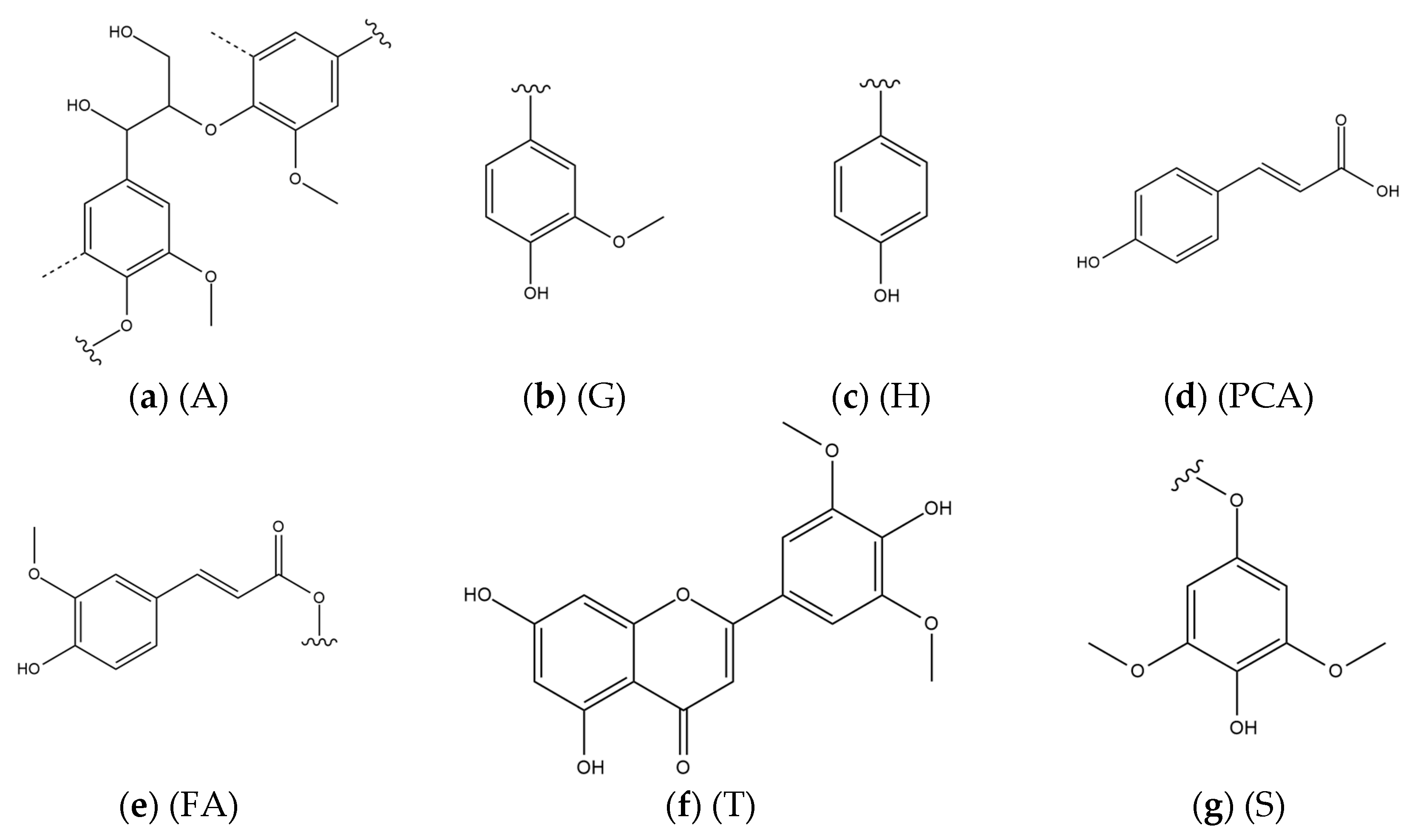
1. Introduction
Coffee is one of the most consumed beverages in the world and as such is becoming a daily commodity in most people’s lives [
1]. Such high consumption of coffee generates increasing amounts of spent coffee grounds (SCG). The United States Department of Agriculture estimate the yearly consumption to be 9.8 million tonnes of coffee beans worldwide [
2], which has been increasing steadily since 2011 [
2,
3,
4]. This demand for coffee demonstrates the lack of seasonality in the supply of coffee beans and the increasing availability of SCG to be industrially used and converted to fuels and higher value chemicals.
