Digital Evaluation of the Accuracy of Computer-Guided Dental Implant Placement: An In Vitro Study
Abstract
:1. Introduction
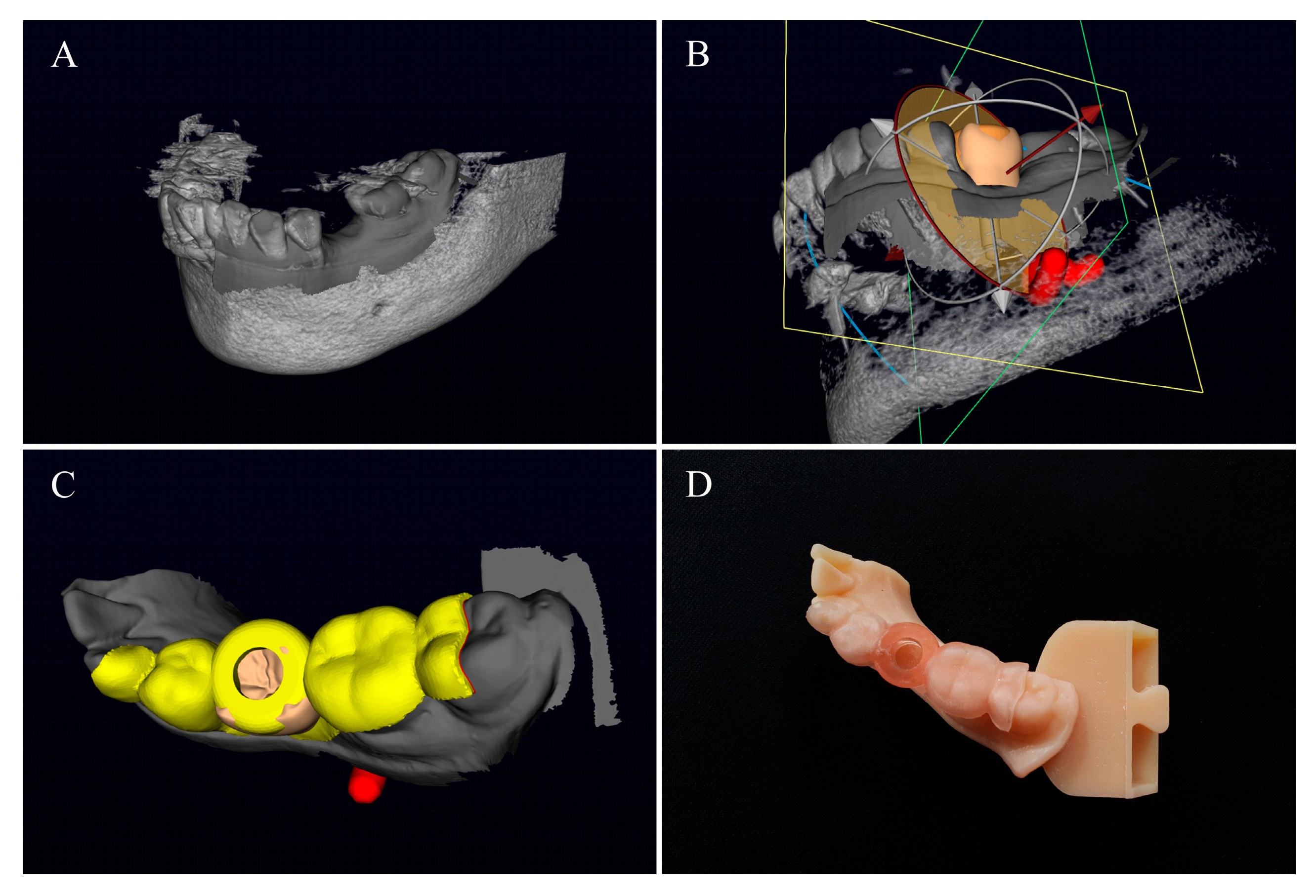
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- The surgical guide fabricated according to the two software programs shows no difference in the positioning accuracy of the implants.
- The accuracy of the personal 3D printed implant surgical guides is in the average range allowed by the dental clinician.
