Emerging Distortion Product Otoacoustic Emission Techniques to Identify Preclinical Warning Signs of Basal Cochlear Dysfunction Due to Ototoxicity
Abstract
:1. Introduction
1.1. Serial Monitoring for Ototoxic Therapies
1.2. Expanding the Bandwidth Beyond 8 kHz
1.3. Advancing Distortion Product Otoacoustic Emission (DPOAE) Paradigms
2. Materials and Methods
2.1. Patients
2.2. Equipment, Software, and Calibration
2.3. Procedures
2.4. Data Analyses
3. Results
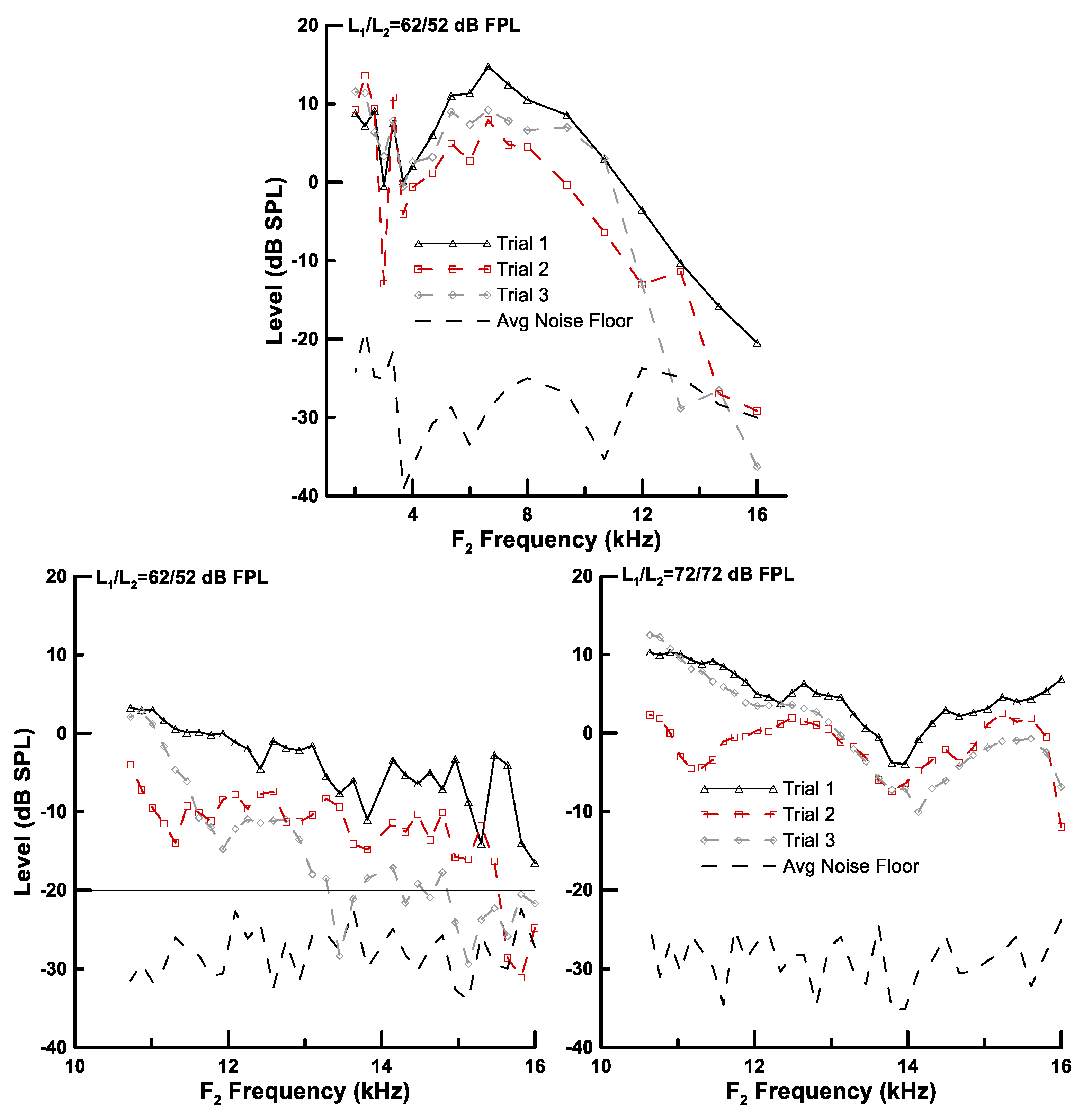
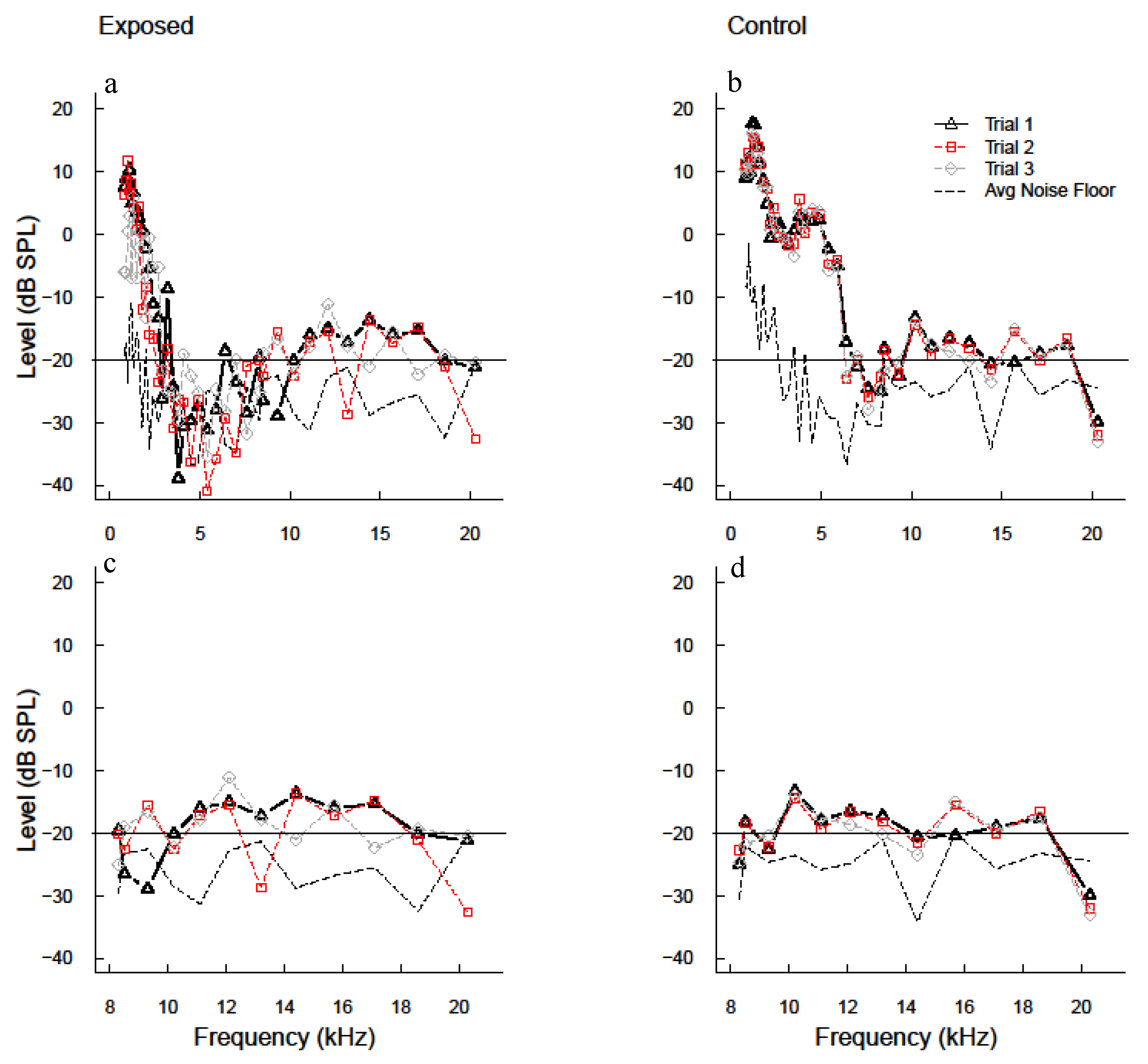
3.1. Advanced DPOAE Paradigms in Exposed Individuals—Frequency Sweeps
3.2. Advanced DPOAE Paradigms in Exposed Individuals—Level Sweeps
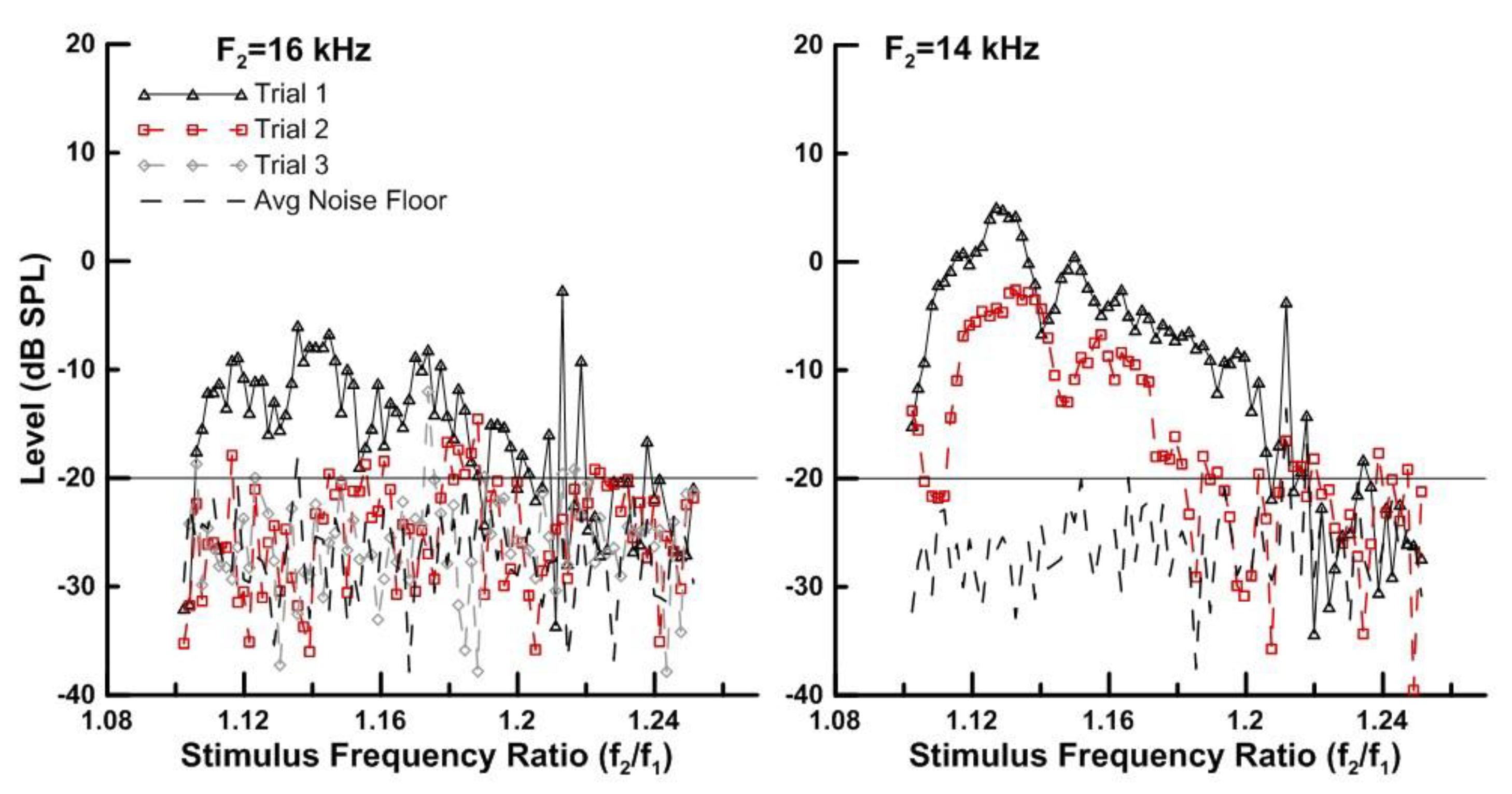
3.3. Advanced DPOAE Paradigms in Exposed Individuals—Ratio Sweeps
3.4. Summary of Results
4. Discussion
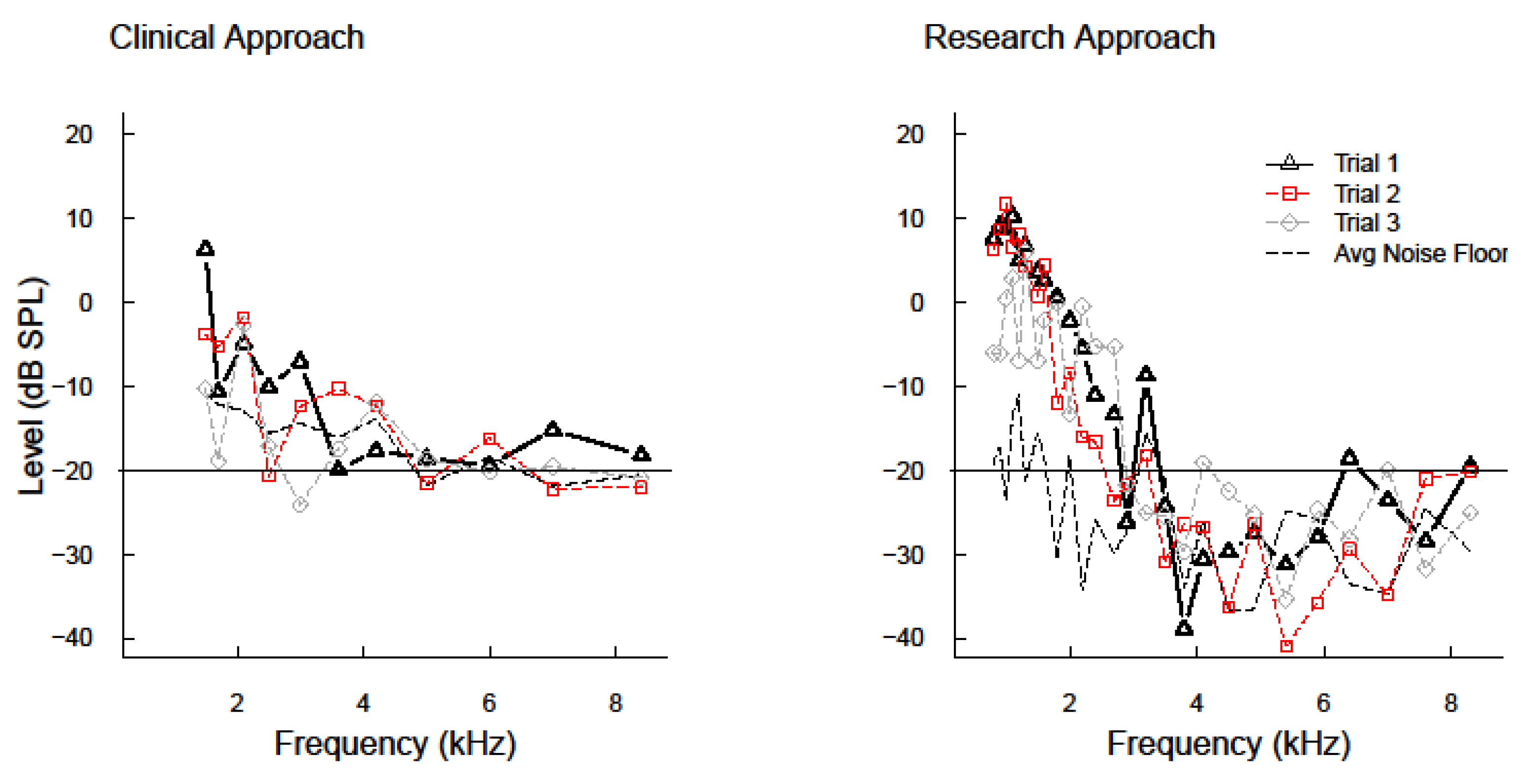
4.1. Serial Measurements of DPOAEs ≤8 kHz
4.2. Serial Measurement of DPOAEs with and without Platinum Derivatives
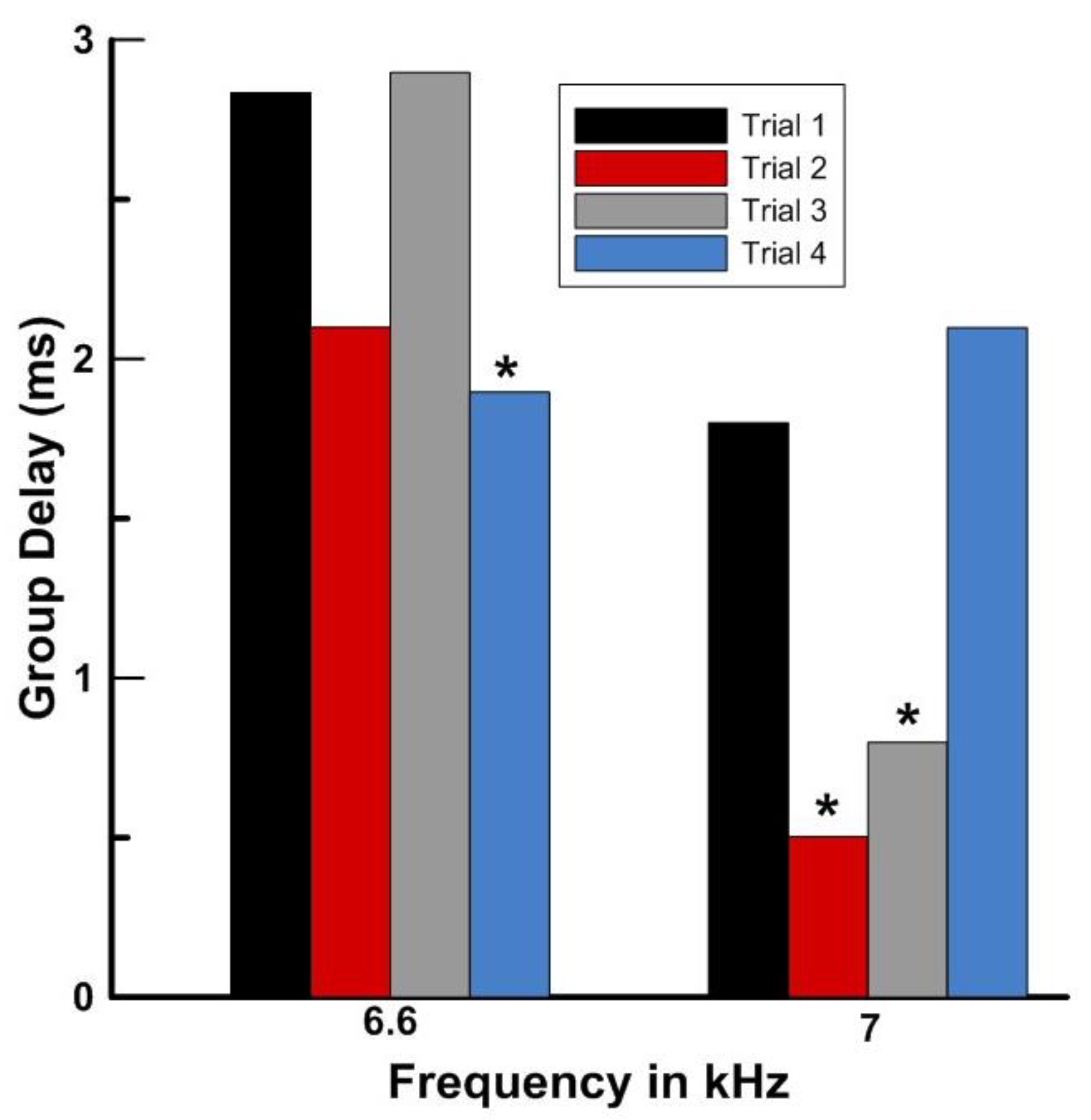
4.3. Advanced DPOAE Paradigm Comparisons (Frequency, Level, and Ratio Sweeps—Group Delays)
4.4. Clinical Implications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Watts, K.L. Ototoxicity: Visualized in Concept Maps. In Seminars in Hearing; Thieme Medical Publishers: Stuttgart, Germany, 2019; Volume 40, pp. 177–187. [Google Scholar]
- Konrad-Martin, D.; Poling, G.L.; Dreisbach, L.E.; Reavis, K.M.; McMillan, G.P.; Lapsley Miller, J.A.; Marshall, L. Serial Monitoring of Otoacoustic Emissions in Clinical Trials. Otol. Neurotol. 2016, 37, e286–e294. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Audiology. American Academy of Audiology’s Position Statement and Practice Guidelines on Ototoxicity Monitoring; American Academy of Audiology: Reston, VA, USA, 2009. [Google Scholar]
- American Speech-Language-Hearing Association. Guidelines for the audiologic management of individuals receiving cochleotoxic drug therapy. ASHA 1994, 36 (Suppl. 12), 11–19. [Google Scholar]
