Separation of Cenospheres from Lignite Fly Ash Using Acetone–Water Mixture
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Cenosphere Recovery and Density
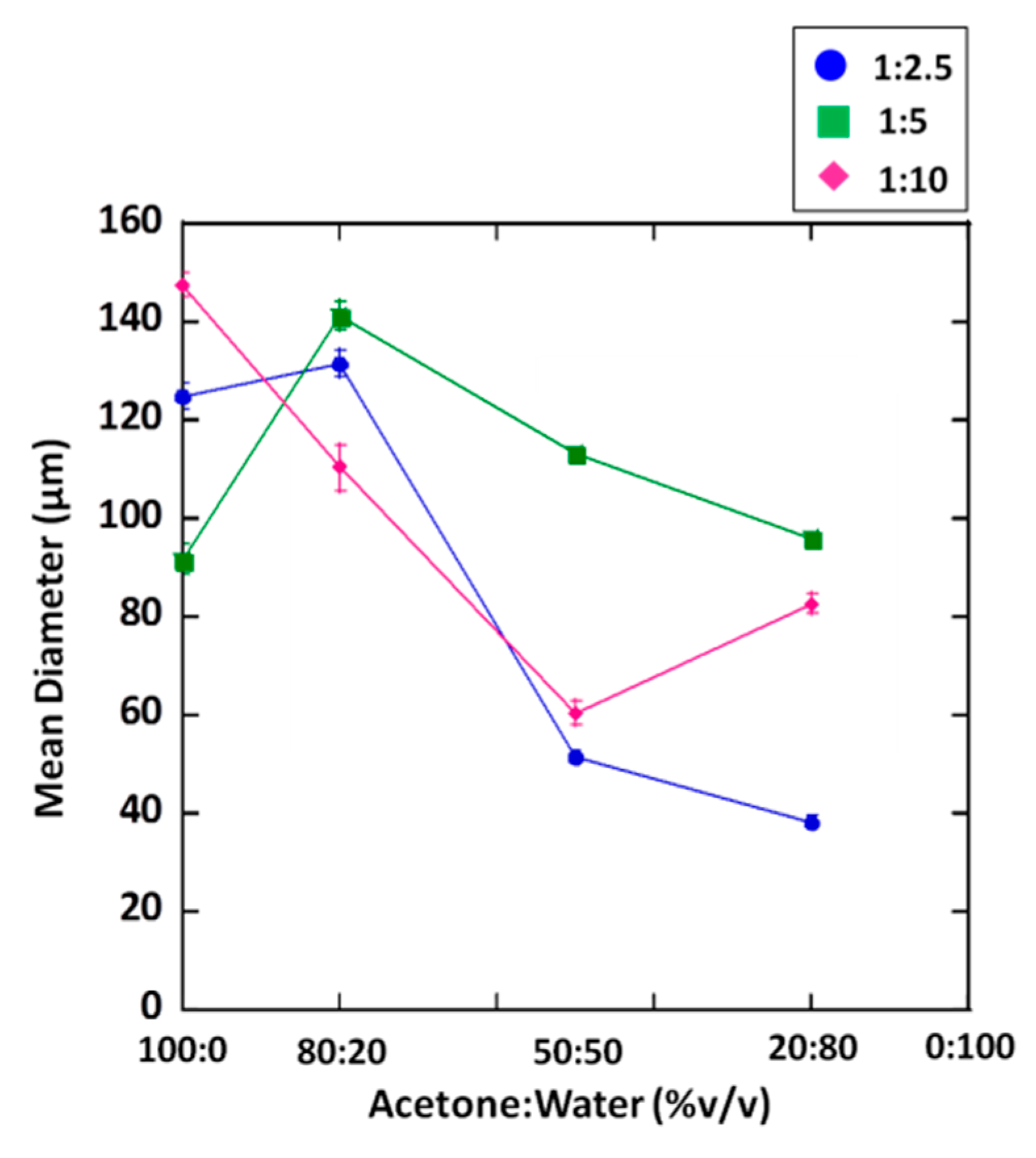
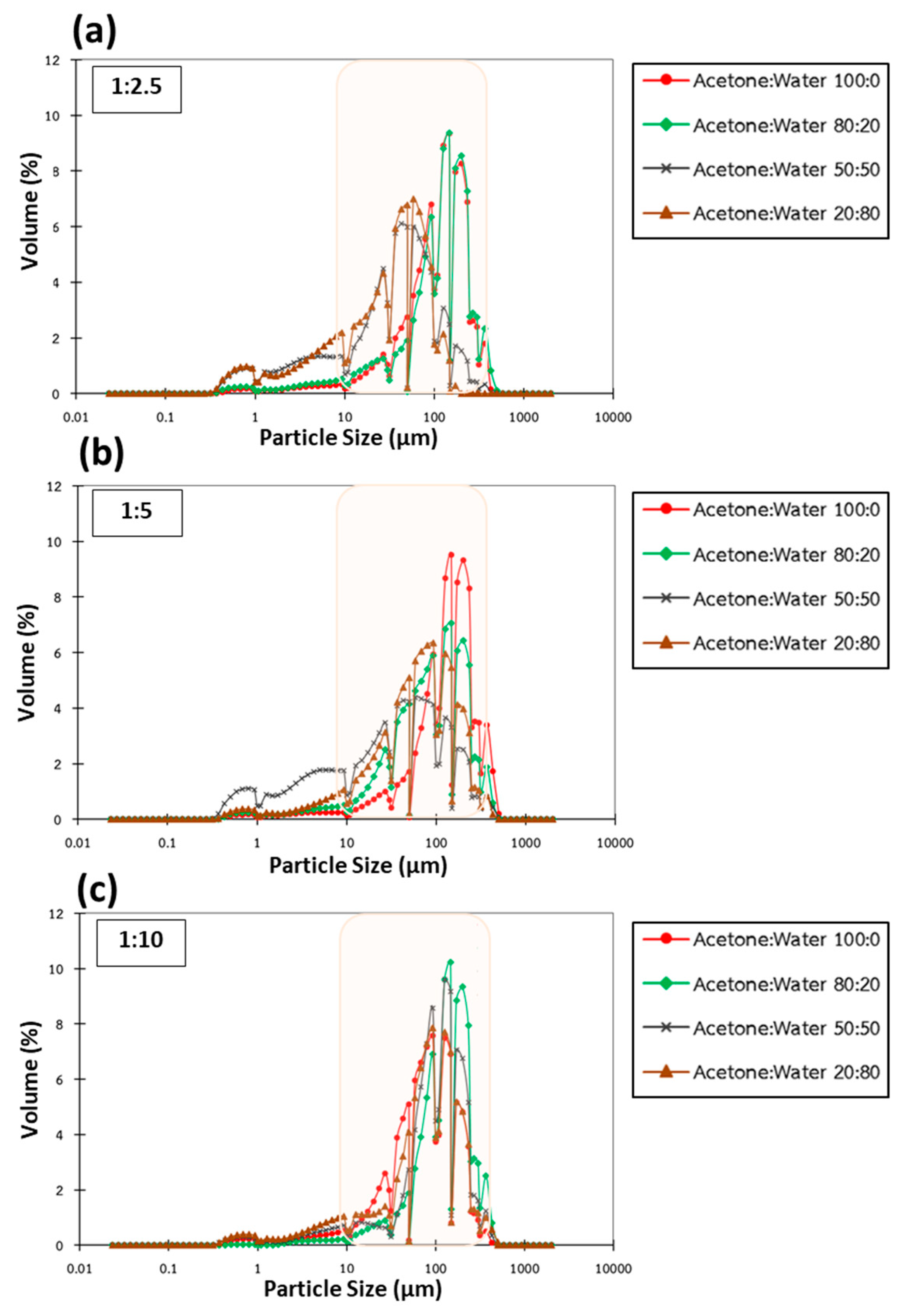
3.2. Particle Size and Distribution
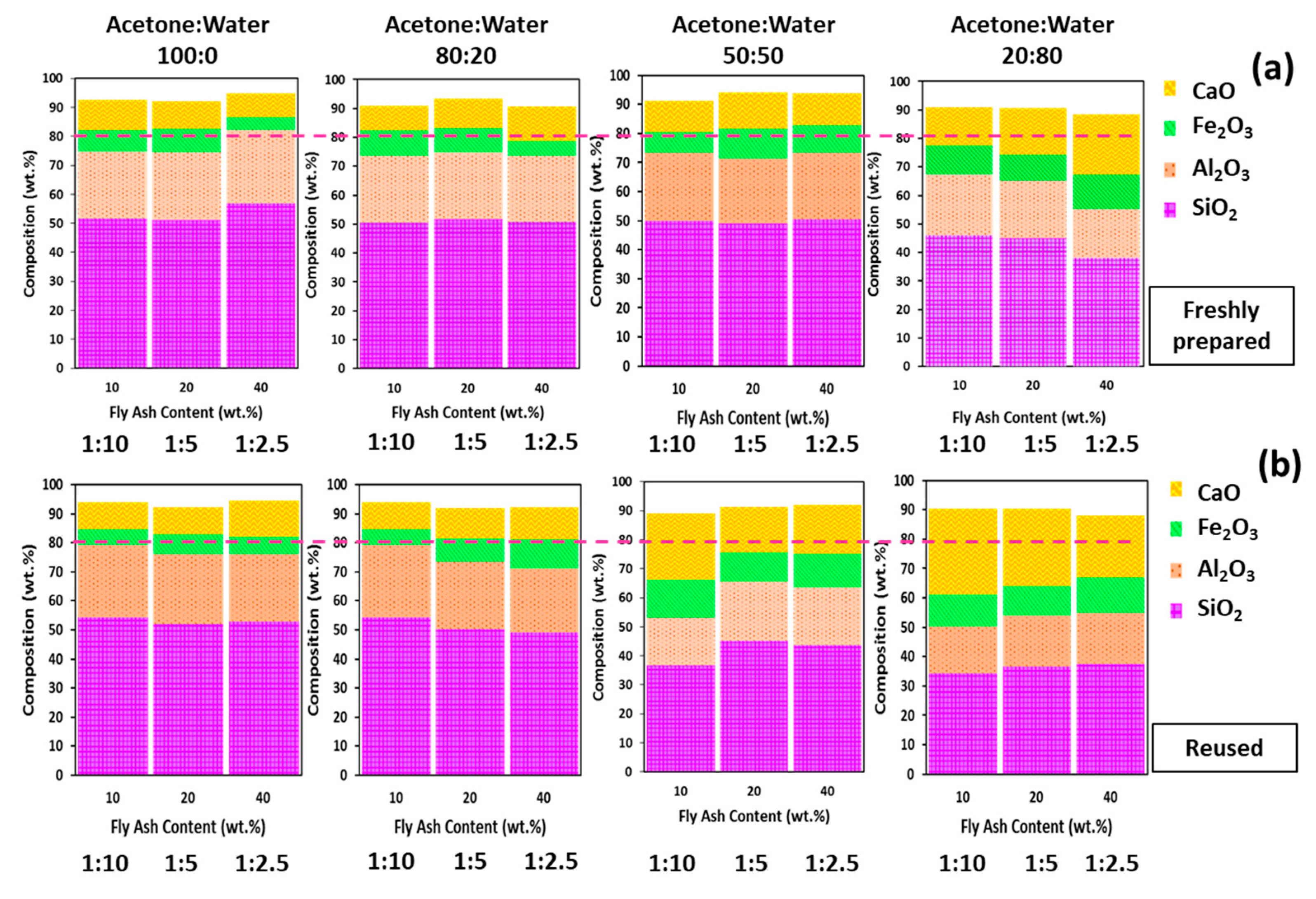
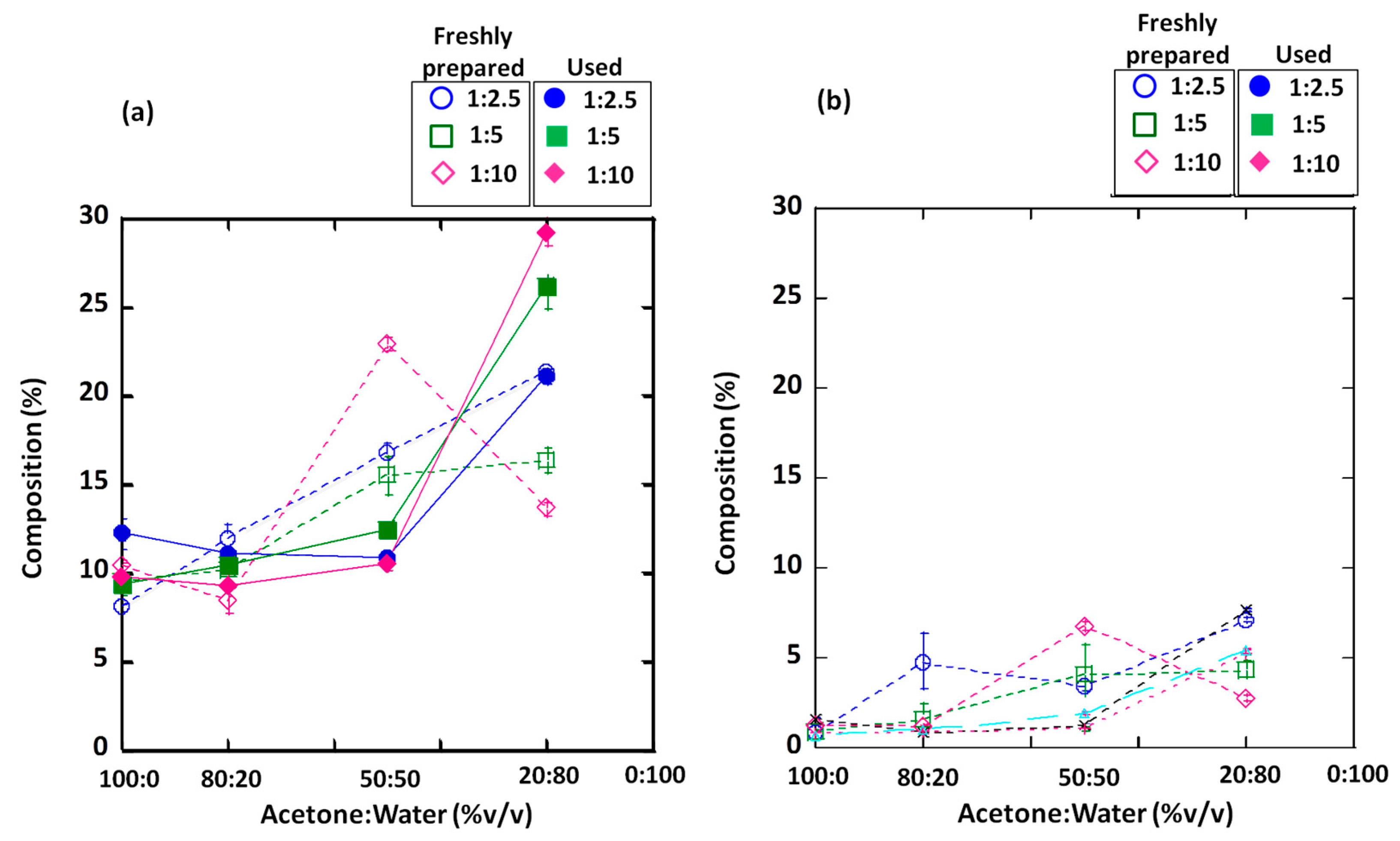
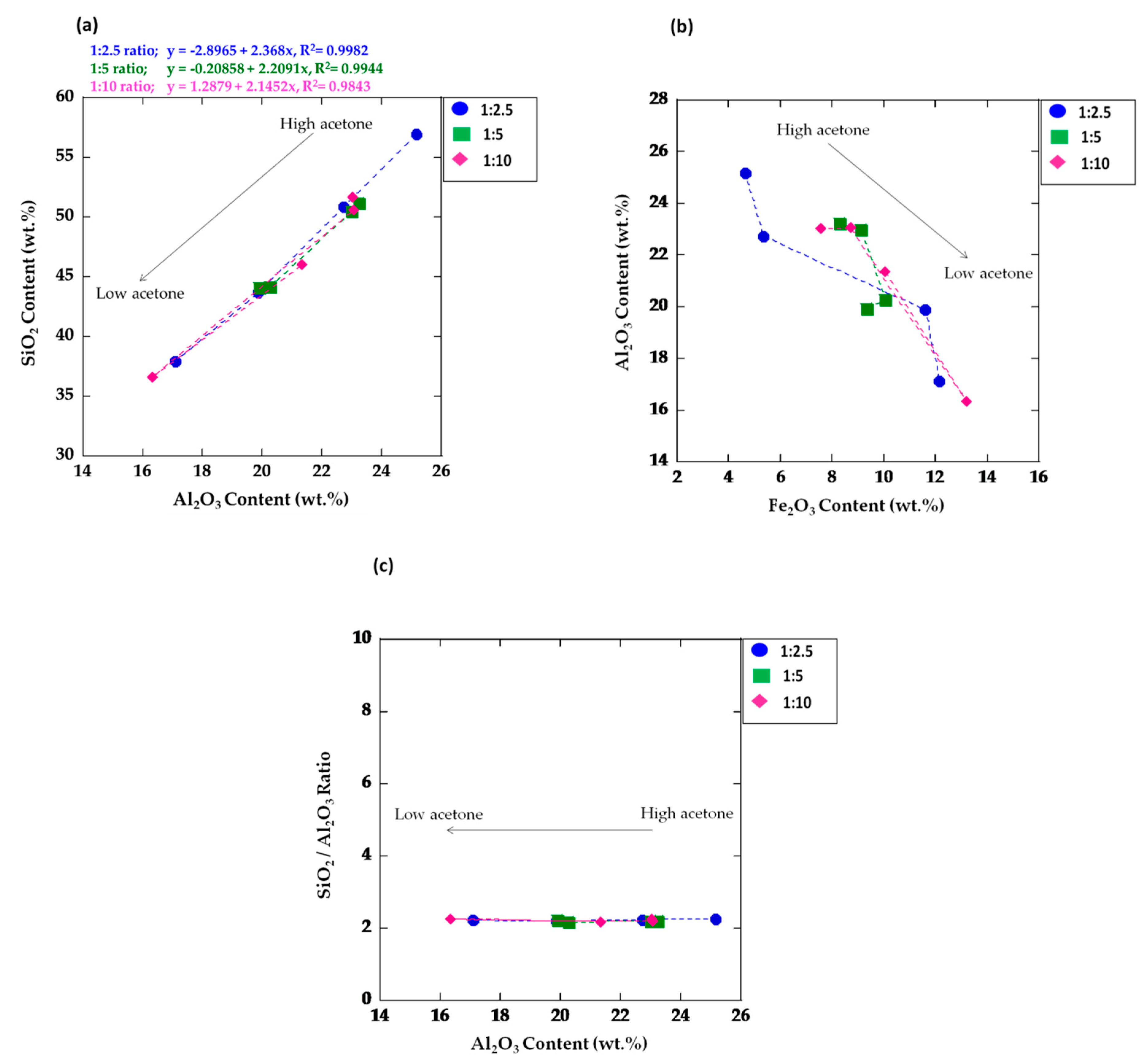
3.3. Chemical Composition
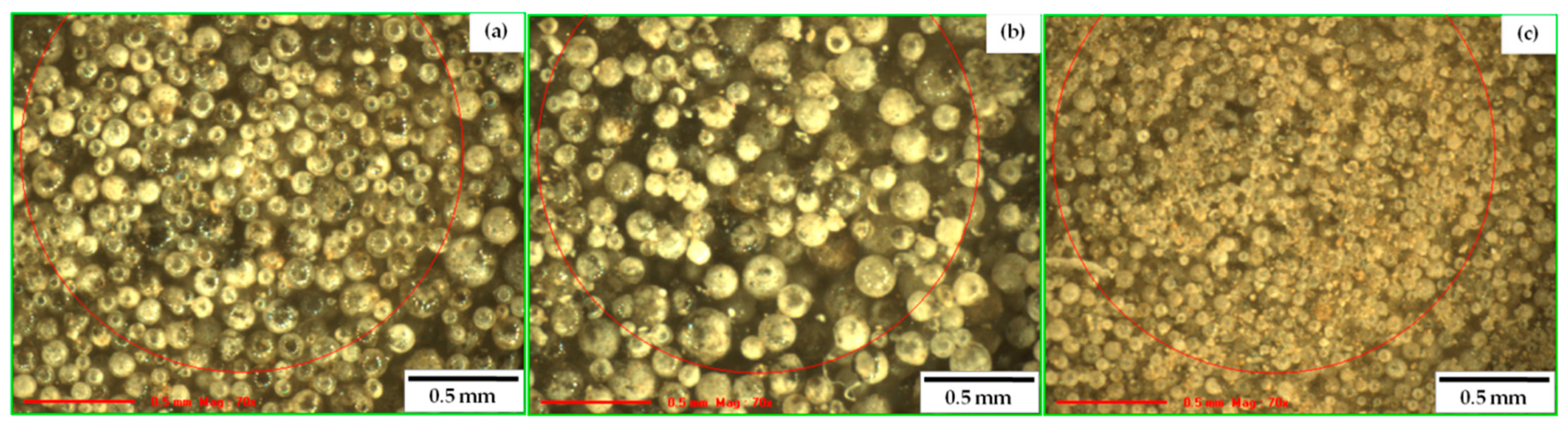
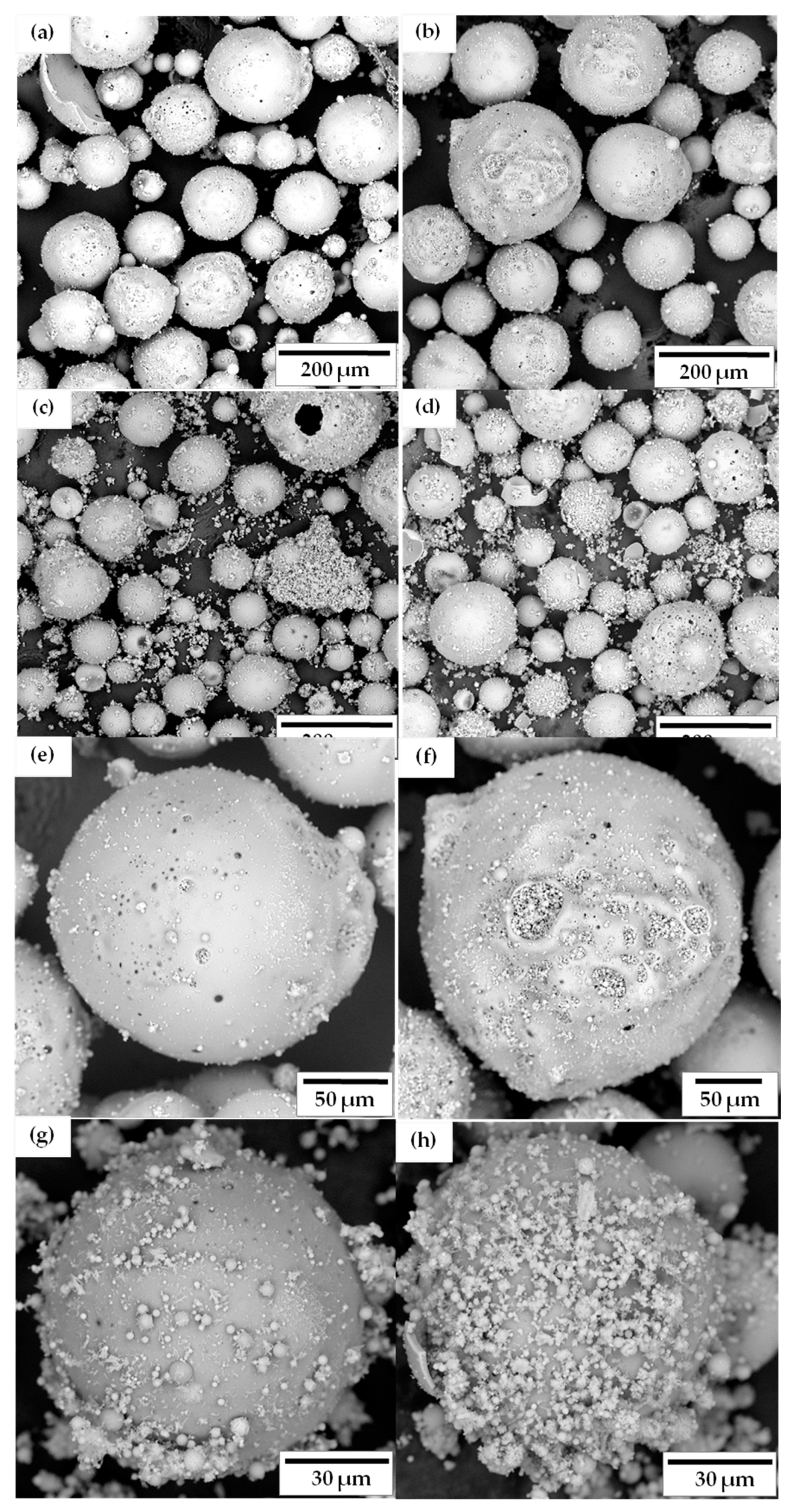
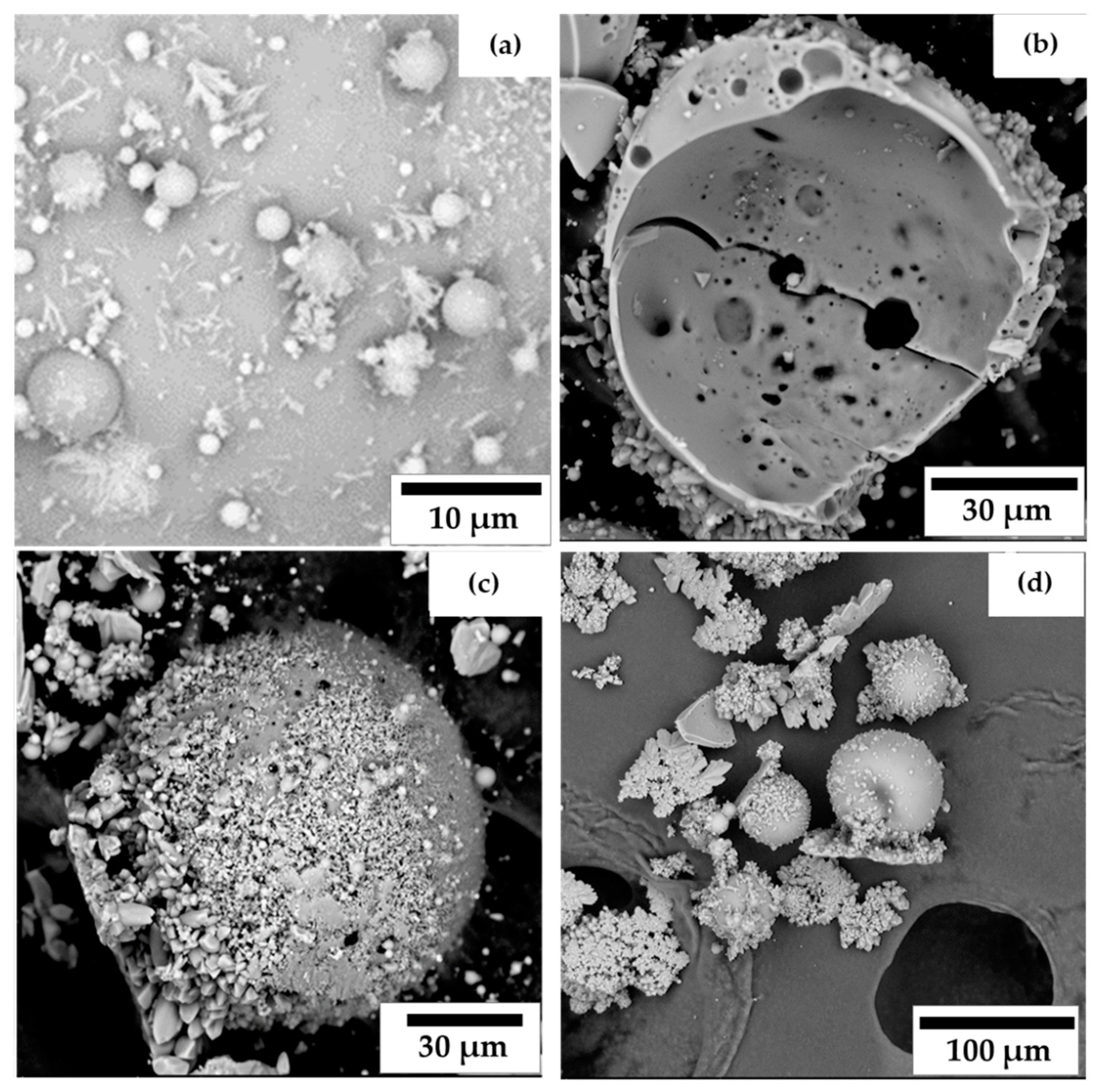
3.4. Morphological Study
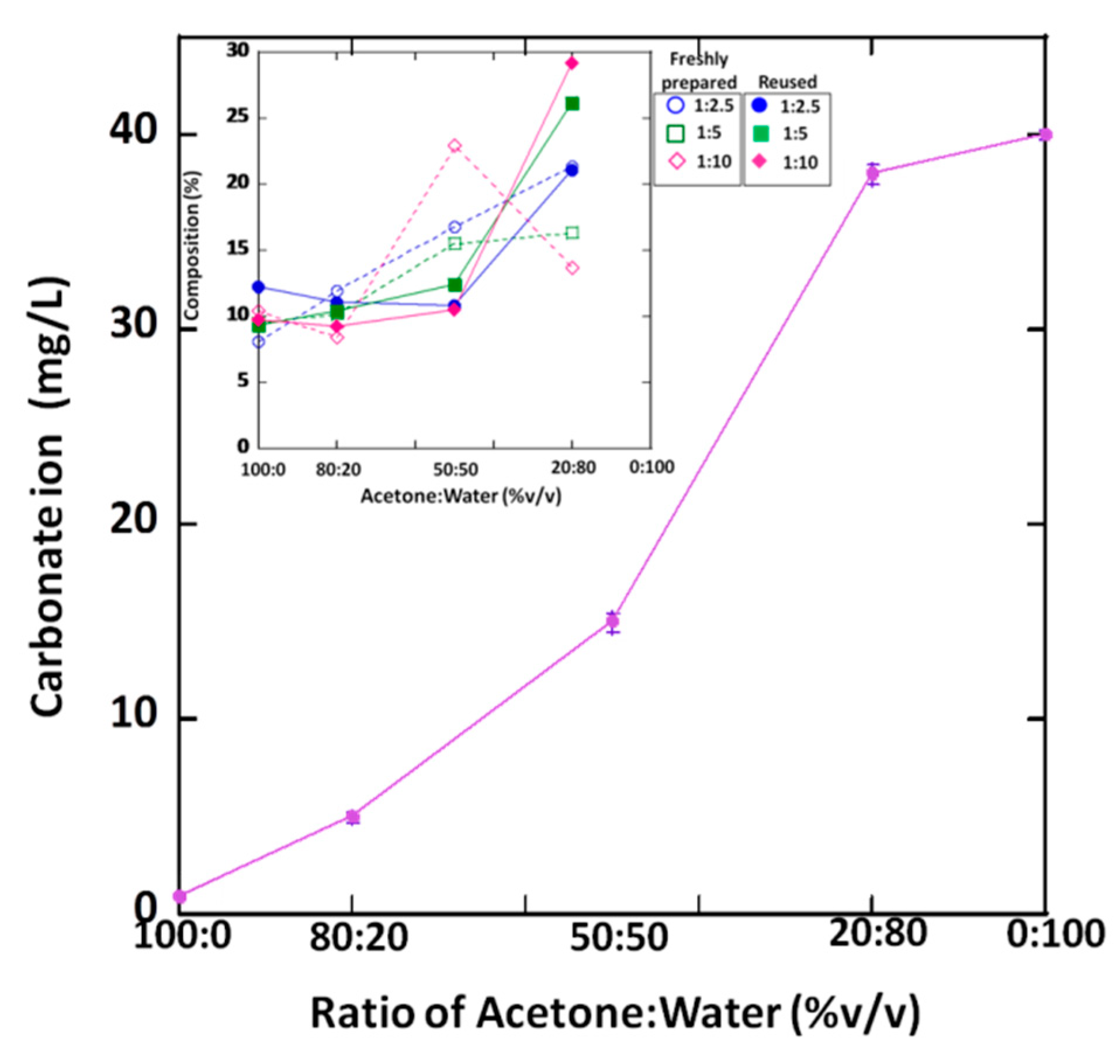
3.5. Carbonate Concentration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hlatshwayo, T.B.; Matjie, R.; Li, Z.; Ward, C.R. Mineralogical characterization of sasol feed coals and corresponding gasification ash constituents. Energy Fuel 2009, 23, 2867–2873. [Google Scholar] [CrossRef]
- Ngernkham, T.P.; Chindaprasirt, P.; Sata, V.; Pangdaeng, S.; Sinsiri, T. Properties of high calcium fly ash geopolymer pastes with Portland cement as an additive. Int. J. Miner. Metall. Mater. 2013, 20, 214–220. [Google Scholar] [CrossRef]
- Kaewmanee, K.; Krammart, P.; Sumranwanich, T.; Choktaweekarn, P.; Tangtermsirikul, S. Effect of free lime content on properties of cement–fly ash mixtures. Constr. Build. Mater. 2013, 38, 829–836. [Google Scholar] [CrossRef]
- Lilkov, V.; Djabarov, N.; Bechev, G.; Kolev, K. Properties and hydration products of lightweight and expansive cements Part I: Physical and mechanical properties. Cem. Concr. Res. 1999, 29, 1635–1640. [Google Scholar] [CrossRef]
- Lilkov, V.; Djabarov, N.; Bechev, G.; Petrov, O. Properties and hydration products of lightweight and expansive cements Part II: Hydration products. Cem. Concr. Res. 1999, 29, 1641–1646. [Google Scholar] [CrossRef]
- Chalivendra, V.B.; Shukla, A.; Bose, A.; Parameswaran, V. Processing and mechanical characterization of lightweight polyurethane composites. J. Mater. Sci. 2003, 38, 1631–1643. [Google Scholar] [CrossRef]
