Comparison of Feature Vector Compositions to Enhance the Performance of NIRS-BCI-Triggered Robotic Hand Orthosis for Post-Stroke Motor Recovery
Abstract
:1. Introduction
2. Materials and Methods
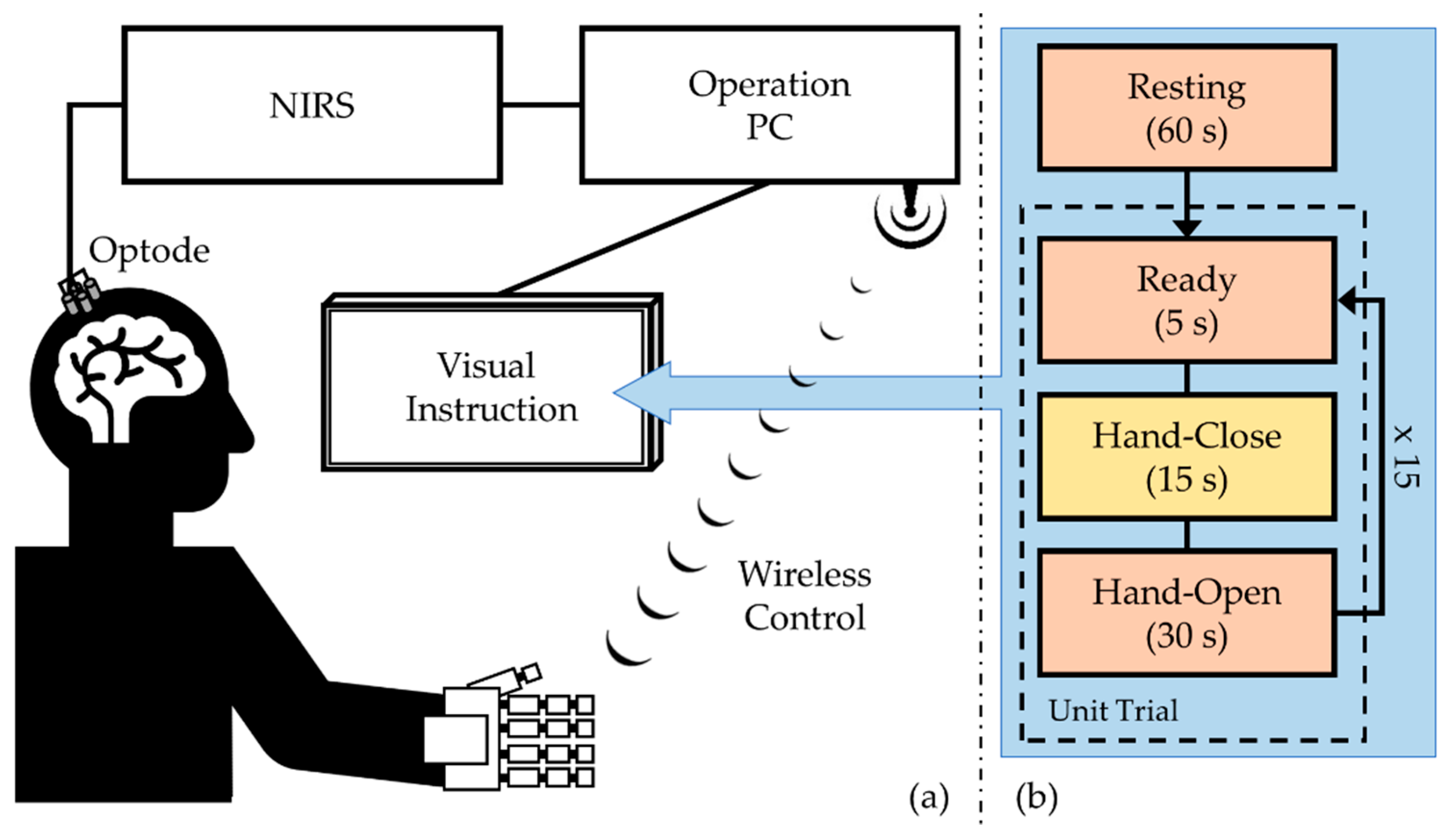
2.1. System and Elements
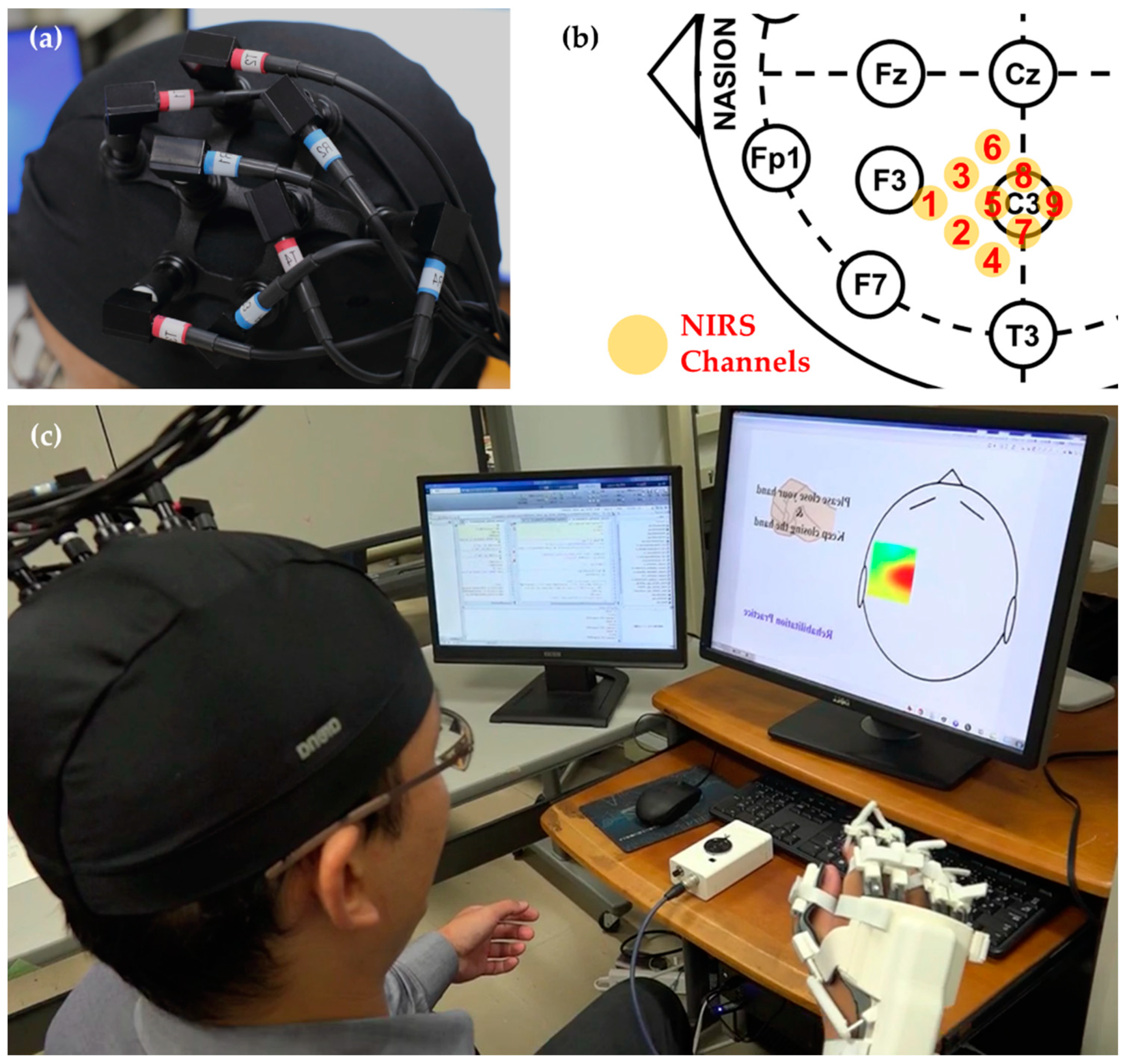
2.1.1. NIRS Measurement
2.1.2. Robotic Hand Orthosis
2.1.3. Wireless Transmission
2.2. Experimental Evaluation
2.2.1. Participants
2.2.2. Experimental Protocol
2.2.3. NIRS Channel Selection
2.2.4. Preprocessing
2.2.5. Binary Classification
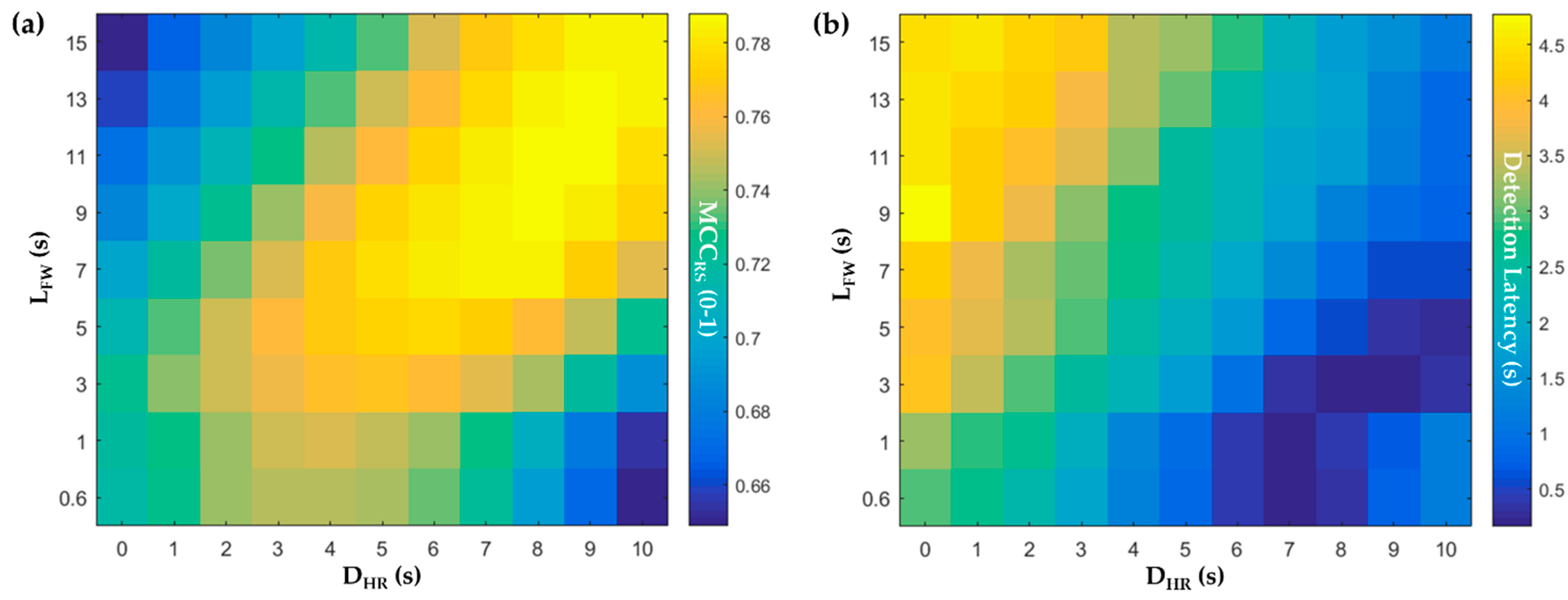
2.2.6. Different Lengths of the Feature Window
2.2.7. Feature Vector Composition
2.2.8. Classification Evaluation
3. Results
3.1. Classifier Training with Different Delays and Window Lengths
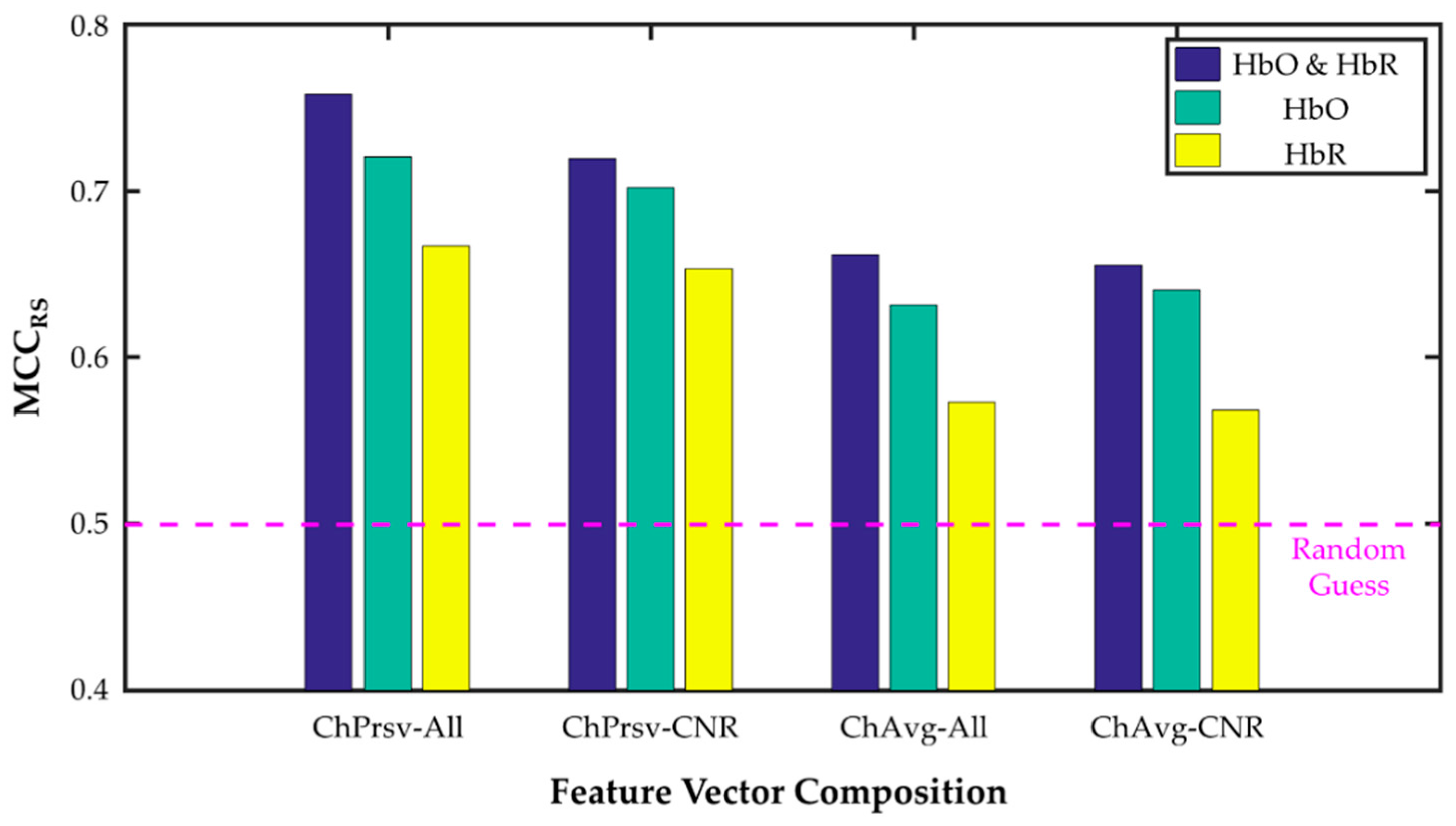
3.2. Channnel-Preserving vs. Channel-Averaging
3.3. [HbO], [HbR], and [HbO & HbR]
3.4. All- and CNR-Selected Channels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wolf, S.L.; Winstein, C.J.; Miller, J.P.; Taub, E.; Uswatte, G.; Morris, D.; Giuliani, C.; Light, K.E.; Nichols-Larsen, D.; for the EXCITE Investigators. Effect of Constraint-Induced Movement Therapy on Upper Extremity Function 3 to 9 Months After Stroke: The EXCITE Randomized Clinical Trial. JAMA 2006, 296, 2095. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.; Braun, C.; Birbaumer, N.; Anders, S.; Cohen, L.G. Motor Learning Elicited by Voluntary Drive. Brain 2003, 126, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Carod-Artal, F.J.; Egido, J.A. Quality of Life after Stroke: The Importance of a Good Recovery. Cerebrovasc. Dis. 2009, 27, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R.; Birbaumer, N.; Heetderks, W.J.; McFarland, D.J.; Peckham, P.H.; Schalk, G.; Donchin, E.; Quatrano, L.A.; Robinson, C.J.; Vaughan, T.M. Brain-Computer Interface Technology: A Review of the First International Meeting. IEEE Trans. Rehabil. Eng. 2000, 8, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Silvoni, S.; Ramos-Murguialday, A.; Cavinato, M.; Volpato, C.; Cisotto, G.; Turolla, A.; Piccione, F.; Birbaumer, N. Brain-Computer Interface in Stroke: A Review of Progress. Clin. EEG Neurosci. 2011, 42, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Soekadar, S.R.; Birbaumer, N.; Slutzky, M.W.; Cohen, L.G. Brain–Machine Interfaces in Neurorehabilitation of Stroke. Neurobiol. Dis. 2015, 83, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y. Functional Near-Infrared Spectroscopy: Current Status and Future Prospects. J. Biomed. Opt. 2007, 12, 062106. [Google Scholar] [CrossRef]
