Surface, Volumetric, and Wetting Properties of Oleic, Linoleic, and Linolenic Acids with Regards to Application of Canola Oil in Diesel Engines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
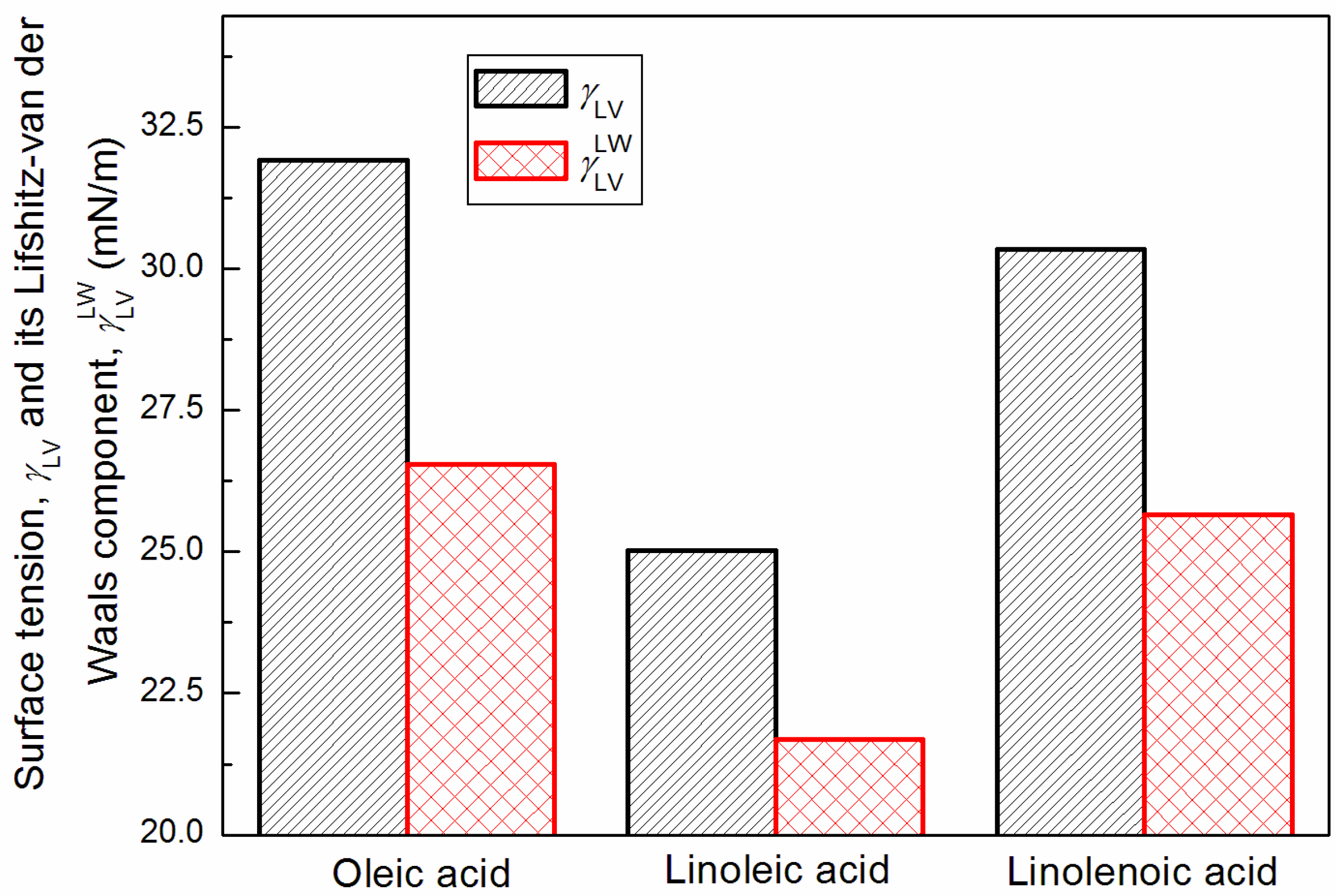
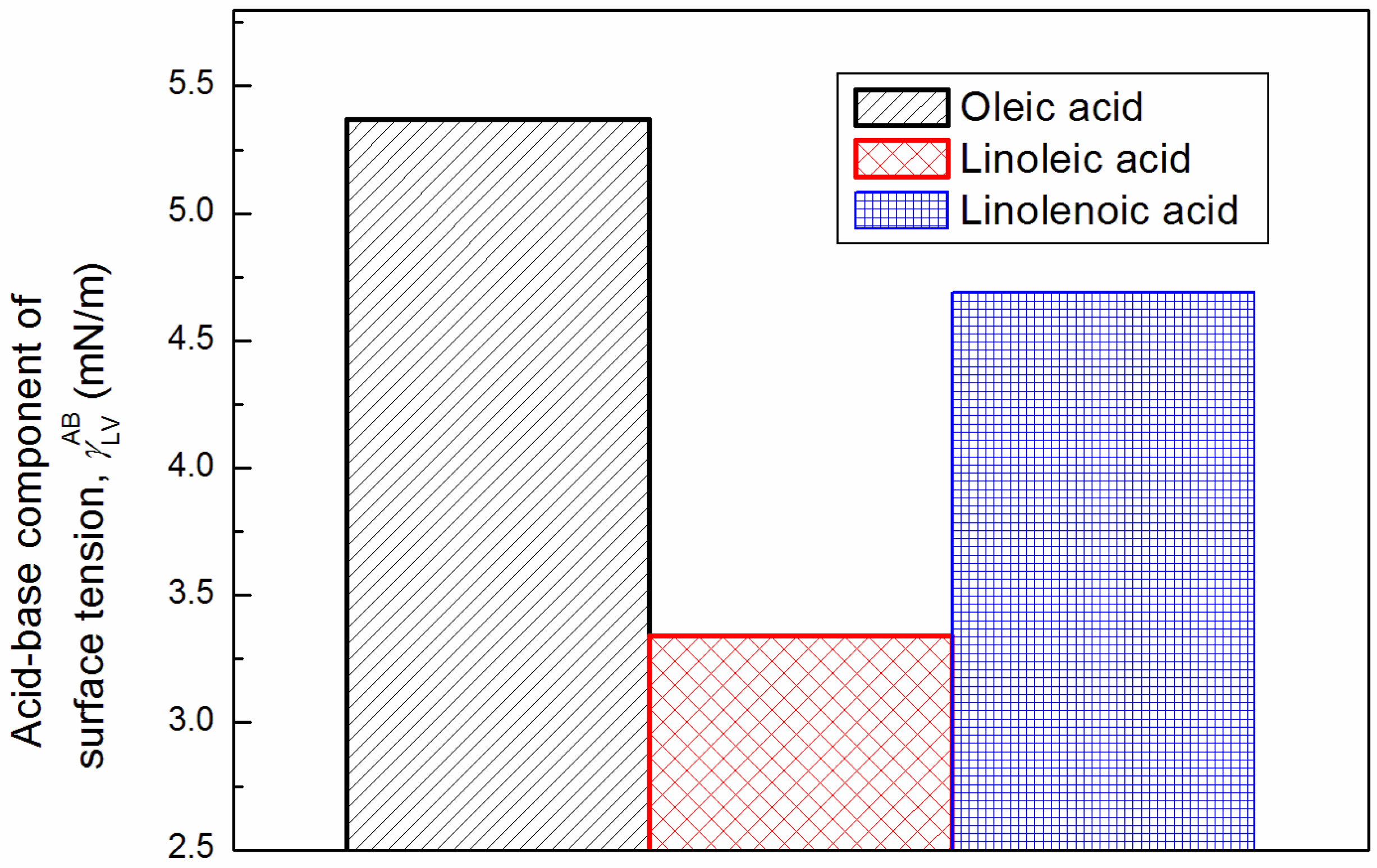
3.1. Surface Tension of Unsaturated Fatty Acids
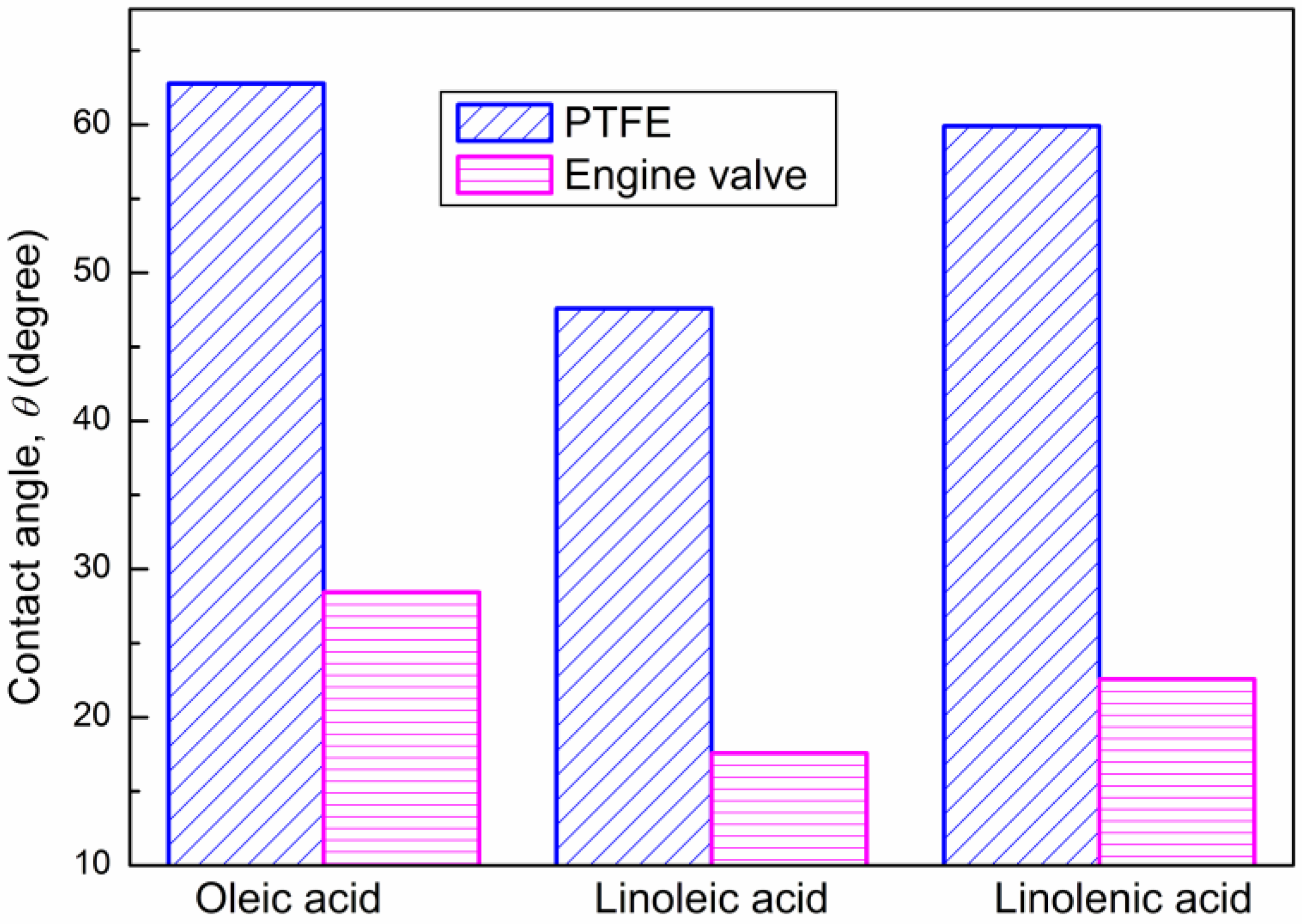
3.2. Wetting Properties of Unsaturated Fatty Acids
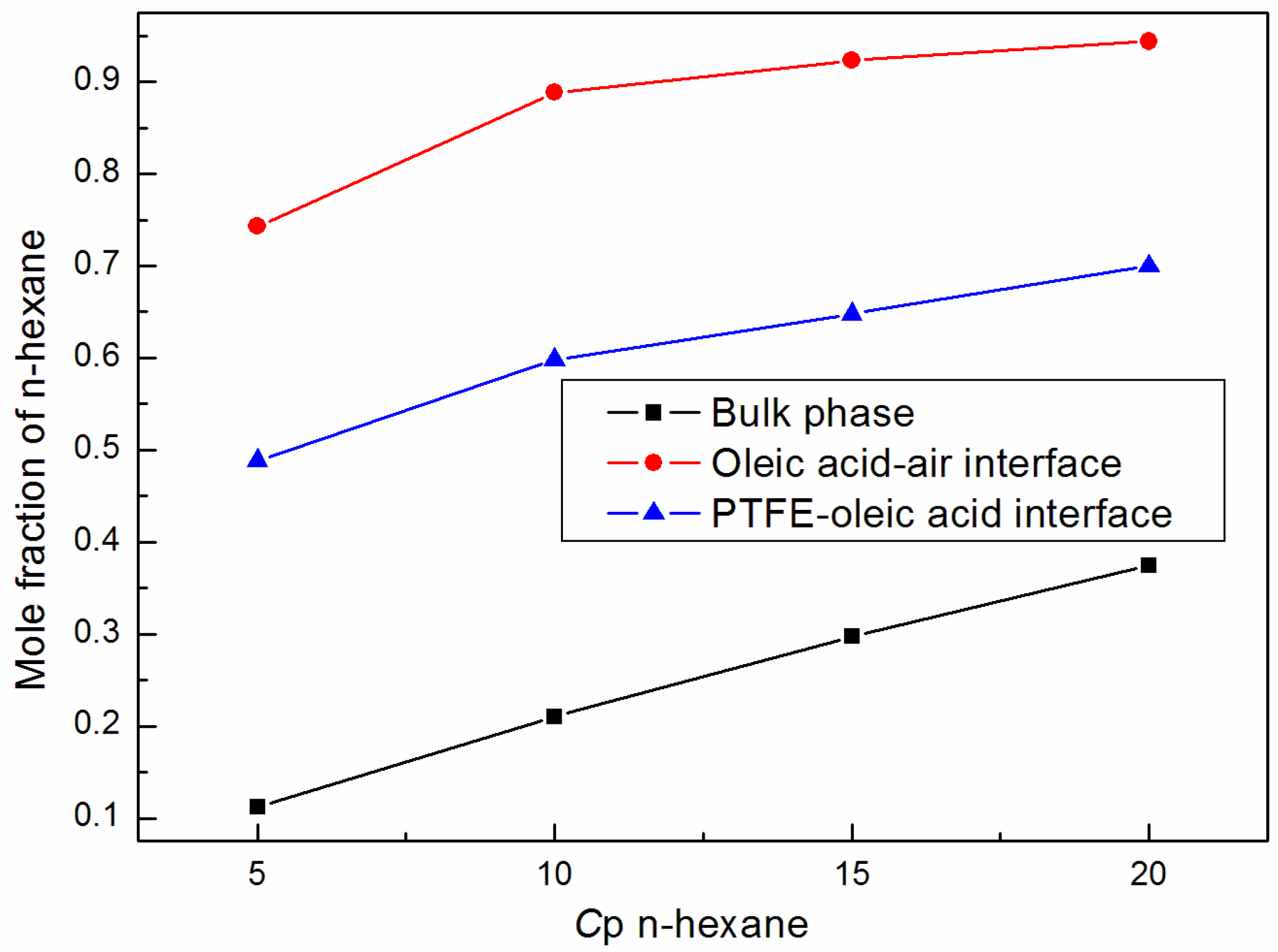
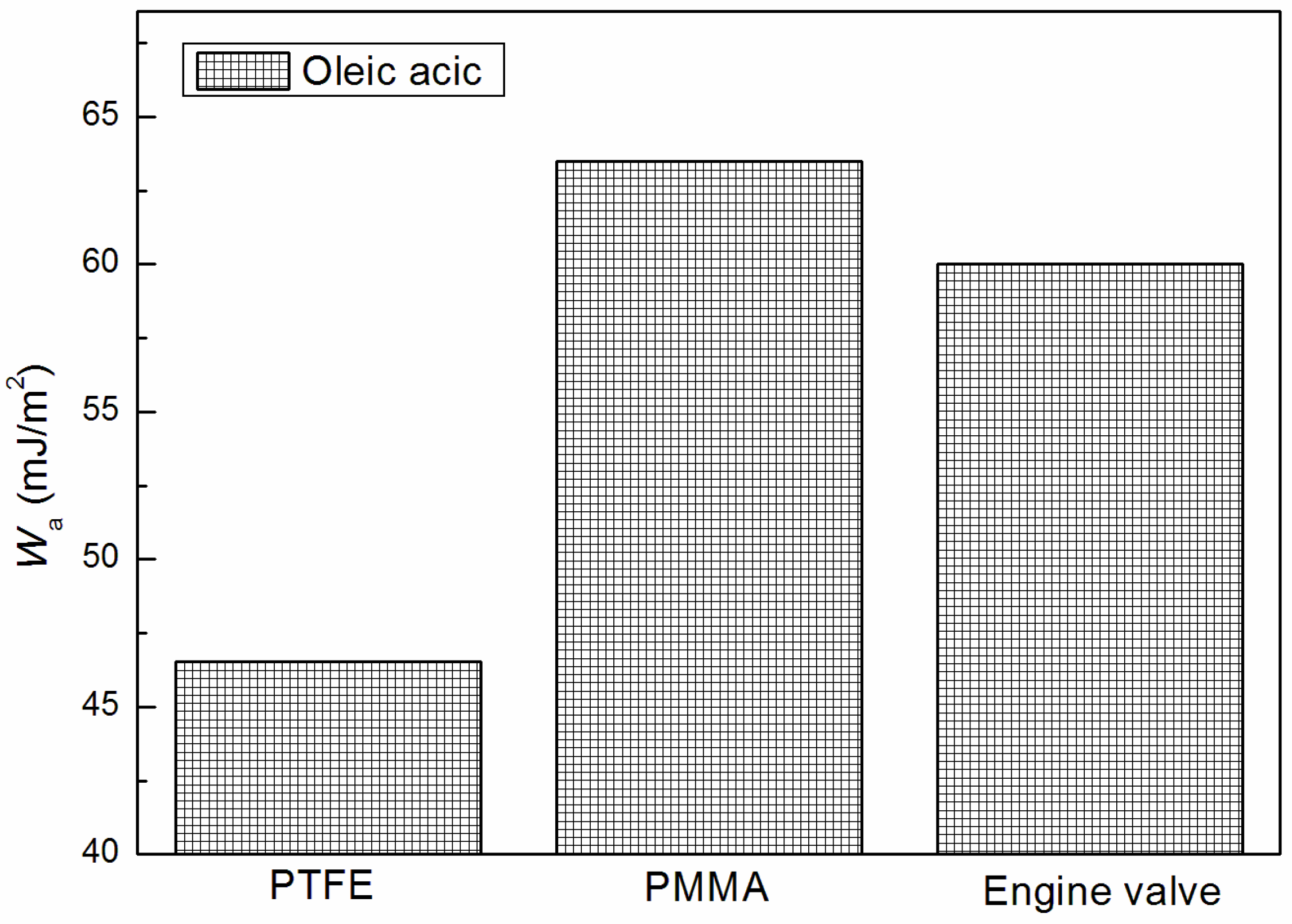
3.3. Adhesion Properties of Unsaturated Fatty Acids
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gunstone, F.D. Rapeseed and Canola Oil: Production, Processing, Properties and Uses; Wiley-Blackwell: Hoboken, NJ, USA, 2009; ISBN 978-1-405-14792-7. [Google Scholar]
- Lapuerta, M.; Armas, O.; García-Contreras, R. Stability of diesel–bioethanol blends for use in diesel engines. Fuel 2007, 86, 1351–1357. [Google Scholar] [CrossRef]
- Longwic, R.; Lotko, W.; Swat, M. The Effect of Rape Oil-Diesel Oil Mixture Composition on Particulate Matter Emission Level in Diesel Engine; Paper Number: 2001-01-3388; SAE: Warrendale, PA, USA, 2001. [Google Scholar]
- Longwic, R.; Lotko, W. Selected Aspects of Reformed Diesel Oil Combustion Process; Paper Number: 2002-01-2220; SAE: Warrendale, PA, USA, 2002. [Google Scholar]