- Cardoso, R.J.; Shukla, A.; Bose, A. Effect of particle size and surface treatment on constitutive properties of polyester-cenosphere composites. J. Mater. Sci. 2002, 37, 603–613. [Google Scholar] [CrossRef]
- Parameswaran, V.; Shukla, A. Processing and characterization of a model functionally gradient material. J. Mater. Sci. 2000, 35, 21–29. [Google Scholar] [CrossRef]
- Shutov, F. Syntactic polymeric foams. In Handbook of Polymeric Foams and Foam Technology; Hanser Publishers: New York, NY, USA, 1991. [Google Scholar]
- Wasekar, P.A.; Kadam, P.G.; Mhaske, S.T. Effect of cenosphere concentration on the mechanical, thermal, rheological and morphological properties of nylon 6. J. Miner. Mater. Charact. Eng. 2012, 11, 807. [Google Scholar] [CrossRef]
- Jha, N.; Badkul, A.; Mondal, D.; Das, S.; Singh, M. Sliding wear behaviour of aluminum syntactic foam: A comparison with Al–10wt% SiC composites. Tribol. Int. 2011, 44, 220–231. [Google Scholar] [CrossRef]
- Chávez-Valdez, A.; Arizmendi-Morquecho, A.; Vargas, G.; Almanza, J.M.; Alvarez-Quintana, J. Ultra-low thermal conductivity thermal barrier coatings from recycled fly-ash cenospheres. Acta Mater. 2011, 59, 2556–2562. [Google Scholar] [CrossRef]
- Ozcivici, E.; Singh, R.P. Fabrication and characterization of ceramic foams based on silicon carbide matrix and hollow alumino-silicate spheres. J. Am. Ceram. Soc. 2005, 88, 3338–3345. [Google Scholar] [CrossRef]
- Rohatgi, P.K.; Gupta, N.; Schultz, B.F.; Luong, D.D. The synthesis, compressive properties, and applications of metal matrix syntactic foams. JOM 2011, 63, 36–42. [Google Scholar] [CrossRef]
- Shunmugasamy, V.C.; Gupta, N.; Nguyen, N.Q.; Coelho, P.G. Strain rate dependence of damage evolution in syntactic foams. Mater. Sci. Eng. 2010, 527, 6166–6177. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Hu, C.; Li, Z. Influence of cenospheres on properties of magnesium oxychloride cement-based composites. Mater. Struct. 2016, 49, 1319–1326. [Google Scholar] [CrossRef]