- Iadecola, C. Neurovascular Regulation in the Normal Brain and in Alzheimer’s Disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef]
- Villringer, A.; Dirnagl, U. Coupling of Brain Activity and Cerebral Blood Flow: Basis of Functional Neuroimaging. Cerebrovasc. Brain Metab. Rev. 1995, 6, 240–276. [Google Scholar]
- Naseer, N.; Hong, K.-S. FNIRS-Based Brain-Computer Interfaces: A Review. Front. Hum. Neurosci 2015, 9. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; Birbaumer, N.; McFarland, D.J.; Pfurtscheller, G.; Vaughan, T.M. Brain–Computer Interfaces for Communication and Control. Clin. Neurophysiol. 2002, 113, 767–791. [Google Scholar] [CrossRef]
- Mihara, M.; Hattori, N.; Hatakenaka, M.; Yagura, H.; Kawano, T.; Hino, T.; Miyai, I. Near-Infrared Spectroscopy–Mediated Neurofeedback Enhances Efficacy of Motor Imagery–Based Training in Poststroke Victims: A Pilot Study. Stroke 2013, 44, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Mukae, N.; Arata, J.; Iwata, H.; Iramina, K.; Iihara, K.; Hashizume, M. A Multichannel-near-Infrared-Spectroscopy-Triggered Robotic Hand Rehabilitation System for Stroke Patients. In Proceedings of the 2017 IEEE International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 158–163. [Google Scholar] [CrossRef]