- Vojtisek-Lom, M.; Pechout, M.; Mazac, M. Experimental Investigation of Rapeseed Oil Combustion in a Modern Common-Rail Diesel Engine; Paper Number: 2011-24-0104; SAE: Warrendale, PA, USA, 2011. [Google Scholar]
- Nishi, K.; Korematsu, K.; Tanaka, J. Potential of Rapeseed Oil as Diesel Engine Fuel; Paper Number: 2004-01-1858; SAE: Warrendale, PA, USA, 2004. [Google Scholar]
- Demirbas, A. Political, economic and environmental impacts of biofuels: A review. Appl. Energy 2009, 86, 108–117. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Progress in biodiesel processing. Appl. Energy 2010, 87, 1815–1835. [Google Scholar] [CrossRef]
- Roy, M.M.; Wang, W.; Bujold, J. Biodiesel production and comparison of emissions of a DI diesel engine fueled by biodiesel–diesel and canola oil–diesel blends at high idling operations. Appl. Energy 2013, 106, 198–208. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 2005, 84, 1059–1065. [Google Scholar] [CrossRef]
- Lozano, R.; Pechout, M.; Vojtisek-Lom, M. Performance of a Diesel Engine Fueled by Rapeseed Oil Heated to Different Temperatures; Paper Number: 2011-24-0107; SAE: Warrendale, PA, USA, 2011. [Google Scholar]
- Sapit, A.; Nagayasu, S.; Tsuboi, Y.; Nada, Y.; Kidoguchi, Y. A Study on Improvement of Diesel Spray Characteristics Fueled by Rape-Seed Oil; Paper Number: 2011-32-0561; SAE: Warrendale, PA, USA, 2011. [Google Scholar]