- Konrad-Martin, D.; Poling, G.L.; Garinis, A.C.; Ortiz, C.E.; Hopper, J.; O’Connell Bennett, K.; Dille, M.F. Applying US national guidelines for ototoxicity monitoring in adult patients: Perspectives on patient populations, service gaps, barriers and solutions. Int. J. Audiol. 2017, 57, S3–S18. [Google Scholar] [CrossRef] [PubMed]
- Dreschler, W.A.; van der Hulst, R.J.; Tange, R.A.; Urbanus, N.A. Role of high-frequency audiometry in the early detection of ototoxicity: II. Clinical Aspects. Audiology 1989, 28, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Ress, B.D.; Sridhar, K.S.; Balkany, T.J.; Waxman, G.M.; Stagner, B.B.; Lonsbury-Martin, B.L. Effects of cis-platinum chemotherapy on otoacoustic emissions: The development of an objective screening protocol. Third place—Resident Clinical Science Award 1998. Otolaryngol. Head Neck Surg. 1999, 121, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Fausti, S.A.; Frey, R.H.; Henry, J.A.; Robertson, P.G.; Hertert, R.S. Portable stimulus generator for obtaining high-frequency (8–14 kHz) auditory brainstem responses. J. Am. Acad. Audiol. 1992, 3, 166–175. [Google Scholar] [PubMed]
- Bielefeld, E.C.; Henderson, D. Mechanisms of Cisplatin Ototoxicity and Routes for Intervention. Perspect. Hear. Hear. Disord. Res. Diagn. 2011, 15, 3–14. [Google Scholar] [CrossRef]
- Komune, S.; Aakuma, S.; Snow, J.B. Pathophysiology of the ototoxicity of cis-diamminedichloroplatinum. Otolaryngol. Head Neck Surg. 1981, 89, 275–282. [Google Scholar] [CrossRef]
- Dammeyer, P.; Hellberg, V.; Wallin, I.; Laurell, G.; Shoshan, M.; Ehrsson, H.; Arnér, E.S.; Kirkegaard, M. Cisplatin and oxaliplatin are toxic to cochlear outer hair cells and both target thioredoxin reductase in organ of Corti cultures. Acta Otolaryngol. 2014, 134, 448–454. [Google Scholar] [CrossRef]
- Hofstetter, P.; Ding, D.; Powers, N.; Salvi, R.J. Quantitative relationship of carboplatin dose to magnitude of inner and outer hair cell loss and the reduction in distortion product otoacoustic emission amplitude in chinchillas. Hear. Res. 1997, 112, 199–215. [Google Scholar] [CrossRef]