- Vikrant, T.; Arun, S.; Bose, A. Acoustic properties of cenosphere reinforced cement and asphalt concrete. Appl. Acoust. 2004, 65, 263–275. [Google Scholar] [CrossRef]
- Blanco, F.; Garcı’a, P.; Mateos, P.; Ayala, J. Characteristics and properties of lightweight concrete manufactured with cenospheres. Cem. Concr. Res. 2000, 30, 1715–1722. [Google Scholar] [CrossRef]
- McBride, S.P.; Shukla, A.; Bose, A. Processing and characterization of a lightweight concrete using cenospheres. J. Mater. Sci. 2002, 37, 4217–4225. [Google Scholar] [CrossRef]
- Ferdous, W.; Manalo, A.; Aravinthan, T.; Fam, A. Flexural and shear behaviour of layered sandwich beams. Constr. Build. Mater. 2018, 173, 429–442. [Google Scholar] [CrossRef]
- Ferdous, W.; Manalo, A.; Van Erp, G.; Aravinthan, T.; Ghabraie, K. Evaluation of an Innovative Composite Railway Sleeper for a Narrow-Gauge Track under Static Load. J. Compos. Constr. 2018, 22, 1–13. [Google Scholar] [CrossRef]
- Acar, İ.; Atalay, M.U. Recovery potentials of cenospheres from bituminous coal fly ashes. Fuel 2016, 180, 97–105. [Google Scholar] [CrossRef]
- Sarkar, A.; Rano, R.; Mishra, K.; Mazumder, A. Characterization of cenospheres collected from ash-pond of a super thermal power plant. Energy Sources Part A Recovery Util. Environ. Eff. 2007, 30, 271–283. [Google Scholar] [CrossRef]
- Kolay, P.K.; Bhusal, S. Recovery of hollow spherical particles with two different densities from coal fly ash and their characterization. Fuel 2014, 117, 118–124. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Solovyov, L.A.; Mikhaylova, O.A.; Anshits, A.G. Composition and morphology of fly ash cenospheres produced from the combustion of kuznetsk coal. Energy Fuel 2013, 27, 5440–5448. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Mineralogy of combustion wastes from coal-fired power stations. Fuel Process. Technol. 1996, 47, 261–280. [Google Scholar] [CrossRef]