- Arata, J.; Ohmoto, K.; Gassert, R.; Lambercy, O.; Fujimoto, H.; Wada, I. A New Hand Exoskeleton Device for Rehabilitation Using a Three-Layered Sliding Spring Mechanism. In Proceedings of the 2013 IEEE International Conference on Robotics and Automation, Karlsruhe, Germany, 6–10 May 2013; pp. 3902–3907. [Google Scholar] [CrossRef]
- Zhang, Y.; Brooks, D.H.; Franceschini, M.A.; Boas, D.A. Eigenvector-Based Spatial Filtering for Reduction of Physiological Interference in Diffuse Optical Imaging. J. Biomed. Opt. 2005, 10, 011014. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Bray, S.; Reiss, A.L. Speeded Near Infrared Spectroscopy (NIRS) Response Detection. PLoS ONE 2010, 5, e15474. [Google Scholar] [CrossRef] [PubMed]
- Durantin, G.; Scannella, S.; Gateau, T.; Delorme, A.; Dehais, F. Moving Average Convergence Divergence Filter Preprocessing for Real-Time Event-Related Peak Activity Onset Detection: Application to FNIRS Signals. In Proceedings of the 2014 IEEE 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 2107–2110. [Google Scholar] [CrossRef]
- Gateau, T.; Durantin, G.; Lancelot, F.; Scannella, S.; Dehais, F. Real-Time State Estimation in a Flight Simulator Using FNIRS. PLoS ONE 2015, 10, e0121279. [Google Scholar] [CrossRef] [PubMed]
- Tachtsidis, I.; Scholkmann, F. False Positives and False Negatives in Functional Near-Infrared Spectroscopy: Issues, Challenges, and the Way Forward. Neurophoton 2016, 3, 031405. [Google Scholar] [CrossRef] [PubMed]
- Lyons, R.G. Understanding Digital Signal Processing; Prentice Hall: Upper Saddle River, NJ, USA, 2011. [Google Scholar]
- Theodoridis, S.; Koutroumbas, K. Pattern Recognition, 4th ed.; Elsevier/Acad. Press: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Fazli, S.; Mehnert, J.; Steinbrink, J.; Curio, G.; Villringer, A.; Müller, K.-R.; Blankertz, B. Enhanced Performance by a Hybrid NIRS–EEG Brain Computer Interface. NeuroImage 2012, 59, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kwon, J.; Choi, J.; Im, C.-H. Performance Enhancement of a Brain-Computer Interface Using High-Density Multi-Distance NIRS. Sci. Rep. 2017, 7, 16545. [Google Scholar] [CrossRef]
- Buch, E.; Weber, C.; Cohen, L.G.; Braun, C.; Dimyan, M.A.; Ard, T.; Mellinger, J.; Caria, A.; Soekadar, S.; Fourkas, A.; et al. Think to Move: A Neuromagnetic Brain-Computer Interface (BCI) System for Chronic Stroke. Stroke 2008, 39, 910–917. [Google Scholar] [CrossRef]
