coupled with antibody-conjugated nanoparticles: A new weapon against cancer. J. Phys. D 2008, 10, 032005. [Google Scholar] [CrossRef]
- Rivie, A.; Martus, K.; Menon, J. Atmospheric pressure plasma accelerates tail regeneration in tadpoles Xenopus laevis. J. Eur. Phys. J. Spec. Top. 2017, 226, 2859–2871. [Google Scholar] [CrossRef]
- Haertel, B.; Von Woedtke, T.; Weltmann, K.D.; Lindequist, U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol. Ther. 2014, 22, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.M.; Shi, X.M.; Cai, J.F.; Chen, S.L.; Li, P.; Yao, C.W.; Chang, Z.S.; Zhang, G.J. Dual effects of atmospheric pressure plasma jet on skin wound healing of mice. Wound Repair Regen. 2015, 23, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Fathollah, S.; Mirpour, S.; Mansouri, P.; Dehpour, A.R.; Ghoranneviss, M.; Rahimi, N.; Safaie Naraghi, Z.; Chalangari, R.; Chalangari, K.M. Investigation on the effects of the atmospheric pressure plasma on wound healing in diabetic rats. Sci. Rep. 2016, 6, 19144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCallion, R.L.; Ferguson, M.W.J. Fetal wound healing. In The Molecular and Cellular Biology of Wound Repair; Clark, R., Ed.; Springer: Boston, MA, USA, 1988; pp. 561–600. ISBN 978-1-4899-0185-9. [Google Scholar]
- Dudas, M.; Wysocki, A.; Gelpi, B.; Tuan, T.L. Memory encoded throughout our bodies: Molecular and cellular basis of tissue regeneration. Pediatr. Res. 2008, 63, 502–512. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef]
- Bielefeld, K.A.; Amini-Nik, S.; Alman, B.A. Cutaneous wound healing: Recruiting developmental pathways for regeneration. Cell. Mol. Life Sci. 2013, 10, 2059–2081. [Google Scholar] [CrossRef]
- Beck, C.W.; Christen, B.; Slack, J.M. Beyond early development: Xenopus as an emerging model for the study of regenerative mechanisms. Dev. Dyn. 2009, 238, 1226–1248. [Google Scholar] [CrossRef]
- Demarquoy, J.; Le Borgne, F. Crosstalk between mitochondria and peroxisomes. World J. Biol. Chem. 2015, 6, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Schrader, M.; Yoon, Y.T. Mitochondria and peroxisomes: Are the “Big Brother” and the “Little Sister” closer than assumed? BioEssays 2007, 29, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Oxidative stress in apoptosis and cancer: An update. Arch. Toxicol. 2012, 11, 1649–1665. [Google Scholar] [CrossRef] [PubMed]
- Bonekamp, N.A.; Völkl, A.; Fahimi, H.D.; Schrader, M. Reactive oxygen species and peroxisomes: Struggling for balance. BioFactors 2009, 35, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, A.Y.; Kushnareva, Y.E.; Starkov, A.A. Mitochondrial metabolism of reactive oxygen species. Biochemistry 2005, 70, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Salas-Vidal, E.; Lomeli, H.; Castro-Obregon, S.; Cuervo, R.; Escalante-Alcalde, D.; Covarrubias, L. Reactive oxygen species participate in the control of mouse embryonic cell death. Exp. Cell Res. 1998, 238, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Duchen, M.R. Roles of mitochondria in health and disease. Diabetes 2004, 53, S96–S102. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.K.; Gerasimenko, J.V.; Thorne, C.; Ferdek, P.; Pozzan, T.; Tepikin, A.V.; Petersen, O.H.; Sutton, R.; Watson, A.J.; Gerasimenko, O.V. Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrialpermeability transition pore (mPTP) opening. J. Biol. Chem. 2009, 284, 20796–20803. [Google Scholar] [CrossRef]
- Bonora, M.; Wieckowski, M.R.; Chinopoulos, C.; Kepp, O.; Kroemer, G.; Galluzzi, L.; Pinton, P. Molecular mechanisms of cell death: Central implication of ATP synthase in mitochondrial permeability transition. Oncogene 2015, 34, 1475–1486. [Google Scholar] [CrossRef]
- Morciano, G.; Giorgi, C.; Bonora, M.; Punzetti, S.; Pavasini, R.; Wieckowski, M.R.; Campo, G.; Pinton, P. Molecular identity of the mitochondrial permeability transition pore and its role in ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2015, 78, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, F.; Trachootham, D.; Huang, P. Preferential killing of cancer cells with mitochondrial dysfunction by natural compounds. Mitochondrion 2011, 10, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Zhivotovsky, B.; Orrenius, S. Calcium and cell death mechanisms. A perspective from the cell death community. Cell Calcium 2011, 50, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407–6418. [Google Scholar] [CrossRef]