- Hu, J.; Du, Z.; Li, C.; Min, E. Study on the lubrication properties of biodiesel as fuel lubricity enhancers. Fuel 2005, 84, 1601–1606. [Google Scholar] [CrossRef]
- Van Gerpen, J.H.; Soylu, S.; Chang, D.Y.Z. Evaluation of the Lubricity of Soybean Oil-Based Additives in Diesel Fuel. Prepared for The United Soybean Board; Mechanical Engineering Department, Iowa State University: Ames, IA, USA, 1998. [Google Scholar]
- Drown, D.C.; Harper, K.; Frame, E.A. Screening vegetable oil alcohol esters as fuel lubricity enhancers. J. Am. Oil Chem. Soc. 2001, 78, 579–584. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Lois, E.; Serdari, A.; Zannikos, F.; Stournas, S.; Kalligeros, S. Lubrication properties of low-sulfur diesel fuels in the presence of specific types of fatty acid derivatives. Energy Fuels 2001, 15, 106–112. [Google Scholar] [CrossRef]
- Dmytryshyn, S.L.; Dalai, A.K.; Chaudhari, S.T.; Mishra, H.K.; Reaney, M.J. Synthesis and characterization of vegetable oil derived esters: Evaluation for their diesel additive properties. Bioresour. Technol. 2004, 92, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.P.; Goodrum, J.W. Effects of specific fatty acid methyl esters on diesel fuel lubricity. Fuel 2004, 83, 2351–2356. [Google Scholar] [CrossRef]
- Stoldt, S.H.; Dave, H.; Heights, H. Esters Derived from Vegetable Oils Used as Additives for Fuels. U.S. Patent 5,730,029, 24 March 1998. [Google Scholar]
