Fire-Induced Changes in Soil and Implications on Soil Sorption Capacity and Remediation Methods
Abstract
1. Introduction
2. Factors Which Determine the Effect of Fire Events on Soil Properties
2.1. Type of Vegetation
2.2. Fire Intensity and Duration
2.3. Soil Properties
3. Fire-Induced Changes in Soil Properties and the Implications on Soil Sorption Capacity
3.1. Fire-Induced Changes in Soil Textural Properties and Implications on Soil Sorption
3.2. Fire-Induced Changes in Soil Mineralogical Properties and the Implications on Soil Sorption Capacity
3.3. Fire-Induced Changes in Soil Organic Matter Content and the Implications on Soil Sorption Capacity
- Total removal of external oxygen-containing functional groups, leading to the formation of substances with low solubility,
- Decreased aliphatic chain length of humic acids and other hydrocarbons,
- Increased degree of aromaticity in organic matter,
- Structural changes in humic acids, including polymerization,
- Formation of nitrogen-containing heterocycles
- Formation of black carbon.
3.4. Fire-Induced Changes in Soil pH and Implications on Soil Sorption
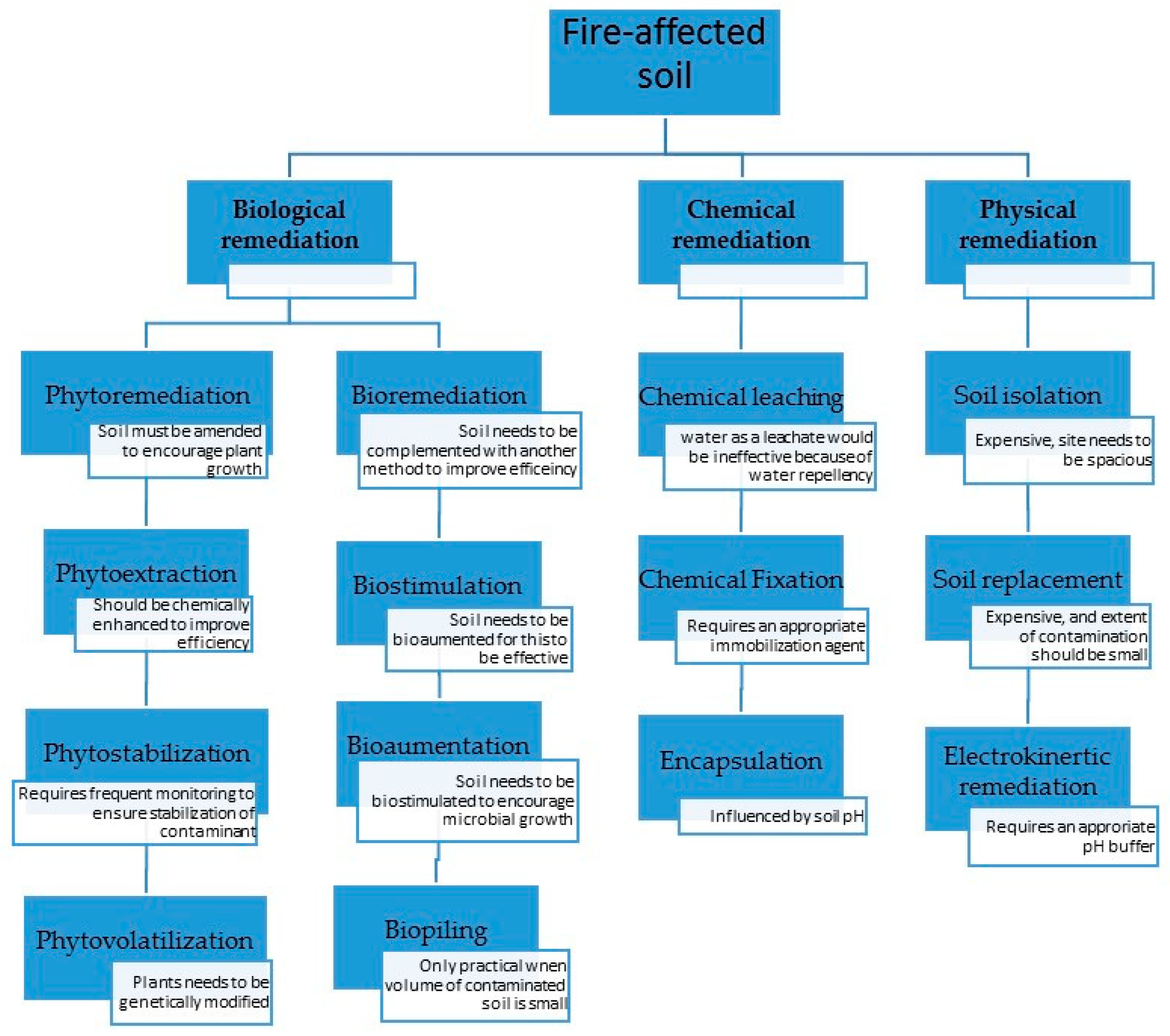
4. Fire-Induced Changes in Soil and Choice of Soil Remediation Technologies
4.1. Physical Remediation Technologies That Could be Used in Fire-Affected Soils
4.1.1. Soil Replacement, Isolation and Importing
4.1.2. Electrokinetic Remediation
4.2. Chemical Remediation Methods
4.2.1. Chemical Leaching
4.2.2. Chemical Fixation
4.2.3. Encapsulation
4.3. Biological Remediation Methods
4.3.1. Phytoremediation
4.3.2. Bioremediation
5. Selection of Remediation Technologies to Use on Fire-Affected Soils
6. Conclusions
Funding
Conflicts of Interest
References
- Huang, Y.; Wu, S.; Kaplan, J.O. Sensitivity of global wildfire occurrences to various factors in the context of global change. Atmos. Environ. 2015, 121, 86–92. [Google Scholar] [CrossRef]
- Marlon, J.R.; Bartlein, P.J.; Carcaillet, C.; Gavin, D.G.; Harrison, S.P.; Higuera, P.E.; Joos, F.; Power, M.J.; Prentice, I.C. Climate and human influences on global biomass burning over the past two millennia. Nat. Geosci. 2008, 1, 697–702. [Google Scholar] [CrossRef]
- Wang, Z.; Chappellaz, J.; Park, K.; Mak, J.E. Large variations in southern hemisphere biomass burning during the last 650 years. Science 2010, 330, 1663–1666. [Google Scholar] [CrossRef] [PubMed]
- North, M.P.; Stephens, S.L.; Collins, B.M.; Agee, J.K.; Aplet, G.; Franklin, J.F.; Fulé, P.Z. Reform forest fire management. Science 2015, 349, 1280–1281. [Google Scholar] [CrossRef]
- Giglio, L.; Randerson, J.T.; van der Werf, G.R. Analysis of daily, monthly, and annual burned area using the fourth-generation global fire emissions database (gfed4). J. Geophys. Res. Biogeosci. 2013, 118, 317–328. [Google Scholar] [CrossRef]
- Plucinski, M.P. A Review of Wildfire Occurrence Research; CSIRO: East Melbourne, Australia, 2012; pp. 1–28. [Google Scholar]
- Cochrane, M.A. Synergistic interactions between habitat fragmentation and fire in evergreen tropical forests. Conserv. Biol. 2002, 15, 1515–1521. [Google Scholar] [CrossRef]
- Lewis, S.L.; Edwards, D.P.; Galbraith, D. Increasing human dominance of tropical forests. Science 2015, 349, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, M.; Spessa, A.; Kaplan, J.O. A model for global biomass burning in preindustrial time: Lpj-lmfire (v1.0). Geosci. Model Dev. 2013, 6, 643–685. [Google Scholar] [CrossRef]
- Santín, C.; Doerr, S.H. Fire effects on soils: The human dimension. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150171. [Google Scholar] [CrossRef] [PubMed]
- José Martínez-Sánchez, J.; Ferrandis, P.; de las Heras, J.; María Herranz, J. Effect of burnt wood removal on the natural regeneration of pinus halepensis after fire in a pine forest in tus valley (se spain). For. Ecol. Manag. 1999, 123, 1–10. [Google Scholar] [CrossRef]
- Vihnanek, R.E.; Ballard, T.M. Slashburning effects on stocking, growth, and nutrition of young douglas-fir plantations in salal-dominated ecosystems of eastern vancouver island. Can. J. For. Res. 1988, 18, 718–722. [Google Scholar] [CrossRef]
- Caldararo, N. Human ecological intervention and the role of forest fires in human ecology. Sci. Total. Environ. 2002, 292, 141–165. [Google Scholar] [CrossRef]
- Scharenbroch, B.C.; Nix, B.; Jacobs, K.A.; Bowles, M.L. Two decades of low-severity prescribed fire increases soil nutrient availability in a midwestern, USA oak (quercus) forest. Geoderma 2012, 183–184, 80–91. [Google Scholar] [CrossRef]
- Kennard, D.K.; Gholz, H.L. Effects of high- and low-intensity fires on soil properties and plant growth in a bolivian dry forest. Plant Soil 2001, 234, 119–129. [Google Scholar] [CrossRef]
- Arocena, J.M.; Opio, C. Prescribed fire-induced changes in properties of sub-boreal forest soils. Geoderma 2003, 113, 1–16. [Google Scholar] [CrossRef]
- De Marco, A.; Gentile, A.E.; Arena, C.; De Santo, A.V. Organic matter, nutrient content and biological activity in burned and unburned soils of a mediterranean maquis area of southern italy. Int. J. Wildland Fire 2005, 14, 365–377. [Google Scholar] [CrossRef]