- The surgical guide fabricated by the method presented in this study can be utilized in dental clinical practice.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van Noort, R. The future of dental devices is digital. Dent. Mater. 2012, 28, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Azari, A.; Nikzad, S. Computer-assisted implantology: Historical background and potential outcomes-a review. Int. J. Med. Robot. 2008, 4, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Joda, T.; Ferrari, M.; Gallucci, G.O.; Wittneben, J.G.; Bragger, U. Digital technology in fixed implant prosthodontics. Periodontology 2000, 73, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Basten, C.H.; Kois, J.C. The use of barium sulfate for implant templates. J. Prosthet. Dent. 1996, 76, 451–454. [Google Scholar] [CrossRef]
- Orentlicher, G.; Abboud, M. Guided surgery for implant therapy. Dent. Clin. North Am. 2011, 55, 715–744. [Google Scholar] [CrossRef] [PubMed]
- Naitoh, M.; Ariji, E.; Okumura, S.; Ohsaki, C.; Kurita, K.; Ishigami, T. Can implants be correctly angulated based on surgical templates used for osseointegrated dental implants? Clin. Oral Implant. Res. 2000, 11, 409–414. [Google Scholar] [CrossRef]
- Edge, M.J. Surgical placement guide for use with osseointegrated implants. J. Prosthet. Dent. 1987, 57, 719–722. [Google Scholar] [CrossRef]
- D’Souza, K.M.; Aras, M.A. Types of implant surgical guides in dentistry: A review. J. Oral Implantol. 2012, 38, 643–652. [Google Scholar] [CrossRef]
- Nickenig, H.J.; Eitner, S. Reliability of implant placement after virtual planning of implant positions using cone beam CT data and surgical (guide) templates. J. Craniomaxillofac. Surg. 2007, 35, 207–211. [Google Scholar] [CrossRef]
- Jung, R.E.; Schneider, D.; Ganeles, J.; Wismeijer, D.; Zwahlen, M.; Hammerle, C.H. Computer technology applications in surgical implant dentistry: A systematic review. Int. J. Oral Maxillofac. Implant. 2009, 24, 92–109. [Google Scholar]
- Tahmaseb, A.; Wismeijer, D.; Coucke, W.; Derksen, W. Computer technology applications in surgical implant dentistry: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Spector, L. Computer-aided dental implant planning. Dent. Clin. North Am. 2008, 52, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Son, K.B.D.; Lee, W.S.; Lee, K.B. Effect of repeated learning for two dental CAD software programs. J. Dent. Rehabil. Appl. Sci. 2017, 33, 88–96. [Google Scholar] [CrossRef]
- Sicilia, A.; Botticelli, D. Computer-guided implant therapy and soft- and hard-tissue aspects. The third EAO consensus conference 2012. Clin. Oral Implant. Res. 2012, 23, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Tahmaseb, A.; Wu, V.; Wismeijer, D.; Coucke, W.; Evans, C. The accuracy of static computer-aided implant surgery: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 416–435. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, Z.; Song, L.; Kuo, C.L.; Shafer, D.M. Clinical factors affecting the accuracy of guided implant surgery-A systematic review and meta-analysis. J. Evid. Based Dent. Pract. 2018, 18, 28–40. [Google Scholar] [CrossRef]
- Son, K.; Huang, M.Y.; Lee, K.B. A method to evaluate the accuracy of dental implant placement without postoperative radiography after computer-guided implant surgery: A dental technique. J. Prosthet. Dent. 2019. [Google Scholar] [CrossRef]
- Martorelli, M. A new approach in CT artifact removal: Three cases study in maxillofacial surgery. Int. J. Interact. Des. Manuf. 2013, 7, 115–124. [Google Scholar] [CrossRef]
- Komiyama, A.; Pettersson, A.; Hultin, M.; Näsström, K.; Klinge, B. Virtually planned and template-guided implant surgery: An experimental model matching approach. Clin. Oral Implant. Res. 2011, 22, 308–313. [Google Scholar] [CrossRef]