- Munson, J.W.; Hertz, P.B.; Dalai, A.K.; Reaney, M.J. Lubricity Survey of Low-Level Biodiesel Fuel Additives Using the Munson ROCLE Bench Test; Paper Number: 1999-01-3590; SAE: Warrendale, PA, USA, 1999. [Google Scholar]
- Lang, X.; Dalai, A.K. Preparation and evaluation of vegetable oil derived biodiesel esters as lubricity additives. Tribo Test J. 2001, 8, 131–150. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Lois, E.; Stournas, S.; Zannikos, F.; Serdari, A. Assessment of the Lubricity of Greek Road Diesel and the Effect of the Addition of Specific Types of Biodiesel; Paper Number: 1999-01-1471; SAE: Warrendale, PA, USA, 1999. [Google Scholar]
- Ball, K.F.; Bostick, J.G.; Brennan, T.J. Fuel Lubricity from Blends of a Diethanolamine Derivative and Biodiesel. U.S. Patent 5,891,203, 6 April 1999. [Google Scholar]
- Tate, R.E.; Watts, K.C.; Allen, C.A.W.; Wilkie, K.I. The viscosities of three biodiesel fuels at temperatures up to 300 C. Fuel 2006, 85, 1010–1015. [Google Scholar] [CrossRef]
- Graboski, M.S.; McCormick, R.L. Combustion of fat and vegetable oil derived fuels in Diesel engines. Prog. Energy Combust. Sci. 1998, 24, 125–164. [Google Scholar] [CrossRef]