- Mbalo, B.A.; Witkowski, E.T.F. Tolerance to soil temperatures experienced during and after the passage of fire in seeds of acacia karroo, a. Tortilis and chromolaena odorata: A laboratory study. South Afr. J. Bot. 1997, 63, 421–425. [Google Scholar] [CrossRef]
- Hardison, J.R. Fire and flame for plant disease control. Annu. Rev. Phytopathol. 1976, 14, 355–379. [Google Scholar] [CrossRef]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Úbeda, X. Effects of prescribed fires on soil properties: A review. Sci. Total. Environ. 2018, 613-614, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Cleland, D.T.; Brosofske, K.D.; Saunders, S.C. Factors influencing modern wildfire occurrence in the mark twain national forest, missouri. South. J. Appl. For. 2007, 31, 73–84. [Google Scholar]
- Cruz, G.M.; Gould, S.J.; Hollis, J.J.; McCaw, L.W. A hierarchical classification of wildland fire fuels for australian vegetation types. Fire 2018, 1, 13. [Google Scholar] [CrossRef]
- Ohlemiller, T.J. Modeling of smoldering combustion propagation. Prog. Energy Combust. Sci. 1985, 11, 277–310. [Google Scholar] [CrossRef]
- Rein, G. Smouldering combustion phenomena in science and technology. Int. Rev. Chem. Eng. 2009, 1, 3–18. [Google Scholar]
- Rein, G.; Garcia, J.; Simeoni, A.; Tihay, V.; Ferrat, L. Smouldering natural fires: Comparison of burning dynamics in boreal peat and mediterranean humus. WIT Trans. Ecol. Environ. 2008, 119, 183–192. [Google Scholar]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Fares, S.; Bajocco, S.; Salvati, L.; Camarretta, N.; Dupuy, J.-L.; Xanthopoulos, G.; Guijarro, M.; Madrigal, J.; Hernando, C.; Corona, P. Characterizing potential wildland fire fuel in live vegetation in the mediterranean region. Ann. For. Sci. 2017, 74, 1. [Google Scholar] [CrossRef]
- Badía, D.; López-García, S.; Martí, C.; Ortíz-Perpiñá, O.; Girona-García, A.; Casanova-Gascón, J. Burn effects on soil properties associated to heat transfer under contrasting moisture content. Sci. Total. Environ. 2017, 601–602, 1119–1128. [Google Scholar] [CrossRef]
- Stoof, C.R.; Moore, D.; Fernandes, P.M.; Stoorvogel, J.J.; Fernandes, R.E.; Ferreira, A.J.; Ritsema, C.J. Hot fire, cool soil. Geophys. Res. Lett. 2013, 40, 1534–1539. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire intensity, fire severity and burn severity: A brief review and suggested usage. Int. J. Wildland Fire 2009, 18, 116–126. [Google Scholar] [CrossRef]
- Reynard-Callanan, J.R.; Pope, G.A.; Gorring, M.L.; Feng, H. Effects of high-intensity forest fires on soil clay mineralogy. Phys. Geogr. 2010, 31, 407–422. [Google Scholar] [CrossRef]
- Janzen, C.; Tobin-Janzen, T. Microbial communities in fireaffected soils. In Microbiology of Extreme Soils, Soil Biology; Dion, P., Nautiyal, C.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 13. [Google Scholar]
- Waldrop, T.A.; Brose, P.H. A comparison of fire intensity levels for stand replacement of table mountain pine (pinus pungens lamb.). For. Ecol. Manag. 1999, 113, 155–166. [Google Scholar] [CrossRef]
- Humphreys, F.R.; Craig, F.G. Effects of Fire on Soil Chemical, Structural and Hydrological Properties; Australian Academy of Science: Canberra, Australia, 1981; p. 124. [Google Scholar]
- Korfmacher, J.L.; Chambers, J.C.; Tausch, R.J.; Roundy, B.A.; Meyer, S.E.; Stanley, K. Technical note: A technique for conducting small-plot burn treatments. J. Range Manag. 2003, 56, 251–254. [Google Scholar] [CrossRef]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliott, P.F. Fire effects on belowground sustainability: A review and synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Bates, J.D.; Davies, K.W.; Sharp, R.N. Shrub-steppe early succession following juniper cutting and prescribed fire. Environ. Manag. 2011, 47, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Úbeda, X.; Outeiro, L. Physical and Chemical Effects of Fire on Soil; Science Publishers, Inc.: Enfield, NH, USA, 2009. [Google Scholar]
- Velasco, A.Y.O. Efecto del Fuego en los Campos de Caña; Academia de Ciencias de Cuba: La Habana, Cuba, 1968; Volume 4. [Google Scholar]
- Cesar, H.L. Efeitos da Quema e Corte Sobre a Vegetacao de um Campo Sujo na Fazenda Agua Limpa-df; University of Brazil: São Paulo, Brazil, 1980. [Google Scholar]
- Cawson, J.; Sheridan, G.; Smith, H.; Lane, P. Surface runoff and erosion after prescribed burning and the effect of different fire regimes in forests and shrublands: A review. Int. J. Wildland Fire 2012, 21, 857–872. [Google Scholar] [CrossRef]
- Bova, A.S.; Dickinson, M.B. Linking surfacefire behavior, stem heating, and tissue necrosis. Can. J. For. Res. 2005, 35, 814–822. [Google Scholar] [CrossRef]
- McArthur, A.G.; Cheney, N.P. The characterization of fires in relation to ecological studies, with an introduction by neil burrows. J. Assoc. Fire Ecol. 2015, 11, 1–9. [Google Scholar] [CrossRef]