- Yan, Y.; Wei, C.; Zhang, W.; Cheng, H.; Liu, J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol. Sin. 2006, 27, 821–826. [Google Scholar] [CrossRef]
- Burraco, P.; Valdés, A.E.; Johansson, F.; Gomez-Mestre, I. Physiological mechanisms of adaptive developmental plasticity in Rana temporaria island populations. BMC Evol. Biol. 2017, 17, 164. [Google Scholar] [CrossRef]
- Gomez-Mestre, I.; Kulkarni, S.; Buchholz, D.R. Mechanisms and consequences of developmental acceleration in tadpoles responding to pond drying. PLoS ONE 2013, 8, e84266. [Google Scholar] [CrossRef]
- Mangel, M.; Munch, S.B. A life-history perspective on short-and long-term consequences of compensatory growth. Am. Nat. 2005, 166, E155–E176. [Google Scholar] [CrossRef]
- Slack, J.M.; Lin, G.; Chen, Y. The Xenopus tadpole: A new model for regeneration research. Cell Mol. Life Sci. 2008, 65, 54–63. [Google Scholar] [CrossRef]
- Love, N.R.; Chen, Y.; Ishibashi, S.; Kritsiligkou, P.; Lea, R.; Koh, Y.; Gallop, J.L.; Dorey, K.; Amaya, E. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat. Cell Biol. 2013, 15, 222–228. [Google Scholar] [CrossRef]
- Beck, C.W.; Christen, B.; Barker, D.; Slack, J.M. Temporal requirement for bone morphogenetic proteins in regeneration of the tail and limb of Xenopus tadpoles. Mech. Dev. 2006, 123, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Nieuwkoop, P.; Faber, J. (Eds.) Normal Table of Xenopus laevis (Daudin): A Systematic and Chronological Survey of the Development from the Fertilized Egg till the End of Metamorphosis; North-Holland Publishing Company: Amsterdam, The Netherlands, 1967; ISBN 978-0815318965. [Google Scholar]
- Denver, R.J.; Mirhadi, N.; Phillips, M. Adaptive plasticity in amphibian metamorphosis: Response of Scaphiopus hammondii tadpoles to habitat desiccation. Ecology 1998, 79, 1859–1872. [Google Scholar] [CrossRef]
- De Block, M.; Stoks, R. Compensatory growth and oxidative stress in a damselfly. Proc. R. Soc. 2008, 275, 781–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denver, R.J. Proximate mechanisms of phenotypic plasticity in amphibian metamorphosis. Integr. Comp. Biol. 1997, 37, 172–184. [Google Scholar] [CrossRef]
- Werner, E.E.; Anholt, B.R. Ecological consequences of the trade-off between growth and mortality rates mediated by foraging activity. Am. Nat. 1993, 142, 242–272. [Google Scholar] [CrossRef] [PubMed]
- Wilbur, H.M.; Collins, J.P. Ecological aspects of amphibian metamorphosis. Science 1973, 182, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Echeverri, K.; Clarke, J.D.; Tanaka, E.M. In vivo imaging indicates muscle fiber dedifferentiation is a major contributor to the regenerating tail blastema. Dev. Biol. 2001, 236, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Schnapp, E.; Kragl, M.; Rubin, L.; Tanaka, E.M. Hedgehog signaling controls dorsoventral patterning, blastema cell proliferation and cartilage induction during axolotl tail regeneration. Development 2005, 132, 3243–3253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whited, J.L.; Tabin, C.J. Regeneration review reprise. J. Biol. 2010, 9, 15. [Google Scholar] [CrossRef]
- Gargioli, C.; Slack, J.M. Cell lineage tracing during Xenopus tail regeneration. Development 2004, 131, 2669–2679. [Google Scholar] [CrossRef]
- Maki, N.; Suetsugu-Maki, R.; Tarui, H.; Agata, K.; Del Rio-Tsonis, K.; Tsonis, P.A. Expression of stem cell pluripotency factors during regeneration in newts. Dev. Dyn. 2009, 238, 1613–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, N.R.; Chen, Y.; Bonev, B.; Gilchrist, M.J.; Fairclough, L.; Lea, R.; Mohun, T.J.; Paredes, R.; Zeef, L.A.; Amaya, E. Genome-wide analysis of gene expression during Xenopus tropicalis tadpole tail regeneration. BMC Dev. Biol. 2011, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Gauron, C.; Rampon, C.; Bouzaffour, M.; Ipendey, E.; Teillon, J.; Volovitch, M.; Vriz, S. Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci. Rep. 2013, 3, 2084. [Google Scholar] [CrossRef] [PubMed]
- Hennings, H.; Michael, D.; Cheng, C.; Steinert, P.; Holbrook, K.; Yuspa, S.H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell 1980, 19, 245–254. [Google Scholar] [CrossRef]