- Sokol, E.; Maksimova, N.; Volkova, N.; Nigmatulina, E.; Frenkel, A. Hollow silicate microspheres from fly ashes of the Chelyabinsk brown coals (South Urals, Russia). Fuel Process. Technol. 2000, 67, 35–52. [Google Scholar] [CrossRef]
- Ramme, B.W.; Noegel, J.J.; Rohatgi, P.K. Separation of Cenospheres from Fly Ash. U.S. Patent No. 8,074,804 B2, 13 December 2011. [Google Scholar]
- Vassilev, S.V.; Menendez, R.; Diaz-Somoano, M.; Martinez-Tarazona, M.R. Phase-mineral and chemical compositon of coal fly ashes as a basis for their multicomponent utilization. 2. Characterization of ceramic cenosphere and salt concentrates. Fuel 2004, 83, 585–603. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C. Cenospheres: A review. Fuel 2017, 207, 1–12. [Google Scholar] [CrossRef]
- Anshits, N.N.; Mikhailova, O.A.; Ssalanov, A.N.; Anshits, A.G. Chemical compostion and structure of the shell of fly ash non-perforated cenospheres produced from the combustion of the Kuznetsk coal (Russia). Fuel 2010, 89, 1849–1862. [Google Scholar] [CrossRef]
- Ngu, L.; Wu, H.; Zhang, D. Characterization of ash cenospheres in fly ash from Australian power station. Energy Fuel 2007, 21, 3437–3445. [Google Scholar] [CrossRef]
- Ghosal, S.; Self, S.A. Particle size–density relation and cenosphere content of coal fly ash. Fuel 1995, 74, 522–529. [Google Scholar] [CrossRef]
- Goodarzi, F. Characteristics and composition of fly ash from Canadian coal-fired power plants. Fuel. 2006, 85, 1418–1427. [Google Scholar] [CrossRef]
- Borm, P.J.A. Toxicity and occupational health hazards of coal fly ash (CFA). A review of data and comparison to coal mine dust. Ann. Occup. Hyg. 1997, 41, 659–676. [Google Scholar] [CrossRef]
- Meij, R.; Vredenbregt, L.H.; Winkel, H.T. The fate and behavior of mercury in coalfired power plants. J. Air Waste Manag. Assoc. 2002, 52, 912–917. [Google Scholar] [CrossRef]
- Hirajima, T.; Petrus, H.; Oosako, Y.; Nonaka, M.; Sasaki, K.; Ando, T. Recovery of cenospheres from coal fly ash using a dry separation process: Separation estimation and potential application. Int. J. Miner. Process. 2010, 95, 18–24. [Google Scholar] [CrossRef]