- Ngole-Jeme, V.M. Changes in the mineralogy and geochemistry of mine tailings contaminated soil as a result of fire events and the implications on soil sorption properties. In Proceedings of the 2017 International Conference on Environmental Pollution Control, Vancouver, BC, Canada, 8–12 October 2017; International Society for Environmental Sciences: Regina, SK, Canada, 2017. [Google Scholar]
- Nikiforova, T.; Savytskyi, M.; Limam, K.; Bosschaerts, W.; Belarbi, R. Methods and results of experimental researches of thermal conductivity of soils. Energy Procedia 2013, 42, 775–783. [Google Scholar] [CrossRef]
- Yun, T.S.; Santamarina, J.C. Fundamental study of thermal conduction in dry soils. Granul. Matter 2007, 10, 197. [Google Scholar] [CrossRef]
- Nusier, O.; Abu-Hamdeh, N. Laboratory techniques to evaluate thermal conductivity for some soils. Heat Mass Transf. 2003, 39, 119–123. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Doerr, S.H. Hydrophobicity and aggregate stability in calcareous topsoils from fire-affected pine forests in southeastern spain. Geoderma 2004, 118, 77–88. [Google Scholar] [CrossRef]
- Badía, D.; Martí, C. Plant ash and heat intensity effects on chemicaland physical properties of two contrasting soils. Arid. Land Res. Manag. 2003, 17, 23–41. [Google Scholar] [CrossRef]
- Zihms, S.G.; Switzer, C.; Karstunen, M.; Tarantino, A. Understanding the Effects of high temperature processes on the engineering properties of soils. In Proceedings of the 18th International Conference on Soil Mechanics and Geotechnical Engineering, Paris, France, 2–6 September 2013; pp. 3427–3430. [Google Scholar]
- Araya, S.N.; Meding, M.; Berhe, A.A. Thermal alteration of soil physico-chemical properties: A systematic study to infer response of sierra nevada climosequence soils to forest fires. Soil 2016, 2, 351–366. [Google Scholar] [CrossRef]
- Midttømme, K.; Roaldset, E.; Aagaard, P. Thermal conductivity claystones and mudstones of selected from england. Claymin 1998, 33, 131–145. [Google Scholar] [CrossRef]
- Giovannini, G.; Lucchesi, S.; Giachetti, M. Effect of heating on some physical and chemical parameters related to soil aggregation and erodibility. Soil Sci. 1988, 146, 255–261. [Google Scholar] [CrossRef]
- Mehdi, H.; Ali, S.; Ali, M.; Mostafa, A. Effects of different fire severity levels on soil chemical and physical properties in zagros forests of western iran. Folia For. Pol. 2012, 54, 241–250. [Google Scholar]
- Jõeleht, A.; Kirsimäe, K.; Shogenova, A.; Sliaupa, S.; Kukkonen, I.; Rastenienè, V.; Zabele, A. Thermal conductivity of cambrian siliciclastic rocks from the baltic basin. Proc. Estonian Acad. Sci. Geol. 2002, 51, 5–15. [Google Scholar]
- Doerr, S.H.; Llewellyn, C.T.; Douglas, P.; Morley, C.P.; Mainwaring, K.A.; Haskins, C.; Johnsey, L.; Ritsema, C.J.; Stagnitti, F.; Allinson, G.; et al. Extraction of compounds associated with water repellency in sandy soils of different origin. Soil Res. 2005, 43, 225–237. [Google Scholar] [CrossRef]
- Dekker, L.W.; Oostindie, K.; Ritsema, C.J. Exponential increase of publications related to soil water repellency. Aust. J. Soil Res. 2005, 43, 403–441. [Google Scholar]
- Zavala, L.M.; Granged, A.J.P.; Jordán, A.; Bárcenas-Moreno, G. Effect of burning temperature on water repellency and aggregate stability in forest soils under laboratory conditions. Geoderma 2010, 158, 366–374. [Google Scholar] [CrossRef]
- Bryant, R.; Doerr, S.H.; Helbig, M. Effect of oxygen deprivation on soil hydrophobicity during heating. Int. J. Wildland Fire 2005, 14, 449–455. [Google Scholar] [CrossRef]
- DeBano, L.F. The role of fire and soil heating on water repellency in wildland environments: A review. J. Hydrol. 2000, 231-232, 195–206. [Google Scholar] [CrossRef]
- Zavala, L.M.; De Celis, R.; JordÁN, A. How wildfires affect soil properties. A brief review. Geogr. Res. Lett. 2014, 40, 311–331. [Google Scholar] [CrossRef]
- Letey, J. Causes and consequences of fire-induced soil water repellency. Hydrol. Process. 2001, 15, 2867–2875. [Google Scholar] [CrossRef]
- Stavi, I.; Barkai, D.; Knoll, Y.M.; Glion, H.A.; Katra, I.; Brook, A.; Zaady, E. Fire impact on soil-water repellency and functioning of semi-arid croplands and rangelands: Implications for prescribed burnings and wildfires. Geomorphology 2017, 280, 67–75. [Google Scholar] [CrossRef]
- DeBano, L.F.; Krammes, J.S. Water repellent soils and their relation to wildfire temperatures. Hydrol. Sci. J. 1966, 11, 14–19. [Google Scholar] [CrossRef]
- MacDonald, L.H.; Huffman, E.L. Post-fire soil water repellency. Soil Sci. Soc. Am. J. 2004, 68, 1729–1734. [Google Scholar] [CrossRef]
- Ulery, A.L.; Graham, R.C. Forest fire effects on soil color and texture. Soil Sci. Soc. Am. J. 1993, 57, 135–140. [Google Scholar] [CrossRef]
- Ketterings, Q.M.; Bigham, J.M.; Laperche, V. Changes in soil mineralogy and texture caused by slash-and-burn fires in sumatra, indonesia. Soil Sci. Soc. Am. J. 2000, 64, 1108–1117. [Google Scholar] [CrossRef]
- Neary, D.G.; Ryan, K.C.; DeBano, L.F. Wildland Fire in Ecosystems: Effects of Fire on Soils and Water; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2005; Volume 4, p. 250.