- Ding, D.L.; Wang, J.; Salvi, R.; Henderson, D.; Hu, B.H.; McFadden, S.L.; Mueller, M. Selective loss of inner hair cells and type-I ganglion neurons in carboplatin-treated chinchillas. Mechanisms of damage and protection. Ann. N. Y. Acad. Sci. 1999, 884, 152–170. [Google Scholar] [CrossRef]
- Kraus, K.S.; Ding, D.; Jiang, H.; Kermany, M.H.; Mitra, S.; Salvi, R.J. Up-regulation of GAP-43 in the chinchilla ventral cochlear nucleus after carboplatin-induced hearing loss: Correlations with inner hair cell loss and outer hair cell loss. Hear. Res. 2013, 302, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Ding, D.; Allman, B.L.; Salvi, R. Review: Ototoxic characteristics of platinum antitumor drugs. Anat. Rec. 2012, 295, 1851–1867. [Google Scholar] [CrossRef]
- Dreisbach, L.; Ho, M.; Reid, E.; Siegel, J. Effects of Oxaliplatin, Carboplatin, and Cisplatin Across Treatment on High-Frequency Objective and Subjective Auditory Measures in Adults. Perspect. ASHA Spec. Interest Groups 2017, 2, 17–36. [Google Scholar] [CrossRef]
- Brown, A.M.; McDowell, B.; Forge, A. Acoustic distortion products can be used to monitor the effects of chronic gentamicin treatment. Hear. Res. 1989, 42, 143–156. [Google Scholar] [CrossRef]
- Scheperle, R.A.; Neely, S.T.; Kopun, J.G.; Gorga, M.P. Influence of in situ, sound-level calibration on distortion-product otoacoustic emission variability. J. Acoust. Soc. Am. 2008, 124, 288–300. [Google Scholar] [CrossRef]
- Scheperle, R.A.; Goodman, S.S.; Neely, S.T. Further assessment of forward pressure level for in situ calibration. J. Acoust. Soc. Am. 2011, 130, 3882–3892. [Google Scholar] [CrossRef] [Green Version]
- Souza, N.N.; Dhar, S.; Neely, S.T.; Siegel, J.H. Comparison of nine methods to estimate ear-canal stimulus levels. J. Acoust. Soc. Am. 2014, 136, 1768. [Google Scholar] [CrossRef]
- Poling, G.L.; Siegel, J.H.; Lee, J.; Dhar, S. Characteristics of the 2f(1)-f(2) distortion product otoacoustic emission in a normal hearing population. J. Acoust. Soc. Am. 2014, 135, 287–299. [Google Scholar] [CrossRef]