SCG were initially considered a waste, being landfilled or disposed in the sea [
5,
6]. However, the compositional analysis reveals the presence of carbohydrates (42–55%
w/w), lignin (0–25%
w/w), lipids (2–24%
w/w), protein (10–18%
w/w), caffeine (0–0.4%
w/w), chlorogenic acids (1–3%
w/w) and ash (1–2%
w/w) [
7,
8,
9,
10,
11,
12]. This composition lends itself to the production of fuels and higher value chemicals, and as such is a highly suitable feedstock to be used in the biorefinery industry.
Initially, as the calorific value of SCG were considered to be similar to agro-chemical residues, SCG were used directly for heat generation in industrial boilers [
13]. However, due to the increased heteroatom content, emissions from direct combustion are particularly hazardous. More suitably SCG have been pyrolyzed, yielding between 55% and 85% bio-oil, depending on the reaction conditions and moisture content [
14]. The pyrolysis process is particularly attractive and as such higher value compounds such as diterpenes can be extracted from the bio-oil. To this end, Peshev et al. added a downstream nanofiltration process that used the permeate and retentate in the production of further products [
15,
16].
The pyrolysis yields are so high as SCG contain up to 15% triglyceride oil. Alternative work has therefore focused on the extraction and transesterification of these lipids into biodiesel [
14,
17,
18,
19]. However, the low triglyceride oil content in SCG requires large amounts of SCG to produce considerable amounts of biodiesel, and as such cannot be produced as economically as from other glyceride sources.
Due to the high carbohydrate content, SCG have also been used in the production of ethanol through fermentation. Mussatto et al. used an acid pretreatment of the SCG, followed by enzymatic step to release the monosaccahrides. The authors compared three different yeast strains on both SCG and coffee silverskin. The SCG used led to much higher ethanol productions (11.7 g/L) compared to the coffee silverskin (producing less than 1 g/L) [
20]. Rocha et al., developed a biorefinery approach through fermentation of the SCG after the extraction of oil. This new design led to higher ethanol concentrations with a final titer of 19 g/L, while the extracted oil was used in the production of biodiesel.
However, with the unique diversity of molecules present in SCG there is also a large potential to produce higher value chemicals alongside fuels. Burniol-Figols et al. developed a biorefinery design to produce chlorogenic acid and bioethanol [
21]. The process included the extraction of phenolics to be converted into chlorogenic acid where the extraction residues were submitted to an acid hydrolysis, to depolymerize the sugars into monosaccharides, followed by fermentation to ethanol. Karmee evaluated a wide range of processes to come up with SCG-based biorefinery design [
10]. The evaluated processes included: different oil extraction processes, the production of biodiesel through base, acid, lipase catalysis or in-situ transesterification of the extracted oils, the catalytic upgrading of the oils to renewable diesel, production of bioethanol through hydrolysis and fermentation of the carbohydrates. The author also reported additional products such as carotenoids, antioxidants and polymers, such as polyols, polylactic acid and polyhydroxyalkanoates.
Caetano et al. presented a design composed of two initial extractions, one aqueous and one lipophilic, followed by the fermentation of the remaining solid waste [
22]. The first extraction was intended to remove high value extracts for upgrading to pharmaceuticals or for the cosmetic industry, while the product of the second extraction were triglycerides, used in the production of biodiesel. Using a similar design, Mata et al. replaced the fermentation process by pyrolysis and torrefaction to produce biochar and bio-oil [
23].
While the C6 sugars can be fermented into a range of products, another application is in the acid catalyzed dehydration to produce 5-hydroxymethylfurfural (HMF) [
24]. HMF can be used in a wide range of applications such as polymers and biofuels. Two of the most common applications include dimethylfuran (DMF) and 2,5-furandicarboxylic acid (FDCA). While DMF is known for being a potential biofuel with comparable energy density to gasoline, FDCA is a building block used in the production of polyesters, one of which, PEF, is a potential replacement for PET [
25,
26]. To date, however, there are no reports of HMF being produced from SCG.