- Begum, A.; Ramaiah, M.; Khan, I.; Veena, K. Heavy metal pollution and chemical profile of cauvery river water. E-J. Chem. 2009, 6, 47–52. [Google Scholar] [CrossRef]
- Xue, L.; Li, Q.; Chen, H. Effects of a wildfire on selected physical, chemical and biochemical soil properties in a pinus massoniana forest in south china. Forests 2014, 5, 2947–2966. [Google Scholar] [CrossRef]
- Ponder, F.; Tadros, M.; Loewenstein, E.F. Microbial properties and litter and soil nutrients after two prescribed fires in developing savannas in an upland missouri ozark forest. For. Ecol. Manag. 2009, 257, 755–763. [Google Scholar] [CrossRef]
- Granged, A.J.P.; Jordán, A.; Zavala, L.M.; Muñoz-Rojas, M.; Mataix-Solera, J. Short-term effects of experimental fire for a soil under eucalyptus forest (se australia). Geoderma 2011, 167-168, 125–134. [Google Scholar] [CrossRef]
- Terefe, T.; Mariscal-Sancho, I.; Peregrina, F.; Espejo, R. Influence of heating on various properties of six mediterranean soils. A laboratory study. Geoderma 2008, 143, 273–280. [Google Scholar] [CrossRef]
- Úbeda, X.; Lorca, M.; Outeiro, L.R.; Bernia, S.; Castellnou, M. Effects of prescribed fire on soil quality in mediterranean grassland (prades mountains, north-east spain). Int. J. Wildland Fire 2005, 14, 379–384. [Google Scholar] [CrossRef]
- Etiégni, L.; Campbell, A.G. Physical and chemical characteristics of wood ash. Bioresour. Technol. 1991, 37, 173–178. [Google Scholar] [CrossRef]
- Hosking, J.S. The ignition at low temperatures of the organic matter in soils. J. Agric. Sci. 1938, 28, 393–400. [Google Scholar] [CrossRef]
- Tiedemann, A.R. Combustion losses of sulfur from forest foliage and litter. For. Sci. 1987, 33, 216–223. [Google Scholar]
- Raison, R.J.; Khanna, P.K.; Woods, P.V. Mechanisms of element transfer to the atmosphere during vegetation fires. Can. J. For. Res. 1985, 15, 132–140. [Google Scholar] [CrossRef]
- Rabenhorst, M.C. Determination of organic and carbonate carbon in calcareous soils using dry combustion. Soil Sci. Soc. Am. J. 1988, 52, 965–968. [Google Scholar] [CrossRef]
- Chandler, C.; Cheney, P.; Thomas, P.; Trabaud, L.; Williams, D. Fire in Forestry. Volume 1. Forest Fire Behavior and Effects; John Wiley & Sons: New York, NY, USA, 1983. [Google Scholar]
- González-Pérez, J.A.; González-Vila, F.J.; Almendros, G.; Knicker, H. The effect of fire on soil organic matter—A review. Environ. Int. 2004, 30, 855–870. [Google Scholar] [CrossRef] [PubMed]
- Almendros, G.; Gonzalez-Vila, F.J.; Martin, F. Fire-induced transformation of soil organic matter from an oak forest: An experimental approach to the effects of fire on humic substances. Soil Sci. 1990, 149, 158–168. [Google Scholar] [CrossRef]
- González-Vila, F.J.; Tinoco, P.; Almendros, G.; Martin, F. Pyrolysis−gc−ms analysis of the formation and degradation stages of charred residues from lignocellulosic biomass. J. Agric. Food Chem. 2001, 49, 1128–1131. [Google Scholar] [CrossRef]
- Almendros, G.; Martín, F.; González-Vila, F.J. Effects of fire on humic and lipid fractions in a dystric xerochrept in spain. Geoderma 1988, 42, 115–127. [Google Scholar] [CrossRef]
- González-Vila, F.J.; González, J.A.; Polvillo, O.; Almendros, G.; Knicker, H. Nature of refractory forms of organic carbon in soils affected by fires. Pyrolytic and spectroscopic approaches. In Proceedings of the IV International Conference on Forest Fire Research and Wildland Fire Safety, Rottadam, The Netherlands, 18–23 November 2002; Viegas, D.X., Ed.; Millpress: Rottadam, The Netherlands; pp. 226–230. [Google Scholar]
- Bodí, M.B.; Martin, D.A.; Balfour, V.N.; Santín, C.; Doerr, S.H.; Pereira, P.; Cerdà, A.; Mataix-Solera, J. Wildland fire ash: Production, composition and eco-hydro-geomorphic effects. Earth-Sci. Rev. 2014, 130, 103–127. [Google Scholar] [CrossRef]
- Kovacic, D.A.; Swift, D.M.; Ellis, J.E.; Hakonson, T.E. Immediate effects of prescribed burning on mineral soil nitrogen in ponderosa pine of new mexico. Soil Sci. 1986, 141, 71–76. [Google Scholar] [CrossRef]
- Schoch, P.; Binkley, D. Prescribed burning increased nitrogen availability in a mature loblolly pine stand. For. Ecol. Manag. 1986, 14, 13–22. [Google Scholar] [CrossRef]
- Covington, W.W.; Sackett, S.S. Soil mineral nitrogen changes following prescribed burning in ponderosa pine. For. Ecol. Manag. 1992, 54, 175–191. [Google Scholar] [CrossRef]
- Bell, R.L.; Binkley, D. Soil nitrogen mineralization and immobilization in response to periodic prescribed fire in a loblolly pine plantation. Can. J. For. Res. 1989, 19, 816–820. [Google Scholar] [CrossRef]
- Knoepp, J.D.; Swank, W.T. Comparison of available soil nitrogen assays in control and burned forested sites. Soil Sci. Soc. Am. J. 1995, 59, 1750–1754. [Google Scholar] [CrossRef]
- Grier, C.C. Wildfire effects on nutrient distribution and leaching in a coniferous ecosystem. Can. J. For. Res. 1975, 5, 559–607. [Google Scholar] [CrossRef]
- DeBano, L.F.; Rice, R.M.; Conrad, C.E. Soil Heating Chaparral Fires: Effects on Soil Properties, Plant Nutrients, Erosion and Runoff; Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: Berkeley, CA, USA, 1979; p. 21.