- Lee, J.; Dhar, S.; Abel, R.; Banakis, R.; Grolley, E.; Lee, J.; Zecker, S.; Siegel, J. Behavioral hearing thresholds between 0.125 and 20 kHz using depth-compensated ear simulator calibration. Ear Hear. 2012, 33, 315–329. [Google Scholar] [CrossRef]
- Kemp, D.T. Evidence of mechanical nonlinearity and frequency selective wave amplification in the cochlea. Arch. Oto-Rhino-Laryngol. 1979, 224, 37–45. [Google Scholar] [CrossRef]
- Dorn, P.A.; Piskorski, P.; Gorga, M.P.; Neely, S.T.; Keefe, D.H. Predicting audiometric status from distortion product otoacoustic emissions using multivariate analyses. Ear Hear. 1999, 20, 149–163. [Google Scholar] [CrossRef]
- Gorga, M.P.; Neely, S.T.; Ohlrich, B.; Hoover, B.; Redner, J.; Peters, J. From laboratory to clinic: A large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear Hear. 1997, 18, 440–455. [Google Scholar] [CrossRef]
- Arnold, D.J.; Lonsbury-Martin, B.L.; Martin, G.K. High-frequency hearing influences lower-frequency distortion-product otoacoustic emissions. Arch. Otolaryngol. Head Neck Surg. 1999, 125, 215–222. [Google Scholar] [CrossRef]
- Reavis, K.M.; Phillips, D.S.; Fausti, S.A.; Gordon, J.S.; Helt, W.J.; Wilmington, D.; Bratt, G.W.; Konrad-Martin, D. Factors affecting sensitivity of distortion-product otoacoustic emissions to ototoxic hearing loss. Ear Hear. 2008, 29, 875–893. [Google Scholar] [CrossRef]
- Dreisbach, L.E.; Siegel, J.H. Distortion-product otoacoustic emissions measured at high frequencies in humans. J. Acoust. Soc. Am. 2001, 110, 2456–2469. [Google Scholar] [CrossRef]
- Dreisbach, L.E.; Long, K.M.; Lees, S.E. Repeatability of high-frequency distortion-product otoacoustic emissions in normal-hearing adults. Ear Hear. 2006, 27, 466–479. [Google Scholar] [CrossRef]
- Dreisbach, L.; Zettner, E.; Chang Liu, M.; Meuel Fernhoff, C.; MacPhee, I.; Boothroyd, A. High-Frequency Distortion-Product Otoacoustic Emission Repeatability in a Patient Population. Ear Hear. 2018, 39, 85–100. [Google Scholar] [CrossRef]
- Dreisbach, L.E.; Siegel, J.H. Level dependence of distortion-product otoacoustic emissions measured at high frequencies in humans. J. Acoust. Soc. Am. 2005, 117, 2980–2988. [Google Scholar] [CrossRef]