In this work, a conceptual HMF biorefinery was investigated with the SCG being fractionated to separate the biomass into three different fractions: a cellulose-enriched fraction (CEF), hemicellulose-enriched fraction (HEF) and lignin-enriched fraction (LEF). This process has been developed for other lignocellulosic biomass, though has again not been demonstrated on SCG, and uses sulfuric acid as a catalyst and a ternary mixture of methyl isobutyl ketone (MIBK), ethanol and water to separate the biomass into the various fractions [
27]. More recent work done by Katahira et al., demonstrated that the replacement of ethanol in the ternary system with acetone leads to a more effective fractionation process [
28]. In this investigation, these solvent systems were then examined for the suitability to produce multiple product streams from SCG, with a focus on the production of HMF.
2. Materials and Methods
2.1. Materials
The SCG were obtained from Bio-bean Ltd. They were stored at 4 °C. SCG are commonly composed by cellulose (10–13%
w/
w), hemicellulose (32–42%
w/
w), lignin (0–25%
w/
w), protein (10–18%
w/
w), lipids (2–24%
w/
w), ash (1–2%
w/
w), caffeine (0–0.4%
w/
w) and chlorogenic acids (1–3%
w/
w) [
8,
9,
10,
11,
12]. Pistachio hull was supplied by the Wonderful Company (Los Angeles, CA, USA), the compositional analysis is given in the
Appendix A (
Table A1). All the chemicals used in this study were purchased from Sigma Aldrich (Gillingham, UK), and used without further purification.
2.2. Clean Fractionation
The fractionation process was carried out in a 300 mL Parr reactor (Parr Company Moline, IL, USA, 4560 mini reactors). In this process, 10 g of biomass were fractionated in three different solvent systems: (1) methyl isobutyl ketone (MIBK), ethanol and water (16/34/50 g/g/g; here forth this solvent system is denominated MEW); (2) MIBK, acetone and water (11/44/44 g/g/g, denominated MAW); (3) MIBK and water (16/84 g/g; MW). In all these systems, sulfuric acid was used as a catalyst in a concentration of 0.1 M. After loading both the biomass and solvent system, agitation was started, temperature increased to 140 °C and the reaction was carried out for 1 h. After the reaction, the reactor was cooled down to 30 °C. The obtained suspension was filtered and washed, initially with 200 mL of the same solvent system used in the fractionation, and secondly, with 650 mL of water to remove any soluble component present amidst the solids. The solid residue after filtration was denominated the cellulose-enriched fraction (CEF) and was dried at room temperature for 24 h. Then, 50 mL of MIBK was added to the filtrate in a separation funnel. The solution was mixed and left to rest until two distinct phases were observed (an aqueous and an organic phase). Once the phases were separated, 50 mL of MIBK was added to the aqueous phase for a second extraction. The solution was mixed, left to rest and the two distinct phases were separated. The obtained aqueous phase was denominated the hemicellulose-enriched fraction (HEF). Both organic phases (obtained from first and second extraction processes) were combined and the solvent removed under vacuum. The dried solids obtained from this process were further dried in an oven at 40 °C for 4 days, this was designated the lignin-enriched fraction (LEF).
2.3. Fermentation
The pH of the HEF fraction was increased to 4. This solution was then used in the preparation of four diluted solutions with deionized water: 25% HEF concentrated, 50% HEF concentrated, 75% HEF concentrated and a fully concentrated solution (100%). A control media of 30 g/L of peptone from soybean meal enzymatic digest and 25 g/L of malt extract was also prepared and autoclaved. All these solutions (4 dilutions from HEF and the control) were then inoculated with Metschnikowia pulcherrima (National Culture of Yeast Collection, NCYC4331) to make solutions with 500,000 cells/mL and a total volume of 20 mL in Erlenmeyer flasks. The flasks were sealed and placed in incubators at a temperature of 25 °C and 230 rpm for 3 days.