- Winnick, J.; Kong, J. Excess volumes of mixtures containing polar liquids. Ind. Eng. Chem. Fundam. 1974, 13, 292–293. [Google Scholar] [CrossRef]
- Birla, S.; Mondal, D.P.; Das, S.; Khare, A.; Singh, J.P. Effect of cenosphere particle size and relative density on the compressive deformation behavior of aluminum-cenosphere hybrid foam. Mater. Des. 2017, 117, 168–177. [Google Scholar] [CrossRef]
- ASTM. ASTM C 618-03 Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- MorenoN, Q.; Andres, J.M.; Stanton, K.; Towler, M.; Nugteren, H.; Janssen-Jurkovicova, J.; Jones, R. Physico-chemical characteristics of European pulverized coal combustion fly ashes. Fuel 2005, 84, 1351–1363. [Google Scholar] [CrossRef]
- Ferdous, M.W.; Kayali, O.; Khennane, A. A detailed procedure of mix design for fly ash based geopolymer concrete. In Proceeding of the Fourth Asia-Pacific Conference on FRP in Structures (APFIS 2013), Melbourne, Australia, 11–13 December 2013. [Google Scholar]
- Bediako, M.; Amankwah, E.O. Analysis of Chemical Composition of Portland Cement in Ghana: A Key to Understand the Behavior of Cement. Adv. Mater. Sci. Eng. 2015, 2015, 349401. [Google Scholar] [CrossRef]
- Nyambura, M.G.; Mugera, G.W.; Felicia, P.L.; Gathura, N.P. Carbonation of brine impacted fractionated coal fly ash: Implications for CO2 sequestration. J. Environ. Manag. 2011, 92, 655–664. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Vereshchagina, T.; Anshits, N.; Maksimov, N.; Vereshchagin, S.; Bayukov, O.; Anshits, A. The nature and properties of iron-containing nanoparticles dispersed in an aluminosilicate matrix of cenospheres. Glass Phys. Chem. 2004, 30, 247–256. [Google Scholar] [CrossRef]
- Anshits, N.; Vereshchagina, T.; Bayukov, O.; Salanov, A.; Anshits, A. The nature of nanoparticles of crystalline phases in cenospheres and morphology of their shells. Glass Phys. Chem. 2005, 31, 306–315. [Google Scholar] [CrossRef]