- Robles, L.; Ruggero, M.A. Mechanics of the mammalian cochlea. Physiol. Rev. 2001, 81, 1305–1352. [Google Scholar] [CrossRef]
- Katbamna, B.; Homnick, D.N.; Marks, J.H. Effects of chronic tobramycin treatment on distortion product otoacoustic emissions. Ear Hear. 1999, 20, 393–402. [Google Scholar] [CrossRef]
- Abdala, C.; Dhar, S. Maturation and Aging of the Human Cochlea: A View through the DPOAE Looking Glass. J. Assoc. Res. Otolaryngol. 2012, 13, 403–421. [Google Scholar] [CrossRef] [Green Version]
- Engdahl, B.; Kemp, D.T. The effect of noise exposure on the details of distortion product otoacoustic emissions in humans. J. Acoust. Soc. Am. 1996, 99, 1573–1587. [Google Scholar] [CrossRef]
- Namyslowski, G.; Morawski, K.; Trybalska, G.; Urbaniec, P. The latencies of the 2f1–f2 DPOAE measured using phase gradient method in young adults and in workers chronically exposed to noise. Otolaryngol. Pol. 2004, 58, 131–138. [Google Scholar]
- Telischi, F.F.; Stagner, B.B.; Widick, M.P.; Balkany, T.J.; Lonsbury-Martin, B.L. Distortion-product otoacoustic emission monitoring of cochlear blood flow. Laryngoscope 1998, 108, 837–842. [Google Scholar] [CrossRef]
- Neely, S.T.; Liu, Z. EMAV: Otoacoustic Emission Averager; Technical Memo No. 17; Boys Town National Research Hospital: Omaha, NE, USA, 1993. [Google Scholar]
- Poling, G.; Lee, J.; Siegel, J.; Dhar, S. Clinical Utilisation of High-frequency DPOAEs. ENT Audiol. News 2012, 21, 1–6. [Google Scholar]
- Heitmann, J.; Waldmann, B.; Schnitzler, H.U.; Plinkert, P.K.; Zenner, H.P. Suppression of distortion product otoacoustic emissions (DPOAE) near 2f1–f2 removes DP-gram fine structure—Evidence for a secondary generator. J. Acoust. Soc. Am. 1998, 103, 1527–1531. [Google Scholar] [CrossRef]
- Talmadge, C.L.; Long, G.R.; Tubis, A.; Dhar, S. Experimental confirmation of the two-source interference model for the fine structure of distortion product otoacoustic emissions. J. Acoust. Soc. Am. 1999, 105, 275–292. [Google Scholar] [CrossRef]