- Giardina, C.P.; Rhoades, C.C. Clear cutting and burning affect nitrogen supply, phosphorus fractions and seedling growth in soils from a wyoming lodgepole pine forest. For. Ecol. Manag. 2001, 140, 19–28. [Google Scholar] [CrossRef]
- Galang, M.A.; Markewitz, D.; Morris, L.A. Soil phosphorus transformations under forest burning and laboratory heat treatments. Geoderma 2010, 155, 401–408. [Google Scholar] [CrossRef]
- Schaller, J.; Tischer, A.; Struyf, E.; Bremer, M.; Belmonte Dácil, U.; Potthast, K. Fire enhances phosphorus availability in topsoils depending on binding properties. Ecology 2015, 96, 1598–1606. [Google Scholar] [CrossRef]
- Tan, K.H.; Hajek, B.F.; Barshad, I. Thermal Analysis Techniques; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1986. [Google Scholar]
- Yusiharni, E.; Robert, J.G. Soil minerals recover after they are damaged by bushfires. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; International Union of Soil Sciences: Vienna, Austria, 2010; pp. 104–107. [Google Scholar]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004. [Google Scholar]
- Frost, R.L.; Horváth, E.; Makó, É.; Kristóf, J.; Rédey, Á. Slow transformation of mechanically dehydroxylated kaolinite to kaolinite—An aged mechanochemically activated formamide-intercalated kaolinite study. Thermochim. Acta 2003, 408, 103–113. [Google Scholar] [CrossRef]
- Richardson, H.M. Phase Changes which Occur on Heating Kaolin Clays. The x-Ray Identification and Crystal Structures of Clay Minerals; Minerals Society Publishing: London, UK, 1972; p. 11. [Google Scholar]
- Prieto-Fernández, A.; Acea, M.J.; Carballas, T. Soil microbial and extractable c and n after wildfire. Biol. Fertil. Soils 1998, 27, 132–142. [Google Scholar] [CrossRef]
- Dumontet, S.; Dinel, H.; Scopa, A.; Mazzatura, A.; Saracino, A. Post-fire soil microbial biomass and nutrient content of a pine forest soil from a dunal mediterranean environment. Soil Biol. Biochem. 1996, 28, 1467–1475. [Google Scholar] [CrossRef]
- Dunn, P.H.; Barro, S.C.; Poth, M. Soil moisture affects survival of microorganisms in heated chaparral soil. Soil Biol. Biochem. 1985, 17, 143–148. [Google Scholar] [CrossRef]
- Matlack, G.R. Factors determining the distribution of soil nematodes in a commercial forest landscape. For. Ecol. Manag. 2001, 146, 129–143. [Google Scholar] [CrossRef]
- Collett, N.G.; Neumann, F.G.; Tolhurst, K.G. Effects of two short rotation prescribed fires in spring on surface-active arthropods and earthworms in dry sclerophyll eucalypt forest of west-central victoria. Aust. For. 1993, 56, 49–60. [Google Scholar] [CrossRef]
- Kim, E.J.; Oh, J.-E.; Chang, Y.-S. Effects of forest fire on the level and distribution of pcdd/fs and pahs in soil. Sci. Total. Environ. 2003, 311, 177–189. [Google Scholar] [CrossRef]
- Fonturbel, M.T.; Vega, J.A.; Bara, S.; Bernardez, I. influence of prescribed burning of pine stands in nw spain on soil microorganisms. Eur. J. Soil Biol. 1995, 31, 13–20. [Google Scholar]
- Choromanska, U.; DeLuca, T.H. Prescribed fire alters the impact of wildfire on soil biochemical properties in a ponderosa pine forest. Soil Sci. Soc. Am. J. 2001, 65, 232–238. [Google Scholar] [CrossRef]
- Thompson, A.; Goyne, K.W. Introduction to the sorption of chemical constituents in soils. Nat. Educ. Knowl. 2012, 4, 7. [Google Scholar]
- Kalembasa, D.; Pakuła, K.; Jaremko, D. Sorption properties of soils in the siedlce upland. Acta Agrophys. 2011, 18, 311–319. [Google Scholar]
- Lair, G.J.; Gerzabek, M.H.; Haberhauer, G. Sorption of heavy metals on organic and inorganic soil constituents. Environ. Chem. Lett. 2007, 5, 23–27. [Google Scholar] [CrossRef]
- Obale-Ebanga, F.; Sevink, J.; de Groot, W.; Nolte, C. Myths of slash and burn on physical degradation of savannah soils: Impacts on vertisols in north cameroon. Soil Use Manag. 2003, 19, 83–86. [Google Scholar] [CrossRef]
- Stoof, C.R.; Wesseling, J.G.; Ritsema, C.J. Effects of fire and ash on soil water retention. Geoderma 2010, 159, 276–285. [Google Scholar] [CrossRef]
- Heydari, M.; Rostamy, A.; Najafi, F.; Dey, D.C. Effect of fire severity on physical and biochemical soil properties in zagros oak (quercus brantii lindl.) forests in iran. J. For. Res. 2017, 28, 95–104. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Giustra, M.G.; Vagliasindi, F.G.A. Soil texture affects adsorption capacity and removal efficiency of contaminants in ex situ remediation by thermal desorption of diesel-contaminated soils. Chem. Ecol. 2011, 27, 119–130. [Google Scholar] [CrossRef]
- Schulten, H.R.; Leinweber, P. New insights into organic-mineral particles: Composition, properties and models of molecular structure. Biol. Fertil. Soils 2000, 30, 399–432. [Google Scholar] [CrossRef]
- Nkedi-Kizza, P.; Rao, P.S.C.; Johnson, J.W. Adsorption of diuron and 2,4,5-t on soil particle-size separates1. J. Environ. Qual. 1983, 12, 195–197. [Google Scholar] [CrossRef]
- Evans, K.M.; Gill, R.A.; Robotham, P.W.J. The pah and organic content of sediment particle size fractions. Water Air Soil Pollut. 1990, 51, 13–31. [Google Scholar] [CrossRef]
- Iturri, L.A.; Buschiazzo, D.E. Cation exchange capacity and mineralogy of loess soils with different amounts of volcanic ashes. Catena 2014, 121, 81–87. [Google Scholar] [CrossRef]
- Martín-García, J.M.; Delgado, G.; Párraga, J.F.; Gámiz, E.; Delgado, R. Chemical, mineralogical and (micro)morphological study of coarse fragments in mediterranean red soils. Geoderma 1999, 90, 23–47. [Google Scholar] [CrossRef]
- Rieuwerts, J.S.; Thornton, I.; Farago, M.E.; Ashmore, M.R. Factors influencing metal bioavailability in soils: Preliminary investigations for the development of a critical loads approach for metals. Chem. Speciat. Bioavailab. 1998, 10, 61–75. [Google Scholar] [CrossRef]
- Hyun, S.; Lee, L.S.; Rao, P.S.C. Significance of anion exchange in pentachlorophenol sorption by variable-charge soils. J. Environ. Qual. 2003, 32, 966–976. [Google Scholar] [CrossRef]
- Wang, H.; Xia, W.; Lu, P. Study on adsorption characteristics of biochar on heavy metals in soil. Korean J. Chem. Eng. 2017, 34, 1867–1873. [Google Scholar] [CrossRef]
- Zhelezova, A.; Cederlund, H.; Stenström, J. Effect of biochar amendment and ageing on adsorption and degradation of two herbicides. Water Air Soil Pollut. 2017, 228, 216. [Google Scholar] [CrossRef]
- Pingree, M.R.A.; DeLuca, T.H. Function of wildfire-deposited pyrogenic carbon in terrestrial ecosystems. Front. Environ. Sci. 2017, 5, 53. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Pingree, M.R.A.; DeLuca, E.E.; Schwartz, D.T.; DeLuca, T.H. Adsorption capacity of wildfire-produced charcoal from pacific northwest forests. Geoderma 2016, 283, 68–77. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Lehmann, J.; Engelhard, M.H. Natural oxidation of black carbon in soils: Changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta 2008, 72, 1598–1610. [Google Scholar] [CrossRef]
- Hammes, K.; Schmidt, M.W.I. Changes of biochar in soil. Biochar Environ. Manag. Sci. Technol. 2009, 1, 169–181. [Google Scholar]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Sherman, L.; Brye, K.R.; Gill, D.E.; Koenig, K.A. Soil Chemistry as Affected by First-Time Prescribed Burning of a Grassland Restoration on a Coastal Plain Ultisol. Soil Sci. 2005, 170, 913–927. [Google Scholar] [CrossRef]
- Jadia, C.D.; Fulekar, M.H. Phytoremediation of heavy metals: Recent techniques. Afr. J. Biotechnol. 2009, 8, 921–928. [Google Scholar]
- USEPA. A Citizon’s Guide to Monitored Natural Attenuation; United States Environmental Protection Agency: Washington, DC, USA, 2012.