- Żyrkowski, M.; Neto, R.C.; Santos, L.F.; Witkowski, K. Characterization of fly-ash cenospheres from coal-fired power plant unit. Fuel 2016, 174, 49–53. [Google Scholar] [CrossRef]
- Vereshchagina, T.A.; Vasilieva, G.N.; Vereshchagin, S.; Paretskov, E.N.; Zykova, I.D.; Kruchek, D.M.; Manakova, L.F.; Tretyakov, A.A.; Anshits, A.G. Porous Materials Based on Cenospheres of Coal Fly Ash for Fixation of Cs-137 and Sr-90 in Mineral-like Aluminosilicates. Mater. Res. Soc. Symp. Proc. 2011, 932, 591–598. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Scientific and Technical Basis for the Geological Disposal of Radioactive Wastes; Technical Reports Series No. 413; IAEA: Vienna, Austria, 2003; pp. 1–80. [Google Scholar]
- Vu, D.H.; Bui, H.B.; Kalantar, B.; Bui, X.N.; Nguyen, D.A.; Le, Q.T.; Do, N.H.; Nguyen, H. Composition and morphology characteristics of magnetic fractions of coal fly ash wastes processed in high-temperature exposure in thermal power plants. Appl. Sci. 2019, 9, 1964. [Google Scholar] [CrossRef]
- Shoumkova, A.S. Magnetic separation of coal fly ash from Bulgarian power plants. Waste Manag. Res. 2011, 29, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Bourliva, A.; Papadopoulou, L.; Aidona, E.; Simeonidis, K.; Vourlias, G.; Devlin, E.; Sanakis, Y. Enrichment and oral bioaccessibility of selected trace elements in fly ash-derived magnetic components. Environ. Sci. Pollut. Res. 2017, 24, 2337–2349. [Google Scholar] [CrossRef] [PubMed]
- Blissett, R.S.; Rowson, N.A. A review of the multi-component utilization of coal fly ash. Fuel 2012, 97, 1–23. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Donatello, S.; Fernández-Jiménez, A.; Palomo, Á. Hydration of Hybrid Alkaline Cement Containing a Very Large Proportion of Fly Ash: A Descriptive Model. Materials 2016, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Molar Mass, Molecular Weight and Elemental Composition Calculator. Available online: https://www.webqc.org/mmcalc.php (accessed on 2 August 2019).