- Soekadar, S.R.; Witkowski, M.; Mellinger, J.; Ramos, A.; Birbaumer, N.; Cohen, L.G. ERD-Based Online Brain–Machine Interfaces (BMI) in the Context of Neurorehabilitation: Optimizing BMI Learning and Performance. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 542–549. [Google Scholar] [CrossRef]
- Ramos-Murguialday, A.; Broetz, D.; Rea, M.; Läer, L.; Yilmaz, Ö.; Brasil, F.L.; Liberati, G.; Curado, M.R.; Garcia-Cossio, E.; Vyziotis, A.; et al. Brain-Machine Interface in Chronic Stroke Rehabilitation: A Controlled Study. Ann. Neurol. 2013, 74, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Shindo, K.; Kawashima, K.; Ota, N.; Ito, M.; Ota, T.; Mukaino, M.; Fujiwara, T.; Kimura, A.; Liu, M.; et al. Brain-Computer Interface with Somatosensory Feedback Improves Functional Recovery from Severe Hemiplegia Due to Chronic Stroke. Front. Neuroeng. 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Sakatani, K.; Hoshino, T.; Fujiwara, N.; Kano, T.; Nakamura, S.; Katayama, Y. Effects of Cerebral Ischemia on Evoked Cerebral Blood Oxygenation Responses and BOLD Contrast Functional MRI in Stroke Patients. Stroke 2006, 37, 2514–2520. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Takikawa, Y.; Kawagoe, R.; Shibuya, S.; Iwano, T.; Kitazawa, S. Influence of Skin Blood Flow on Near-Infrared Spectroscopy Signals Measured on the Forehead during a Verbal Fluency Task. NeuroImage 2011, 57, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Giller, C.A.; Giller, A.M.; Cooper, C.R.; Hatab, M.R. Evaluation of the Cerebral Hemodynamic Response to Rhythmic Handgrip. J. Appl. Physiol. 2000, 88, 2205–2213. [Google Scholar] [CrossRef]
- Saager, R.B.; Telleri, N.L.; Berger, A.J. Two-Detector Corrected Near Infrared Spectroscopy (C-NIRS) Detects Hemodynamic Activation Responses More Robustly than Single-Detector NIRS. NeuroImage 2011, 55, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
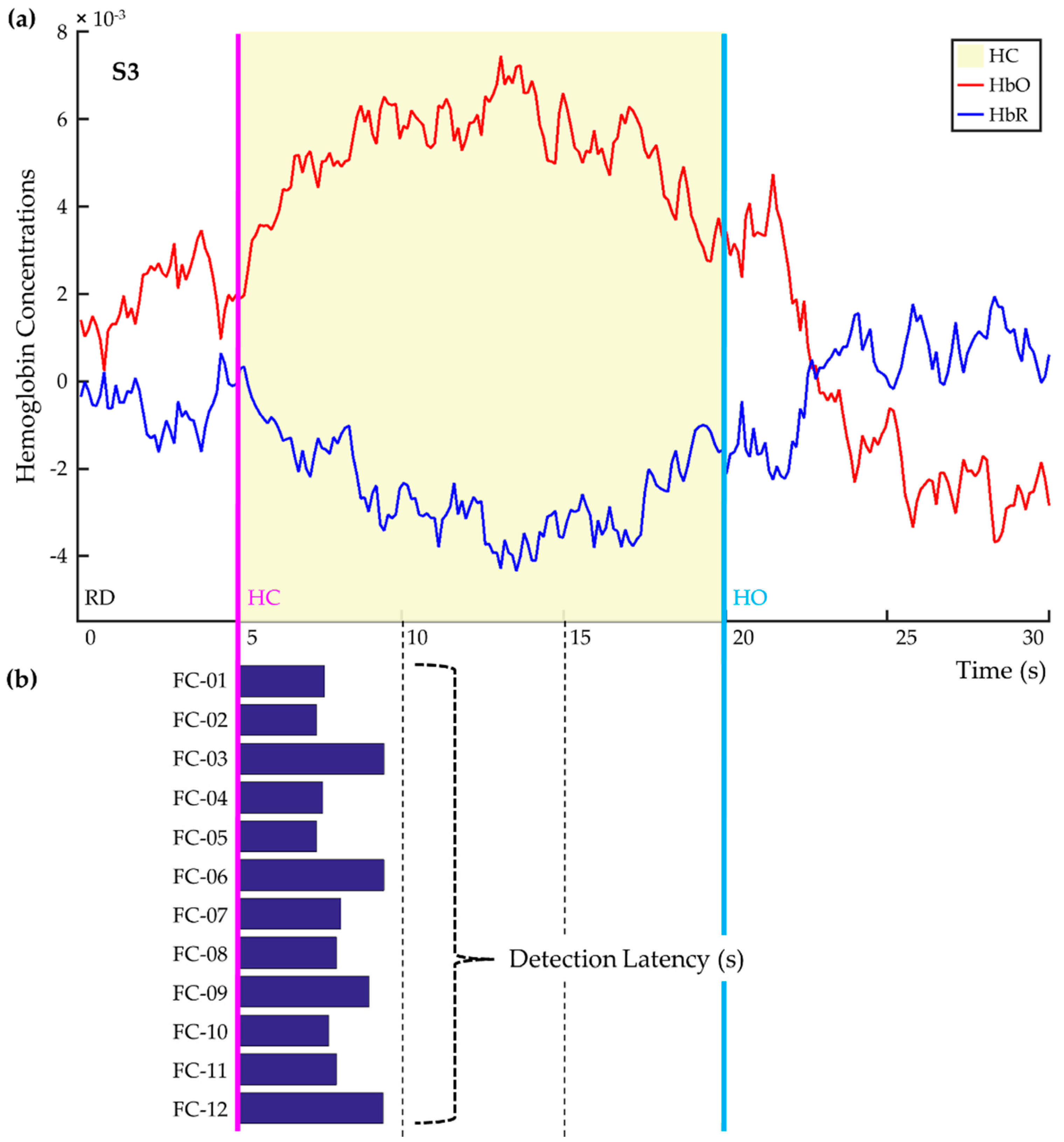
| Label | Composition | Dimension | ||
|---|---|---|---|---|
| Channel | [Hb] | Average | ||
| FC-1 | All | both | - | 36 × 1 |
| FC-2 | [HbO] | - | 18 × 1 | |
| FC-3 | [HbR] | - | 18 × 1 | |
| FC-4 | CNR-selective | both | - | (NCNR-sel × 2 × 2) × 1 |
| FC-5 | [HbO] | - | (NCNR-sel × 2) × 1 | |
| FC-6 | [HbR] | - | (NCNR-sel × 2) × 1 | |
| FC-7 | All | both | y | 4 × 1 |
| FC-8 | [HbO] | y | 2 × 1 | |
| FC-9 | [HbR] | y | 2 × 1 | |
| FC-10 | CNR-selective | both | y | 4 × 1 |
| FC-11 | [HbO] | y | 2 × 1 | |
| FC-12 | [HbR] | y | 2 × 1 | |