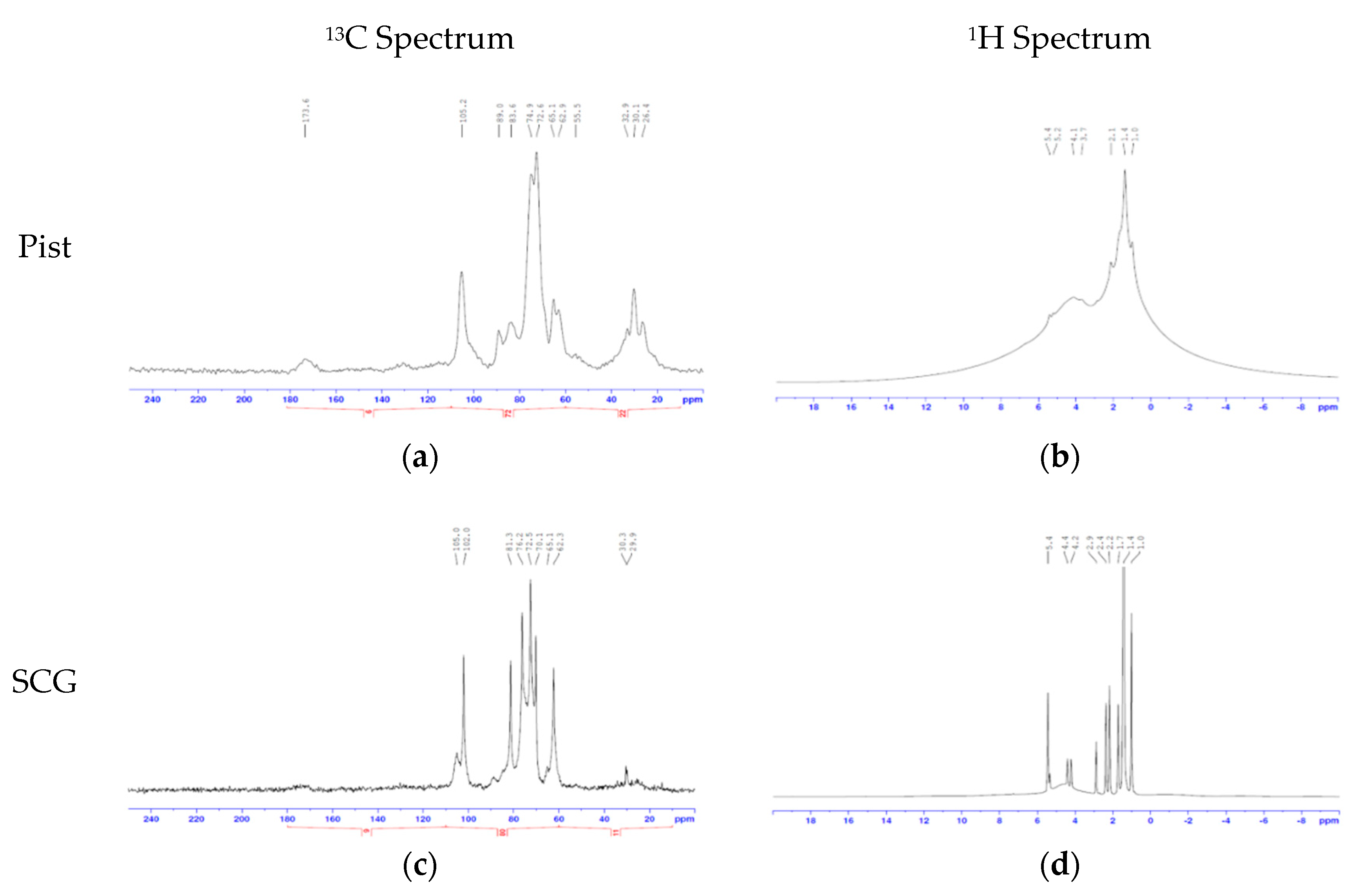
2.4. 2D-NMR
Solution state nuclear magnetic resonance (NMR) spectra were acquired using a Bruker Avance III NMR spectrometer operating at 500.13 MHz for 1 h and 125.77 MHz for 13 C. Samples were investigated in DMSO-d6 at 27 °C unless specified and were referenced to the residual solvent signal at 2.50 ppm (
1H) and 39.52 ppm (
13C). Following this,
1H-
13C correlation spectra were obtained using the “hsqcedetgpsp” pulse sequence, with td = 256 and ns = 128 and a relaxation delay of 1.5 s. Spectra were acquired using Bruker Topspin 2.1 and processed using Bruker Topspin 3.1. Assignment of the individual components was follows previous reports [
28,
29].