- Dhar, S.; Talmadge, C.L.; Long, G.R.; Tubis, A. Multiple internal reflections in the cochlea and their effect on DPOAE fine structure. J. Acoust. Soc. Am. 2002, 112, 2882–2897. [Google Scholar] [CrossRef]
- Conrad, A.; Dreisbach, L. Repeatability of high-frequency DPOAE measures in normal-hearing children. Am. Audit. Soc. Abstr. 2011, 36, 42. [Google Scholar]
- Newman, S.; Dreisbach, L. Repeatability of high-frequency behavioral and DPOAE measures in normal-hearing children. Am. Audit. Soc. Abstr. 2012, 37, 57. [Google Scholar]
- Franklin, D.J.; McCoy, M.J.; Martin, G.K.; Lonsbury-Martin, B.L. Test/retest reliability of distortion-product and transiently evoked otoacoustic emissions. Ear Hear. 1992, 13, 417–429. [Google Scholar] [CrossRef]
- Roede, J.; Harris, F.P.; Probst, R.; Xu, L. Repeatability of distortion product otoacoustic emissions in normally hearing humans. Audiology 1993, 32, 273–281. [Google Scholar] [CrossRef]
- Beattie, R.C.; Kenworthy, O.T.; Luna, C.A. Immediate and short-term reliability of distortion-product otoacoustic emissions. Int. J. Audiol. 2003, 42, 348–354. [Google Scholar] [CrossRef]
- Reavis, K.M.; McMillan, G.; Austin, D.; Gallun, F.; Fausti, S.A.; Gordon, J.S.; Helt, W.J.; Konrad-Martin, D. Distortion-product otoacoustic emission test performance for ototoxicity monitoring. Ear Hear. 2011, 32, 61–74. [Google Scholar] [CrossRef]
- Dille, M.; McMillan, G.; Reavis, K.; Jacobs, P.; Fausti, S.; Konrad-Martin, D. Ototoxicity risk assessment combining distortion product otoacoustic emissions with a cisplatin dose model. J. Acoust. Soc. Am. 2010, 128, 1163–1174. [Google Scholar] [CrossRef]
- Shera, C.A.; Guinan, J.J. Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs. J. Acoust. Soc. Am. 1999, 105, 782–798. [Google Scholar] [CrossRef]
- Martin, G.K.; Stagner, B.B.; Jassir, D.; Telischi, F.F.; Lonsbury-Martin, B.L. Suppression and enhancement of distortion-product otoacoustic emissions by interference tones above f(2). I. Basic findings in rabbits. Hear. Res. 1999, 136, 105–123. [Google Scholar] [CrossRef]