- Schumacher, L.G.; Van Gerpen, J.; Adams, B.T. An ASAE Meeting Presentation; Paper 036036; ASABE (American Society of Agricultural and Biological Engineers): St. Joseph, MI, USA, 2003. [Google Scholar]
- Ge, J.C.; Yoon, S.K.; Kim, M.S.; Choi, N.J. Application of canola oil biodiesel/diesel blends in a common rail diesel engine. Appl. Sci. 2017, 7, 34. [Google Scholar] [CrossRef]
- Górski, K.; Sander, P.; Longwic, R. The assessment of ecological parameters of diesel engine supplied with mixtures of canola oil with n-hexane. In Proceedings of the IOP Conference Series: Materials Science and Engineering 2018, Krakow, Poland, 13–14 September 2018; Volume 421, p. 042025. [Google Scholar]
- Longwic, R.; Sander, P.; Nieoczym, A.; Samociuk, W.; Bąkowski, H. Effect of some properties of hydrocarbon fuels on self-ignition delay. Przem. Chem. 2017, 96, 1123–1127. [Google Scholar]
- Longwic, R.; Sander, P. The course of combustion process under real conditions of work of a traction diesel engine supplied by mixtures of canola oil containing n-hexane. In Proceedings of the IOP Conference Series: Materials Science and Engineering 2018, Krakow, Poland, 13–14 September 2018; Volume 421, p. 042025. [Google Scholar]
- Longwic, R.; Sander, P. The characteristics of the combustion process occurring under real operating conditions of traction. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Krakow, Poland, 22–23 September 2016; Volume 148, p. 012071. [Google Scholar]