- Mattson, M.P. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell. Biol. 2000, 1, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C. Fusion and fission: Interlinked processes critical for mitochondrial health. Ann. Rev. Genet. 2012, 46, 265–287. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Shirihai, O.S. The interplay between mitochondrial dynamics and mitophagy. Antioxid. Redox Signal 2011, 14, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Szabadkai, G.; Simoni, A.M.; Chami, M.; Wieckowski, M.R.; Youle, R.J.; Rizzuto, R. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol. Cell 2004, 16, 59–68. [Google Scholar] [CrossRef]
- Youle, R.J.; Van Der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Rouzier, C.; Bannwarth, S.; Chaussenot, A.; Chevrollier, A.; Verschueren, A.; Bonello-Palot, N.; Fragaki, K.; Cano, A.; Pouget, J.; Pellissier, J.F.; et al. The MFN2 gene is responsible for mitochondrial DNA instability and optic atrophy “plus” phenotype. Brain 2012, 135, 23–34. [Google Scholar] [CrossRef] [PubMed]
- McCarron, J.G.; Wilson, C.; Sandison, M.E.; Olson, M.L.; Girkin, J.M.; Saunter, C.; Chalmers, S. From structure to function: Mitochondrial morphology, motion and shaping in vascular smooth muscle. J. Vasc. Res. 2013, 50, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Solenski, N.J.; diPierro, C.G.; Trimmer, P.A.; Kwan, A.L.; Helms, G.A. Ultrastructural changes of neuronal mitochondria after transient and permanent cerebral ischemia. Stroke 2002, 33, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B. Mitochondrial permeability transition pore: An enigmatic gatekeeper. New Horiz. Sci. Technol. 1015, 1, 47–51. [Google Scholar]
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef] [Green Version]
- van der Zand, A.; Gent, J.; Braakman, I.; Tabak, H.F. Biochemically distinct vesicles from the endoplasmic reticulum fuse to form peroxisomes. Cell 2012, 149, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Barroso, J.B.; del Río, L.A. Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci. 2001, 6, 145–150. [Google Scholar] [CrossRef]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef]
- Tattersall, G.J.; Wright, P.A. The effects of ambient pH on nitrogen excretion in early life stages of the American toad (Bufo americanus). Comp. Biochem. Physiol. A Physiol. 1996, 113, 369–374. [Google Scholar] [CrossRef]
- Willens, S.; Stoskopf, M.K.; Baynes, R.E.; Lewbart, G.A.; Taylor, S.K.; Kennedy-Stoskopf, S. Percutaneous malathion absorption by anuran skin in flow-through diffusion cells. Environ. Toxicol. Pharmacol. 2006, 22, 255–262. [Google Scholar] [CrossRef]
- Sumigray, K.D.; Lechler, T. Cell adhesion in epidermal development and barrier formation. In Current Topics in Developmental Biology; Yap, A., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 383–414. [Google Scholar]

 100 m. However, the size of the blastema was slightly larger in the experimental group compared to the control [33].
100 m. However, the size of the blastema was slightly larger in the experimental group compared to the control [33].
 100 m. However, the size of the blastema was slightly larger in the experimental group compared to the control [33].
100 m. However, the size of the blastema was slightly larger in the experimental group compared to the control [33].
 100 m.
100 m.
 100 m.
100 m.

 100 m.
100 m.
 100 m.
100 m.
 100 m.
100 m.



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holganza, M.V.; Rivie, A.; Martus, K.; Menon, J. Modulation of Metamorphic and Regenerative Events by Cold Atmospheric Pressure Plasma Exposure in Tadpoles, Xenopus laevis. Appl. Sci. 2019, 9, 2860. https://doi.org/10.3390/app9142860
Holganza MV, Rivie A, Martus K, Menon J. Modulation of Metamorphic and Regenerative Events by Cold Atmospheric Pressure Plasma Exposure in Tadpoles, Xenopus laevis. Applied Sciences. 2019; 9(14):2860. https://doi.org/10.3390/app9142860
Chicago/Turabian StyleHolganza, Ma Veronica, Adonis Rivie, Kevin Martus, and Jaishri Menon. 2019. "Modulation of Metamorphic and Regenerative Events by Cold Atmospheric Pressure Plasma Exposure in Tadpoles, Xenopus laevis" Applied Sciences 9, no. 14: 2860. https://doi.org/10.3390/app9142860
APA StyleHolganza, M. V., Rivie, A., Martus, K., & Menon, J. (2019). Modulation of Metamorphic and Regenerative Events by Cold Atmospheric Pressure Plasma Exposure in Tadpoles, Xenopus laevis. Applied Sciences, 9(14), 2860. https://doi.org/10.3390/app9142860