- Rao, A.; Long, G.R. Effects of aspirin on distortion product fine structure: Interpreted by the two-source model for distortion product otoacoustic emissions generation. J. Acoust. Soc. Am. 2011, 129, 792–800. [Google Scholar] [CrossRef]

| Patient Demographics | |||||
|---|---|---|---|---|---|
| Patient | Gender | Age (years) | Platinum Agent | Cumulative Dose (mg) | Highest Frequency DPOAE (kHz) |
| 9 | Female | 33 | Carboplatin | 1455 | 16 |
| 16 | Female | 41 | Oxaliplatin | 1057 | 13.3 |
| 4 | Female | 42 | Oxaliplatin | 873 | 16 |
| 18 | Female | 44 | Carboplatin | 3907 | 13 |
| 8 | Female | 45 | Carboplatin | 3779 | 10.6 |
| 7 | Male | 47 | Oxaliplatin | 1079 | 7.3 |
| 1 | Male | 49 | Oxaliplatin | 519 | 12 |
| 12 | Male | 55 | Cisplatin | 163 | 6 |
| 10 | Female | 56 | Oxaliplatin | 1202 | 9 |
| 6 | Female | 59 | Carboplatin | 2412 | 8 |
| 2 | Male | 61 | Oxaliplatin | 3669 | 5.3 |
| 3 | Female | 79 | Carboplatin | Unknown | 3 |
| Concentrated Frequency Sweep: Mid-Level | ||||||
|---|---|---|---|---|---|---|
| Patient | Platinum Agent | Number of Sessions | Highest Frequency DPOAE (kHz) | Session of Change | Frequencies of Change (kHz) | Direction of Level Change |
| 1 | Oxaliplatin | 2 | 12 | S1 to S2 | 7.9–8.0 | Increase |
| 8.4–8.7, 9.9–10.0, & 10.3–10.4 | Decrease | |||||
| 4 | Oxaliplatin | 5 | 16 | S1 to S2 | 10.7–11.5 | Increase |
| S1 to S3 | 10.7–11.0 | Increase | ||||
| 15.3–15.8 | Decrease | |||||
| S1 to S4 | 10.7–12.3 | Increase | ||||
| S1 to S5 | 10.7–11.9 Hz | Increase | ||||
| 6 | Carboplatin | 4 | 8 | S1 to S2/S3 | 7.9–8.0 Hz | Increase |
| S1 to S4 | 7.8–8.0 | |||||
| 8 | Carboplatin | 4 | 10.6 | S1 to S2 | 7.5–7.6 & 8.5–8.6 | Decrease |
| S1 to S4 | 7.4–7.7, 7.9–8.0, 8.3–9.0, & 10.3–10.7 | |||||
| 9 | Carboplatin | 3 | 16 | S1 to S2 | 10.7–10.3, 12.6–13.1, 14.1–14.3, 14.8–15.0, & 14.6–16.0 | Decrease |
| S1 to S3 | 11.5–15.8 | |||||
| 10 a | Oxaliplatin | 3 | 9 | S1 to S3 | 6.1–6.5 & 7.5–7.6 | Increase |
| 8.6–8.7 | Decrease | |||||
| 16 | Oxaliplatin | 2 | 13.3 | S1 to S2 | 9.4–9.5 & 9.8–11.1 | Increase |