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Yeung, A.T.; Gu, Y.-Y. A review on techniques to enhance electrochemical remediation of contaminated soils. J. Hazard. Mater. 2011, 195, 11–29. [Google Scholar] [CrossRef]
- Alshawabkeh, A.N. Electrokinetic soil remediation: Challenges and opportunities. Sep. Sci. Technol. 2009, 44, 2171–2187. [Google Scholar] [CrossRef]
- Wang, H.; Ma, J.; Fan, X. Research progress on enhancement of in situ remediation of heavy metal by electrokinitics. Ecol. Environ. 2007, 16, 223–227. [Google Scholar]
- Tampouris, S.; Papassiopi, N.; Paspaliaris, I. Removal of contaminant metals from fine grained soils, using agglomeration, chloride solutions and pile leaching techniques. J. Hazard. Mater. 2001, 84, 297–319. [Google Scholar] [CrossRef]
- Ouyang, X.; Chen, J.W.; Zhang, X.G. Advance in supercritical CO2 fluid extraction of contaminants from soil. Geol. Bull. China 2010, 29, 1655–1661. [Google Scholar]
- Abumaizar, R.J.; Smith, E.H. Heavy metal contaminants removal by soil washing. J. Hazard. Mater. 1999, 70, 71–86. [Google Scholar] [CrossRef]
- Derakhshan Nejad, Z.; Jung, M.C.; Kim, K.-H. Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology. Environ. Geochem. Health 2018, 40, 927–953. [Google Scholar]
- Violante, A.; Cozzolino, V.; Perelomov, L.; Caporale, A.G.; Pigna, M. Mobility and bioavailability of heavy metals and metalloids in soil environments. J. Soil Sci. Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, S.; Lee, S.; Khim, Z.G.; Char, K.; Hyeon, T. Synthesis and magnetic studies of uniform iron nanorods and nanospheres. J. Am. Chem. Soc. 2000, 122, 8581–8582. [Google Scholar] [CrossRef]
- Tripp, S.L.; Pusztay, S.V.; Ribbe, A.E.; Wei, A. Self-assembly of cobalt nanoparticle rings. J. Am. Chem. Soc. 2002, 124, 7914–7915. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Gao, S. Monodisperse nickel nanoparticles prepared from a monosurfactant system and their magnetic properties. J. Mater. Chem. 2003, 13, 1510–1512. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Rangel, A.; Castro, P. Remediation of heavy metal contaminated soils: Phytoremediation as a potentially promising clean-up technology. Crit. Rev. Environ. Sci. Technol. 2009, 39, 622–654. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 2011, 20. [Google Scholar] [CrossRef]
- Nowack, B.; Schulin, R.; Robinson, B.H. Critical assessment of chelant-enhanced metal phytoextraction. Environ. Sci. Technol. 2006, 40, 5225–5232. [Google Scholar] [CrossRef]