- Sarıkaya, I.; Bilgen, S.; Akbaş, H. The production of fuel from, and the thermal stability of, sunflower oil, corn oil, and canola oil. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 3514–3520. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.K.; Choi, N.J. Using canola oil biodiesel as an alternative fuel indiesel engines: A Review. Appl. Sci. 2017, 7, 881. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Szymczyk, K.; Jańczuk, B.; Longwic, R.; Sander, P. Surface properties of canola oil and some parts of diesel engine. Int. J. Adhes. Adhes. 2015, 60, 23–30. [Google Scholar] [CrossRef]
- Liu, H.; Przybylski, R.; Dawson, K.; Eskin, N.A.M.C.; Biliaderis, G. Comparison of the composition and properties of canola and sunflower oil sediments with canola seed hull lipids. J. Am. Chem. Soc. 1996, 73, 493–498. [Google Scholar] [CrossRef]
- Chumpitaz, L.D.A.; Coutinho, L.F.; Meirelles, A.J.A. Surface tension of fatty acids and triglycerides. J. Am. Oil Chem. Soc. 1999, 76, 379–382. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; Wiley-Interscience: New York, NY, USA, 1997. [Google Scholar]
- Li, D.; Neumann, A.W. Equation of state for interfacial tensions of solid-liquid systems. Adv. Colloid Interface Sci. 1992, 39, 299–345. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle interpretation in terms of solid surface tension. Colloids Surf. A 2000, 161, 31–48. [Google Scholar] [CrossRef]