2.5. Cellulose Hydrolysis
The enzymatic hydrolysis of cellulose was carried out in 50 mL Falcon tubes in an incubator at 50 °C. Then, 0.5 g of CEF were added to 5 mL of a 0.1 M buffer of sodium acetate and acetic acid with a pH of 4.8. Cellulase (Cellulase from Aspergillus niger sourced from Sigma-Aldrich, UK) was used as enzyme—137.04 FPU/mL. Antibiotics were also added to avoid contamination: 12 mg/L of tetracyclin and 15 mg/L of gentamicin. The reaction was carried out for 72 h. At the end of the reaction, the solution was placed in a hot bath at 85 °C for enzyme deactivation, followed by centrifugation to separate the majority of the solids. High Performance Liquid Chromatography (HPLC) equipped with an Aminex HPX-87H HPLC column from Bio-Rad (heated up to 65 °C, with a flow rate of 0.6 mL/min) and a refractive index detector was used to determine the sugars content in solution.
2.6. Glucose Isomerization
After hydrolysis, the buffer solution had to be neutralized to a pH of 7 as this is the ideal pH for glucose isomerase from Streptomyces murinus (Sigma-Aldrich, UK)—15 FPU of enzyme were added to solution. The reaction was carried out for 24 h at 60 °C. The same conditions (column and detector) used in the glucose analysis were used for fructose quantification. The glucose concentrations in the initial solution and the added volume of sodium acetate (used in neutralization) were taken into account in the fructose yield calculations.
2.7. Dehydration to HMF
The HMF production was carried out in a biphasic system composed of 1.5 g of sodium chloride saturated aqueous solution (5 wt% fructose, 5 mM of AlCl
3, 3.17 mM of HCl) and 3 g of γ-valerolactone (GVL) as the organic solvent. The reaction was performed in glass pressure tubes and heated up to 170 °C. After 20 minutes the pressure tubes were cooled down to room temperature. The two phases were separated using a separating funnel. The HMF present in GVL was extracted from this solvent by mixing 1 part of GVL with 1 part of water and 20 parts of cyclopentane in a first extraction. This led to an aqueous solution containing 90% of the produced HMF and 43% of GVL [
30]. By mixing the resulting aqueous phase with 20 parts of cyclopentane three more times, the percentage of GVL in the aqueous solution is reduced to 0.5% and the percentage of recovered HMF was 99% of the total produced. The same Aminex HPX-87H column was used in the HMF analysis, however a diode array detector was used at a wavelength of 280 nm. The 5 mM sulfuric acid aqueous solvent was set to a flow rate of 0.6 mL/min and the column heated up to 65 °C.
4. Discussion
The similar masses obtained in the fractionation process for SCG and pistachio hull indicate that this process, initially developed for lignocellulosic biomass sources, can be efficiently used in the separation of the components present in SCG. The CEF mass yields obtained were approximately in accordance with the higher content of cellulose present in pistachio hull than in SCG (14% and 12%, respectively). Both the MAW and MEW solvent systems also deposit a range of other components, up to 60%, into this phase. Although this is in accordance with other lignocellulosic biomass separated through this method [
28].
The poor performance of the MW system demonstrates how both ethanol and acetone aid in the fractionation process. When these solvents are not used, most of the lignin remains as a solid species and is deposited elsewhere in the system. This reduces the ability to use LEF for bioenergy. Despite of the better fractionation performance, the presence of ethanol and acetone in the HEF fraction, demonstrated by the increased TOC in the phase, make it unsuitable for fermentation. In contrast, the aqueous fraction produced with MW contained all the micronutrients necessary for growth and could be used to grow the oleaginous yeast M. pulcherrima; interestingly the growth of the yeast was only limited by the sugar present and not inhibited by the presence of anything in the broth. For all the samples, the HEF fraction had a higher total nitrogen content when SCG was used. While this will relate to bioactive compounds such as caffeine as well as the increased protein content, it did not seem to have an inhibitory effect on the yeast.
The sugar content of the HEF fraction showed that, while HEF from pistachio hull can be used in the production of furfural, the absence of C5 sugars in SCG means that furfural could not be produced as a co-product with HMF in a SCG biorefinery. Instead, the presence of C6 sugars, such as galactose and mannose indicate that this fraction could potentially be used in the production of HMF, although with similar issues associated with the conversion of glucose. While the sugars in the HEF fraction could be fermented, to achieve a good fractionation in the overall system, ethanol and acetone must be used—this severely limits the use of these sugars without further costly separations.