- Fashola, M.O.; Ngole-Jeme, V.M.; Babalola, O.O. Heavy metal pollution from gold mines: Environmental effects and bacterial strategies for resistance. Int. J. Environ. Res. Public Health 2016, 13, 1047. [Google Scholar] [CrossRef] [PubMed]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques–classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar] [CrossRef]
- Vogt, C.; Richnow, H.H. Bioremediation via in situ microbial degradation of organic pollutants. In Geobiotechnology II. Advances in Biochemical Engineering/Biotechnology; Schippers, A., Glombitza, F., Sand, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 142, pp. 123–146. [Google Scholar]
- Roy, A.; Dutta, A.; Pal, S.; Gupta, A.; Sarkar, J.; Chatterjee, A.; Saha, A.; Sarkar, P.; Sar, P.; Kazy, S.K. Biostimulation and bioaugmentation of native microbial community accelerated bioremediation of oil refinery sludge. Bioresour. Technol. 2018, 253, 22–32. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total. Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Das, S.; Dash, H.R. 1–microbial bioremediation: A potential tool for restoration of contaminated areas. In Microbial Biodegradation and Bioremediation; Das, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–21. [Google Scholar]
- Silva-Castro, G.A.; Uad, I.; Rodríguez-Calvo, A.; González-López, J.; Calvo, C. Response of autochthonous microbiota of diesel polluted soils to land-farming treatments. Environ. Res. 2015, 137, 49–58. [Google Scholar] [CrossRef]

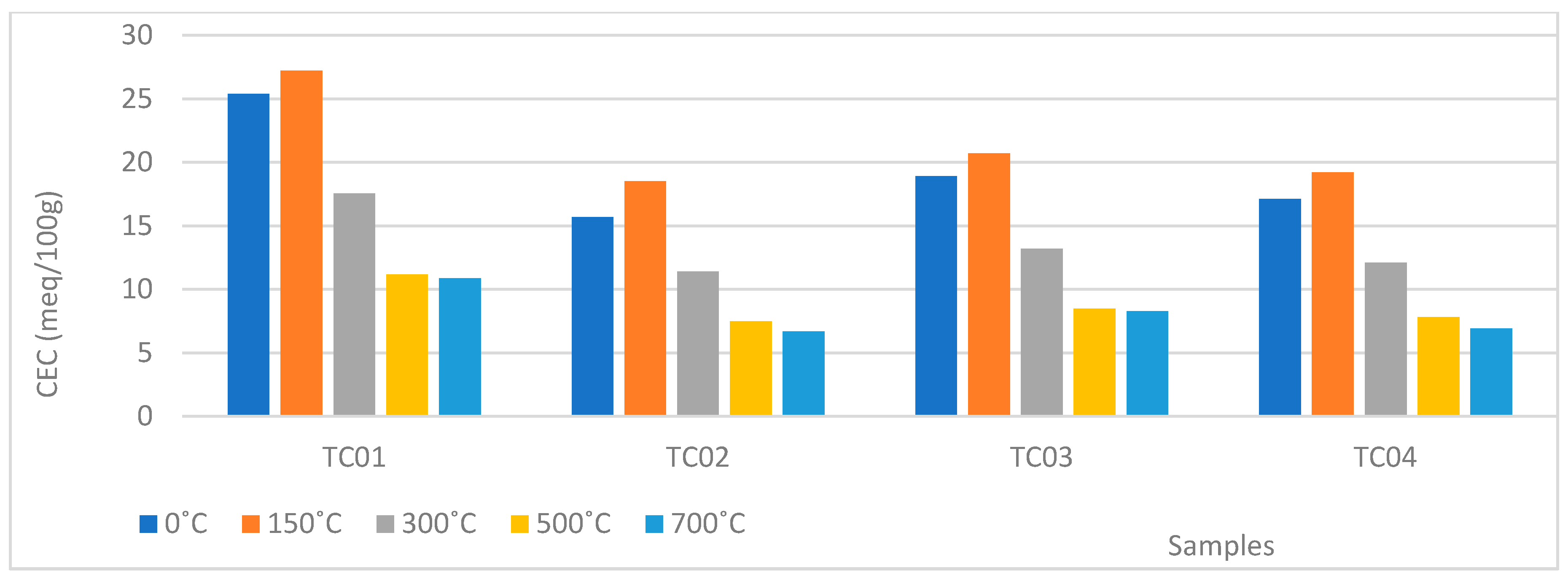
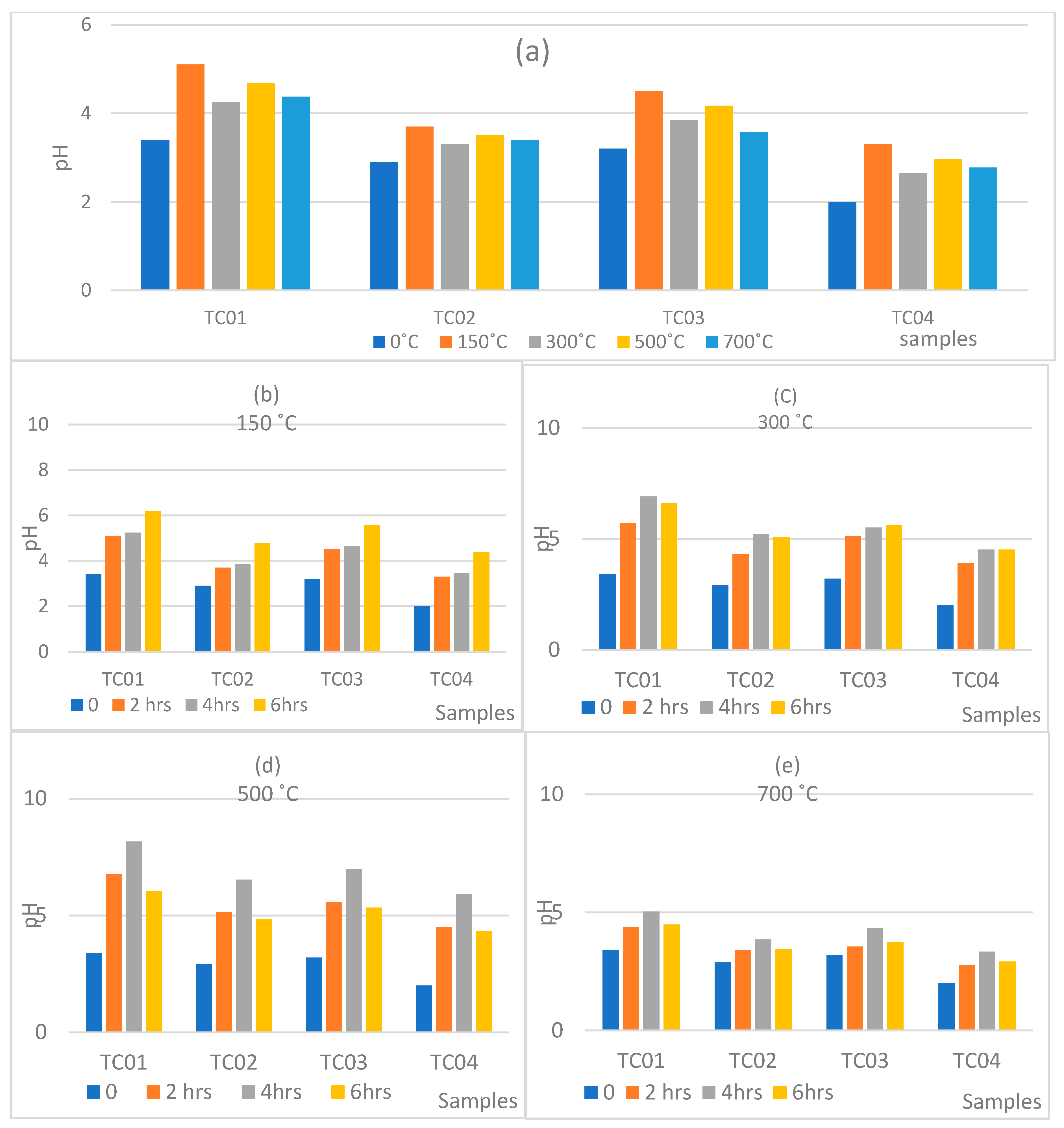
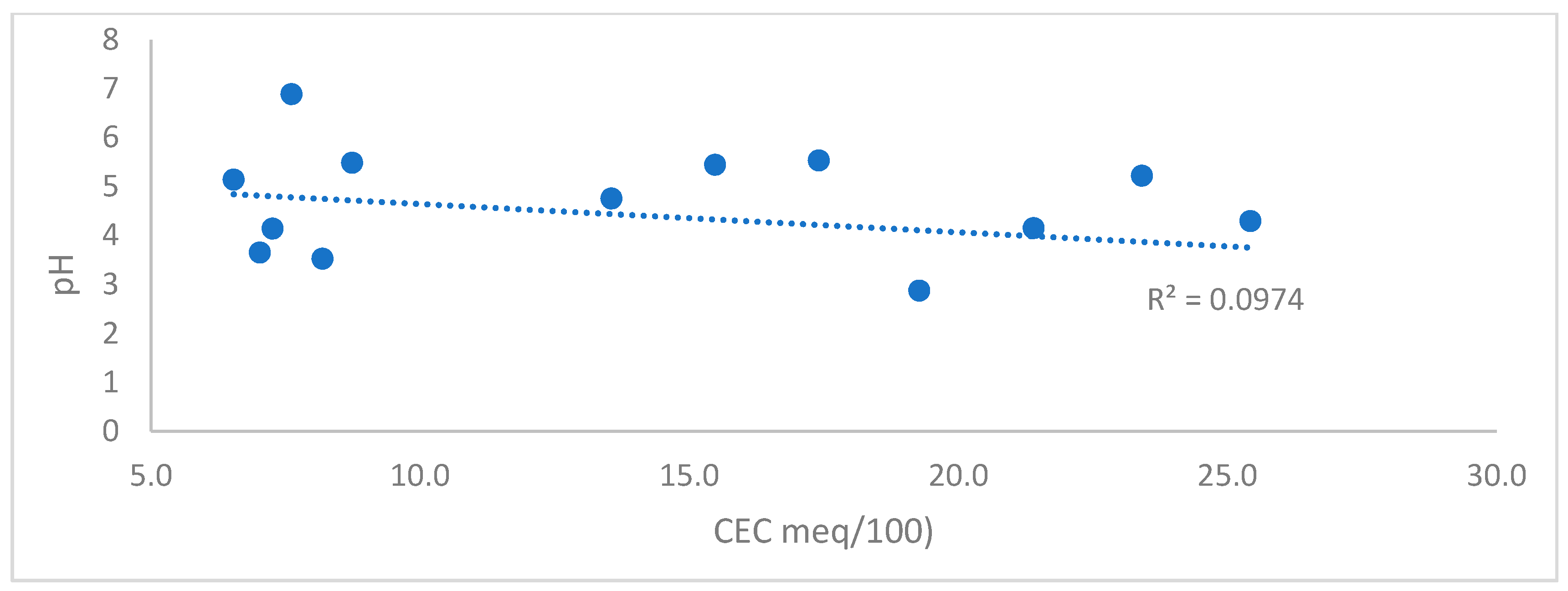
| Soil Particle | Cation Exchange Capacity Range cmol (+)/kg |
|---|---|
| Fine clay | 57.4–81.3 |
| Coarse clay | 33.6–74.9 |
| Whole clay | 20.9–110.0 |
| Fine silt | 6.8–41.4 |
| Medium silt | 63–34.5 |
| Coarse silt | 1.2–12.8 |
| Sand | 1.0–15.6 |
| Temperature of Heating (°C) | Duration of Heating (hrs) | Minerals Composition (%) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCO1 | TCO2 | TCO3 | TCO4 | |||||||||||||||||||||
| Hematite/Goethite | K-Feldspar | Quartz | Kaolinite | Hematite/Goethite | K-Feldspar | Plagioclase | Quartz | Kaolinite | Hematite/Goethite | K-Feldspar | Plagioclase | Quartz | Mica | Kaolinite | Chlorite | Pyrophyllite | Hematite/Goethite | K-Feldspar | Quartz | Mica | Kaolinite | Pyrophyllite | ||