- Jańczuk, B.; Zdziennicka, A.; Wójcik, W. Relationship between wetting of teflon by cetyltrimethylammonium bromide solution and adsorption. Eur. Polym. J. 1997, 33, 1093–1098. [Google Scholar] [CrossRef]
- van Oss, C.J.; Good, R.J. Surface tension and the solubility of polymers and biopolymers: The role of polar and apolar interfacial free energies. J. Macromol. Sci. 1989, 26, 1183–1203. [Google Scholar] [CrossRef]
- van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Monopolar surfaces. Adv. Colloid Interface Sci. 1987, 28, 35–64. [Google Scholar] [CrossRef]
- van Oss, C.J.; Good, R.J.; Chaudhury, M.K. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 1988, 4, 884–891. [Google Scholar] [CrossRef]
- Krawczyk, J.; Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Wettability of polymers by aqueous solution of binary surfactantsmixture with regard to adhesion in polymer-solution system. I—Correlation between the adsorption of surfactants mixture and contact angle. Inter. J. Adhes. Adhes. 2013, 45, 98–105. [Google Scholar] [CrossRef]
- Groszek, A.J. Selective adsorption at graphite/hydrocarbon interfaces. Proc. R. Soc. Lond. A 1970, 314, 473–478. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Szymczyk, K.; Krawczyk, J.; Jańczuk, B. Some remarks on the solid surface tension determination form contact angle measurements. Appl. Surf. Sci. 2017, 405, 88–101. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdziennicka, A.; Szymczyk, K.; Jańczuk, B.; Longwic, R.; Sander, P. Surface, Volumetric, and Wetting Properties of Oleic, Linoleic, and Linolenic Acids with Regards to Application of Canola Oil in Diesel Engines. Appl. Sci. 2019, 9, 3445. https://doi.org/10.3390/app9173445
Zdziennicka A, Szymczyk K, Jańczuk B, Longwic R, Sander P. Surface, Volumetric, and Wetting Properties of Oleic, Linoleic, and Linolenic Acids with Regards to Application of Canola Oil in Diesel Engines. Applied Sciences. 2019; 9(17):3445. https://doi.org/10.3390/app9173445
Chicago/Turabian StyleZdziennicka, Anna, Katarzyna Szymczyk, Bronisław Jańczuk, Rafał Longwic, and Przemysław Sander. 2019. "Surface, Volumetric, and Wetting Properties of Oleic, Linoleic, and Linolenic Acids with Regards to Application of Canola Oil in Diesel Engines" Applied Sciences 9, no. 17: 3445. https://doi.org/10.3390/app9173445
APA StyleZdziennicka, A., Szymczyk, K., Jańczuk, B., Longwic, R., & Sander, P. (2019). Surface, Volumetric, and Wetting Properties of Oleic, Linoleic, and Linolenic Acids with Regards to Application of Canola Oil in Diesel Engines. Applied Sciences, 9(17), 3445. https://doi.org/10.3390/app9173445