The LEF fraction, solubilized in MIBK, obtained using MEW and MAW had a reasonably high HHV (15–26 MJ/kg) when compared to the LEF fraction from MW (approximately 11 MJ/kg). For both the MEW and MAW solvent systems, the LEF fractions from SCG had higher HHV (22–26 MJ/g) than pistachio hull (15–19 MJ/kg). This suggests that this fraction of SCG has higher potential to be used as a biofuel component. However, both the GC-MS and 2D NMR analysis of this fraction demonstrated the presence of depolymerized lignin and fatty acids. Both materials have elevated lipids, though the SCG has more. This indicates that this fraction may also be used in the production of renewable biodiesel and polymers, however, similar to the HEF fraction, the fatty acids would need to be separated from the other components, which could limit the applicability for lower value uses. One possibility would be to extract the lipids prior to the fractionation.
The final fraction contains the solids left over from the process, predominantly cellulose (CEF). The elemental analysis, TGA, ssNMR and FT-IR demonstrated that cellulose is present in both samples, up to approximately 40% of the phase. A large proportion of nitrogen and sulfur were also observed suggesting that protein was present, although there was no clear indication of high levels of lignin in this phase. As with the other phases, the CEF fraction from the MW separation was heavily contaminated for both biomass sources tested. The CEF fraction can be depolymerized to release glucose for either fermentation or further chemical manufacture. For the manufacture of HMF, the cellulose is too stable, and little HMF was produced from any of the CEF fractions. Indeed, it is only the conversion through multiple enzymatic steps to fructose that yielded reasonable HMF concentrations. While only 8.2% of the original starting material of HMF was produced from the SCG, 8.0% HMF was produced from pistachio hull. This is partly due to low percentage of cellulose in CEF and the thermodynamic equilibrium between glucose and fructose which limits the reaction yields [
41]. According to Al-Tai et al. the maximum theoretical yield that can be obtained in the glucose isomerization reaction is approximately 50% [
42]. This was the limiting step and reduces amounts of HMF produced per batch. As such, the yields obtained from SCG and pistachio were highly similar to crystalline cellulose when the lower amount of cellulose was taken into account. This suggests that the fractionation works well, and that there are no inhibitory compounds in this phase that limit further processing.
Considering the entire process of biomass fractionation (using both pistachio and SCG) and the subsequent conversion of CEF to HMF, it was possible to produce approximately 0.37 g of HMF from 10 g of pistachio hull and approximately 0.35 g from SCG. This value of approximately 4% is close to the theoretical possible, considering that there is 14% and 12% of cellulose in pistachio hull and SCG, respectively, that the conversion of cellulose to glucose is limited to between 75% and 95%, the conversion of glucose to fructose is limited by the kinetic equilibrium between these two sugars, which is translated to a maximum theoretical yield obtained is 50% and maximum HMF yield from fructose is 80%.
5. Conclusions
In the paper, a biorefinery based around the organosolv fractionation of spent coffee grounds (SCG) was attempted for the first time. The fractionation of SCG led to similar results to the fractionation of a lignocellulosic material, in this case pistachio hull. These results demonstrate that this organosolv process can effectively separate the components present in SCG into three different enriched fractions: yielding a solubilized fraction predominantly from hemicellulose (HEF), a cellulose enriched solid (CEF) and a lignin enriched organic phase (LEF). The fractionation worked well when ethanol and acetone were used, though both of these solvents partitioned into the aqueous phase, inhibiting the use as a fermentation media. On fractionating with just water and MIBK, the HEF fraction could be used for fermentation and was only limited by the amount of sugars that partitioned there. For the SCG, this was predominantly C6 mono- and disaccharides.
The LEF samples for the SCG had very high HHV and were predominately made up of lignin fragments and fatty acids. Both the MEW and MAW solvent systems were suitable for this fractionation. Finally, the CEF fraction was analyzed and demonstrated to contain large content of cellulose alongside a range of other macromolecules. The CEF fraction was shown to be suitable for the production of HMF, with close to the theoretical yield obtained when depolymerized to glucose and isomerized to fructose prior to conversion. This paper demonstrates that the organosolv process is suitable for fractionating SCG and multiple products can be produced in a biorefinery concept.