- McArthur, H.; Spalding, D. Engineering Materials Science: Properties, Uses, Degradation, Remediation, 1st ed.; Woodhead Publishing: Philadelphia, PA, USA, 2004; p. 608. [Google Scholar]
- Lee, Y.L.; Wang, W.H.; Lin, F.H.; Lin, C.P. Hydration behaviors of calcium silicate-based biomaterials. J. Formos. Med. Assoc. 2017, 116, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yamauchi, K.; Li, Z.; Zhang, X.; Ma, H.; Ge, H. Novel understanding of calcium silicate hydrate from dilute hydration. Cem. Concr. Res. 2017, 99, 95–105. [Google Scholar] [CrossRef]
- Fábián, B.; Jójárt, B.; Horvai, G.; Jedlovszky, P. Properties of the Liquid−Vapor Interface of Acetone−Water Mixtures. A Computer Simulation and ITIM Analysis Study. J. Phys. Chem. 2015, 119, 12473–12487. [Google Scholar] [CrossRef]
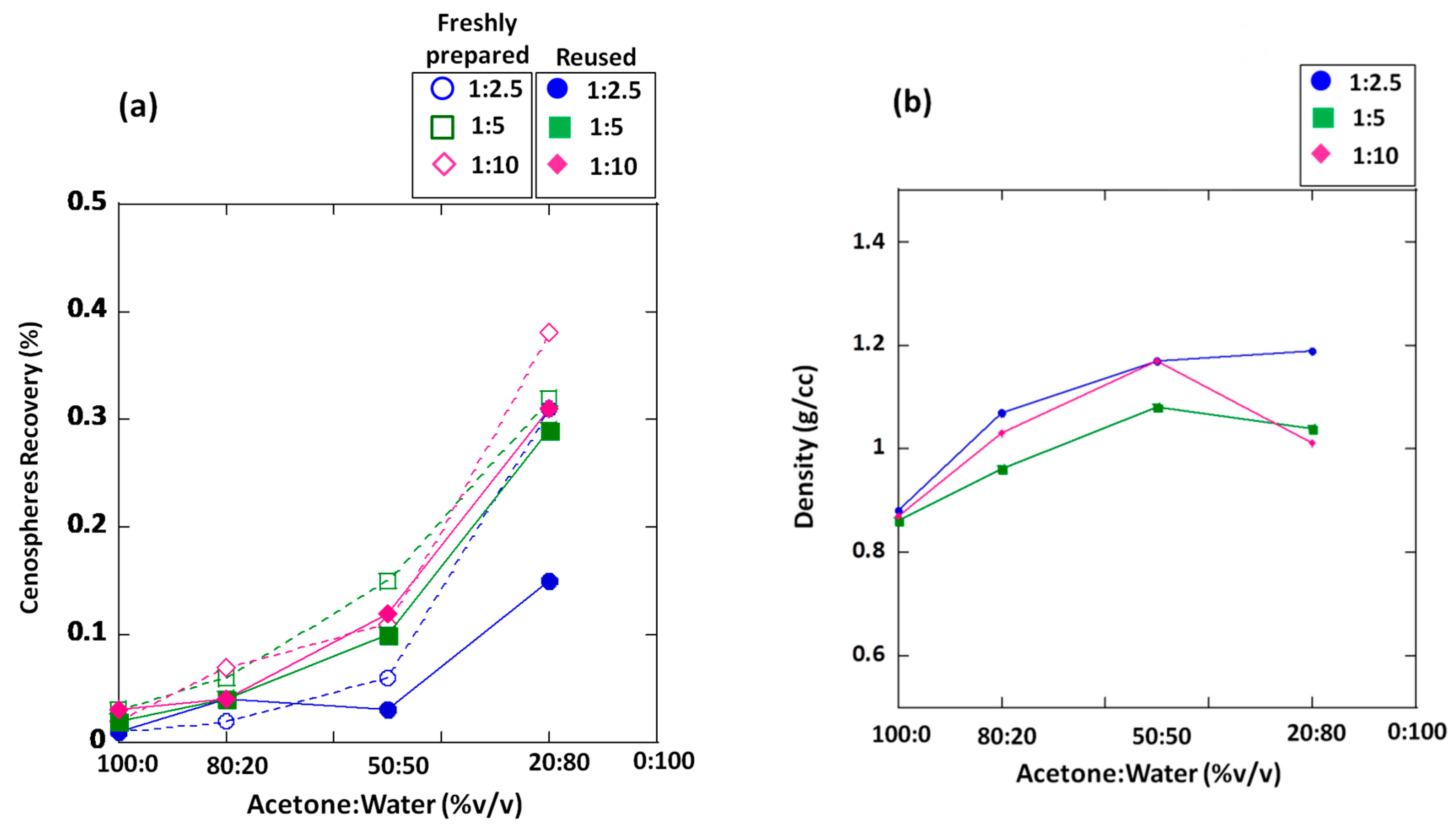
| Acetone/Water Ratio (% v/v) | Calculated Density of Acetone–Water Mixtures | Percentage of Cenosphere Recovery (%) | |||||
|---|---|---|---|---|---|---|---|
| Fly Ash/Medium Ratio | |||||||
| 1:2.5 (Freshly Prepared) | 1:2.5 (Used) | 1:5 (Freshly Prepared) | 1:5 (Used) | 1:10 (Freshly Prepared) | 1:10 (Used) | ||
| 100:0 | 0.79 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.03 ± 0.004 | 0.02 ± 0.002 | 0.02 ± 0.003 | 0.03 ± 0.003 |
| 80:20 | 0.85 | 0.02 ± 0.001 | 0.04 ± 0.001 | 0.06 ± 0.002 | 0.04 ± 0.003 | 0.07 ± 0.003 | 0.04 ± 0.002 |
| 50:50 | 0.90 | 0.06 ± 0.003 | 0.03 ± 0.002 | 0.15 ± 0.020 | 0.10 ± 0.015 | 0.11 ± 0.008 | 0.12 ± 0.005 |
| 20:80 | 0.96 | 0.31 ± 0.005 | 0.15 ± 0.025 | 0.32 ± 0.008 | 0.29 ± 0.014 | 0.38 ± 0.007 | 0.31 ± 0.023 |
| Fly Ash/Medium Ratio | Acetone/Water Ratio | D [4,3] (μm) | Weight Percentage Size Distribution (%) | ||||
|---|---|---|---|---|---|---|---|
| <50 μm | 50–100 μm | 100–150 μm | 150–200 μm | >200 μm | |||
| 1:2.5 | 100:0 | 125.09 ± 2.71 | 18.46 | 24.16 | 23.69 | 16.23 | 17.46 |
| 80:20 | 131.42 ± 2.79 | 18.50 | 21.19 | 23.49 | 16.63 | 20.18 | |
| 50:50 | 51.65 ± 0.67 | 63.09 | 23.02 | 7.66 | 3.24 | 2.99 | |
| 20:80 | 38.17 ± 1.21 | 68.98 | 25.74 | 4.98 | 0.30 | n/a | |
| 1: 5 | 100:0 | 91.56 ± 2.99 | 31.85 | 31.22 | 19.17 | 9.98 | 7.79 |
| 80:20 | 141.21 ± 3.01 | 11.48 | 22.89 | 25.66 | 18.2 | 21.77 | |
| 50:50 | 113.55 ± 1.10 | 18.43 | 30.33 | 24.77 | 13.81 | 12.66 | |
| 20:80 | 96.07 ± 1.33 | 29.80 | 30.96 | 19.62 | 10.01 | 9.61 | |
| 1:10 | 100:0 | 147.76 ± 2.33 | 13.60 | 19.58 | 23.40 | 17.84 | 25.58 |
| 80:20 | 110.57 ± 4.75 | 29.67 | 24.07 | 18.18 | 12.50 | 15.58 | |
| 50:50 | 60.67 ± 2.64 | 60.35 | 19.15 | 9.35 | 5.03 | 6.12 | |
| 20:80 | 82.85 ± 1.86 | 41.18 | 27.56 | 15.24 | 8.11 | 7.90 | |
| Compound wt % | Fly Ash | Recovered Fly Ash | Acetone/Water (% v/v) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100:0 | 80:20 | 50:50 | 20:80 | |||||||||||
| 1:2.5 | 1:5 | 1:10 | 1:2.5 | 1:5 | 1:10 | 1:2.5 | 1:5 | 1:10 | 1:2.5 | 1:5 | 1:10 | |||