- Tianhong, T.; Luman, L.; Zhuoli, H.; Xiaoyu, G.; Xiuyin, Z. Accuracy of the evaluation of completely digital registration implant position using a radiographic method compared with a method. J. Prosthet. Dent. 2019. [Google Scholar] [CrossRef]
- Schubert, C.; van Langeveld, M.C.; Donoso, L.A. Innovations in 3D printing: A 3D overview from optics to organs. Br. J. Ophthalmol. 2014, 98, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Tahayeri, A.; Morgan, M.; Fugolin, A.P.; Bompolaki, D.; Athirasala, A.; Pfeifer, C.S. 3D printed versus conventionally cured provisional crown and bridge dental materials. Dent. Mater. 2018, 34, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Whitley, D.; Eidson, R.S.; Rudek, I.; Bencharit, S. In-office fabrication of dental implant surgical guides using desktop stereolithographic printing and implant treatment planning software: A clinical report. J. Prosthet. Dent. 2017, 118, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Lin, W.S.; Harris, B.T.; Pellerito, J.; Morton, D. Fabrication of an interim complete removable dental prosthesis with an in-office digital light processing three-dimensional printer: A proof-of-concept technique. J. Prosthet. Dent. 2018, 120, 331–334. [Google Scholar] [CrossRef]
- Sotsuka, Y.; Nishimoto, S. Making three-dimensional mandible models using a personal three-dimensional printer. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 576–578. [Google Scholar] [CrossRef]
- Deeb, G.R.; Allen, R.K.; Hall, V.P.; Whitley, D.; Laskin, D.M.; Bencharit, S. How accurate are implant surgical guides produced with desktop stereolithographic 3-dimentional printers? J. Oral Maxillofac. Surg. 2017, 75, 2559.e1–2559.e8. [Google Scholar] [CrossRef]
- Van Assche, N.; Vercruyssen, M.; Coucke, W.; Teughels, W.; Jacobs, R.; Quirynen, M. Accuracy of computer-aided implant placement. Clin. Oral Implant. Res. 2012, 23, 112–123. [Google Scholar] [CrossRef]
- Ma, B.Y.; Park, T.S.; Chun, I.K.; Yun, K.D. The accuracy of a 3D printing surgical guide determined by CBCT and model analysis. J. Adv. Prosthodont. 2018, 10, 279–285. [Google Scholar] [CrossRef]
- Choi, B.; Jeong, S. Digital Flapless Implantology; Ji-Sung Publishing, Co.: Seoul, Korea, 2015; pp. 32–51. [Google Scholar]


| No. | Apical Deviation (mm) | Angular Deviation (°) |
|---|---|---|
| 1 | 0.4264 | 1.378 |
| 2 | 0.9347 | 2.8391 |
| 3 | 0.8911 | 2.3762 |
| 4 | 0.4799 | 3.213 |
| 5 | 0.6554 | 1.102 |
| 6 | 0.6141 | 3.0092 |
| 7 | 0.3532 | 1.4332 |
| 8 | 0.6747 | 2.1232 |
| 9 | 0.4748 | 1.2342 |
| 10 | 0.5325 | 0.9968 |
| Mean | 0.60368 | 1.9704 |
| Standard Deviation | 0.19182 | 0.8465 |
| No. | Apical Deviation (mm) | Angular Deviation (°) |
|---|---|---|
| 1 | 0.6219 | 1.9113 |
| 2 | 0.4728 | 1.3134 |
| 3 | 0.9832 | 2.983 |
| 4 | 0.7261 | 2.0142 |
| 5 | 0.6281 | 1.8602 |
| 6 | 0.3729 | 1.4312 |
| 7 | 0.5812 | 1.9786 |
| 8 | 0.4981 | 1.6823 |
| 9 | 0.3827 | 1.4821 |
| 10 | 0.8273 | 2.5829 |
| Mean | 0.6094 | 1.9239 |
| Standard Deviation | 0.1845 | 0.5207 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-M.; Son, K.; Kim, D.-Y.; Lee, K.-B. Digital Evaluation of the Accuracy of Computer-Guided Dental Implant Placement: An In Vitro Study. Appl. Sci. 2019, 9, 3373. https://doi.org/10.3390/app9163373
Kim S-M, Son K, Kim D-Y, Lee K-B. Digital Evaluation of the Accuracy of Computer-Guided Dental Implant Placement: An In Vitro Study. Applied Sciences. 2019; 9(16):3373. https://doi.org/10.3390/app9163373
Chicago/Turabian StyleKim, Seong-Min, Keunbada Son, Duk-Yeon Kim, and Kyu-Bok Lee. 2019. "Digital Evaluation of the Accuracy of Computer-Guided Dental Implant Placement: An In Vitro Study" Applied Sciences 9, no. 16: 3373. https://doi.org/10.3390/app9163373