| 11.8–11.9 | Decrease | |||||
| 18 | Carboplatin | 2 | 13 | S1 to S2 | 8.0–10.1 & 10.5–10.6 | Decrease |
| 11.6–12.0 | Increase | |||||
| Summary of Results | |||
|---|---|---|---|
| Paradigm Analysis | Patients Tested | Patients with Significant Change | Significant Change Percentage |
| Gross Frequency Sweep | 12 | 1, 4, 6, 8, 9, 10, 16, 18 | 8/12 = 66% |
| Mid-Level Concentrated Sweep | 10 | 1, 4, 6, 8, 9, 10, 16, 18 | 8/10 = 80% |
| High-Level Concentrated Sweep | 12 | 1, 2, 4, 7, 8, 9, 10, 16, 18 | 9/12 = 75% |
| L2 Fixed, L1 Varied at Highest Frequency | 10 | 6, 7, 9, 10 | 4/10 = 40% |
| L2 Fixed, L1 Varied at 2nd Highest Frequency | 9 | 6, 7, 8, 9, 16, 18 | 6/9 = 66% |
| L1 Fixed, L2 Varied at Highest Frequency | 10 | 8, 9, 10, 16, 18 | 5/10 = 50% |
| L1 Fixed, L2 Varied at 2nd Highest Frequency | 10 | 4, 6, 7, 8, 9, 10, 12, 16, 18 | 9/10 = 90% |
| DP Level across Ratio at Highest Frequency | 9 | 4, 6, 7, 8, 9, 16, 18 | 7/9 = 78% |
| DP Level across Ratio at 2nd Highest Frequency | 8 | 4, 6, 7, 8, 9, 10, 18 | 7/8 = 87% |
| Group Delays at Highest Frequency | 9 | 6, 7, 9//6, 7, 9 | 3/9 = 33% |
| Group Delays at 2nd Highest Frequency | 8 | 4, 7, 18//7, 10, 18 | 3/8 = 37.5% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poling, G.L.; Vlosich, B.; Dreisbach, L.E. Emerging Distortion Product Otoacoustic Emission Techniques to Identify Preclinical Warning Signs of Basal Cochlear Dysfunction Due to Ototoxicity. Appl. Sci. 2019, 9, 3132. https://doi.org/10.3390/app9153132
Poling GL, Vlosich B, Dreisbach LE. Emerging Distortion Product Otoacoustic Emission Techniques to Identify Preclinical Warning Signs of Basal Cochlear Dysfunction Due to Ototoxicity. Applied Sciences. 2019; 9(15):3132. https://doi.org/10.3390/app9153132
Chicago/Turabian StylePoling, Gayla L., Brittany Vlosich, and Laura E. Dreisbach. 2019. "Emerging Distortion Product Otoacoustic Emission Techniques to Identify Preclinical Warning Signs of Basal Cochlear Dysfunction Due to Ototoxicity" Applied Sciences 9, no. 15: 3132. https://doi.org/10.3390/app9153132