| SiO2 | 32.12 ± 0.29 | 30.90 ± 0.16 | 56.91 ± 0.55 | 51.20 ± 0.26 | 51.68 ± 0.22 | 50.80 ± 0.52 | 51.63 ± 1.73 | 50.57 ± 0.84 | 43.70 ± 0.73 | 45.02 ± 1.91 | 36.60 ± 0.43 | 37.95 ± 0.20 | 44.88 ± 1.23 | 46.03 ± 0.55 |
| Al2O3 | 13.82 ± 0.19 | 14.08 ± 0.24 | 25.16 ± 0.13 | 23.22 ± 0.16 | 23.02 ± 0.22 | 22.72 ± 0.61 | 23.29 ± 0.29 | 23.08 ± 0.33 | 19.87 ± 0.30 | 20.55 ± 0.77 | 16.34 ± 0.25 | 17.11 ± 0.14 | 20.3 ± 0.37 | 21.34 ± 0.09 |
| Fe2O3 | 14.55 ± 0.27 | 16.21 ± 0.45 | 4.65 ± 0.46 | 8.29 ± 0.17 | 7.55 ± 0.60 | 5.34 ± 0.92 | 8.37 ± 1.12 | 8.74 ± 0.38 | 11.62 ± 0.10 | 10.19 ± 0.15 | 13.19 ± 0.28 | 12.16 ± 0.18 | 9.08 ± 0.88 | 10.04 ± 0.31 |
| CaO | 24.49 ± 0.05 | 25.33 ± 0.63 | 8.10 ± 0.20 | 9.50 ± 0.04 | 10.39 ± 0.37 | 11.90 ± 0.95 | 10.2 ± 0.08 | 8.46 ± 3.81 | 16.79 ± 0.65 | 15.48 ± 1.08 | 22.93 ± 0.46 | 21.34 ± 0.34 | 16.35 ± 0.71 | 13.63 ± 0.45 |
| SO3 | 12.03 ± 0.26 | 10.03 ± 0.27 | 0.81 ± 0.10 | 0.96 ± 0.05 | 1.27 ± 0.03 | 4.77 ± 1.55 | 1.61 ± 0.8 | 1.19 ± 0.05 | 3.46 ± 0.31 | 4.07 ± 1.65 | 6.76 ± 0.22 | 7.12 ± 0.11 | 4.35 ± 0.45 | 2.76 ± 0.17 |
| K2O | 2.31 ± 0.02 | 2.56 ± 0.03 | 3.74 ± 0.03 | 3.66 ± 0.03 | 3.69 ± 0.07 | 3.88 ± 0.03 | 4.05 ± 0.03 | 3.92 ± 0.16 | 3.62 ± 0.06 | 3.81 ± 0.02 | 3.27 ± 0.03 | 3.46 ± 0.03 | 4.1 ± 0.36 | 3.97 ± 0.08 |
| TiO2 | 0.55 ± 0.02 | 0.61 ± 0.01 | 0.63 ± 0.02 | 0.77 ± 0.01 | 0.69 ± 0.04 | 0.59 ± 0.02 | 0.76 ± 0.03 | 4.24 ± 6.02 | 0.76 ± 0.00 | 0.75 ± 0.03 | 0.71 ± 0.01 | 0.67 ± 0.01 | 0.78 ± 0.04 | 0.75 ± 0.02 |
| MnO | 0.55 ± 0.01 | 0.14 ± 0.01 | nd | nd | 0.05 ± 0.00 | nd | nd | 0.06 ± 0.00 | 0.07 ± 0.00 | nd | 0.09 ± 0.00 | 0.10 ± 0.00 | nd | 0.07 ± 0.00 |
| Cr2O3 | nd | 0.55 ± 0.01 | nd | nd | Nd | nd | nd | 0.41 ± 0.00 | 0.06 ± 0.00 | nd | 0.06 ± 0.01 | 0.06 ± 0.00 | nd | 0.05 ± 0.00 |
| CuO | nd | nd | nd | nd | 0.03 ± 0.00 | nd | nd | 0.04 ± 0.00 | 0.04 ± 0.00 | nd | 0.04 ± 0.00 | 0.04 ± 0.00 | nd | 0.04 ± 0.00 |
| MgO | nd | nd | nd | nd | 2.45 ± 0.10 | nd | nd | 2.20 ± 0.00 | nd | nd | nd | nd | nd | 1.98 ± 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoriya, S.; Intana, T.; Tepsri, P. Separation of Cenospheres from Lignite Fly Ash Using Acetone–Water Mixture. Appl. Sci. 2019, 9, 3792. https://doi.org/10.3390/app9183792
Yoriya S, Intana T, Tepsri P. Separation of Cenospheres from Lignite Fly Ash Using Acetone–Water Mixture. Applied Sciences. 2019; 9(18):3792. https://doi.org/10.3390/app9183792
Chicago/Turabian StyleYoriya, Sorachon, Tanasan Intana, and Phattarathicha Tepsri. 2019. "Separation of Cenospheres from Lignite Fly Ash Using Acetone–Water Mixture" Applied Sciences 9, no. 18: 3792. https://doi.org/10.3390/app9183792
APA StyleYoriya, S., Intana, T., & Tepsri, P. (2019). Separation of Cenospheres from Lignite Fly Ash Using Acetone–Water Mixture. Applied Sciences, 9(18), 3792. https://doi.org/10.3390/app9183792