| 0 | 0 | 1 | - | 97 | 2 | 1 | - | - | 97 | 2 | 1 | - | 1 | 89 | 2 | - | 4 | 1 | 1 | - | 96 | 1 | 2 | - |
| 150 | 2 | 1 | 1 | 96 | 3 | 1 | 1 | - | 96 | 2 | 1 | 1 | - | 89 | 3 | - | 5 | 2 | 2 | 1 | 95 | - | 2 | - |
| 4 | 1 | - | 96 | 3 | 1 | - | 1 | 96 | 2 | 1 | - | - | 90 | 2 | - | 5 | 2 | 2 | - | 96 | - | 2 | - | |
| 6 | 1 | - | 97 | 2 | 1 | - | - | 98 | 1 | 1 | - | - | 94 | 1 | 4 | - | - | 1 | - | 97 | - | 2 | - | |
| 300 | 2 | 1 | - | 96 | 3 | 1 | - | - | 97 | 2 | 1 | - | 1 | 89 | 2 | - | 5 | 2 | 2 | - | 96 | - | 2 | - |
| 4 | 2 | - | 95 | 3 | 1 | - | - | 97 | 2 | 1 | - | - | 94 | 2 | 3 | - | - | 2 | - | 95 | - | 2 | 1 | |
| 6 | 1 | - | 97 | 2 | 1 | - | - | 99 | - | 1 | - | - | 96 | - | 3 | - | - | 1 | - | 98 | - | 1 | - | |
| 500 | 2 | 2 | - | 98 | - | 1 | - | - | 99 | - | 1 | - | - | 93 | 2 | - | 2 | 2 | 2 | - | 98 | - | - | - |
| 4 | 2 | - | 98 | - | 1 | - | - | 98 | 1 | 1 | - | - | 94 | 2 | 3 | - | - | 1 | - | 99 | - | - | - | |
| 6 | 1 | - | 99 | - | 1 | - | - | 99 | - | 1 | - | - | 95 | 1 | 2 | - | 1 | 1 | - | 99 | - | - | - | |
| 750 | 2 | 2 | 1 | 97 | - | 1 | - | - | 99 | - | 2 | - | - | 97 | 1 | - | - | - | 3 | - | 97 | - | - | - |
| 4 | 2 | - | 98 | - | 1 | - | - | 99 | - | 1 | - | - | 99 | - | - | - | - | 1 | - | 99 | - | - | - | |
| 6 | 1 | - | 99 | - | 1 | - | - | 99 | - | 1 | - | - | 99 | - | - | - | - | 2 | - | 98 | - | - | - | |
| Soil Mineral | Cation Exchange Capacity (Meq/100 g) |
|---|---|
| Montmorillonite (smectites) | 80–50 |
| Vermicullite | 100–150 |
| Illite | 20–40 |
| Kaolinite | 3–15 |
| Allophane | 5–30 |
| Gibbsite | 0–4 |
| Hematite/goethite | 4–100 |
| Halloysite | 5–50 |
| glauconite | 5–40 |
| Sesquioxides | 0–1 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngole-Jeme, V.M. Fire-Induced Changes in Soil and Implications on Soil Sorption Capacity and Remediation Methods. Appl. Sci. 2019, 9, 3447. https://doi.org/10.3390/app9173447
Ngole-Jeme VM. Fire-Induced Changes in Soil and Implications on Soil Sorption Capacity and Remediation Methods. Applied Sciences. 2019; 9(17):3447. https://doi.org/10.3390/app9173447
Chicago/Turabian StyleNgole-Jeme, Veronica M. 2019. "Fire-Induced Changes in Soil and Implications on Soil Sorption Capacity and Remediation Methods" Applied Sciences 9, no. 17: 3447. https://doi.org/10.3390/app9173447
APA StyleNgole-Jeme, V. M. (2019). Fire-Induced Changes in Soil and Implications on Soil Sorption Capacity and Remediation Methods. Applied Sciences, 9(17), 3447. https://doi.org/10.3390/app9173447