| Channel Use | Channel Selection | [HbO] & [HbR] | [HbO] | [HbR] | Mean | |
|---|---|---|---|---|---|---|
| MCCRS | Preserving | All | 0.7586 | 0.7207 | 0.6669 | 0.7154 |
| CNR-sel. | 0.7196 | 0.7023 | 0.6532 | 0.6917 | ||
| Averaging | All | 0.6617 | 0.6316 | 0.5732 | 0.6222 | |
| CNR-sel. | 0.6554 | 0.6406 | 0.5685 | 0.6215 | ||
| Mean | 0.6988 | 0.6738 | 0.6154 | - | ||
| Detection Latency (s) | Preserving | All | 2.4015 | 2.9464 | 3.2066 | 2.8515 |
| CNR-sel. | 2.9184 | 3.3637 | 3.4257 | 3.2359 | ||
| Averaging | All | 3.7349 | 3.4492 | 3.2491 | 3.4778 | |
| CNR-sel. | 4.2665 | 4.4143 | 6.6126 | 5.0978 | ||
| Mean | 3.3303 | 3.5434 | 4.1235 | - | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Mukae, N.; Arata, J.; Iihara, K.; Hashizume, M. Comparison of Feature Vector Compositions to Enhance the Performance of NIRS-BCI-Triggered Robotic Hand Orthosis for Post-Stroke Motor Recovery. Appl. Sci. 2019, 9, 3845. https://doi.org/10.3390/app9183845
Lee J, Mukae N, Arata J, Iihara K, Hashizume M. Comparison of Feature Vector Compositions to Enhance the Performance of NIRS-BCI-Triggered Robotic Hand Orthosis for Post-Stroke Motor Recovery. Applied Sciences. 2019; 9(18):3845. https://doi.org/10.3390/app9183845
Chicago/Turabian StyleLee, Jongseung, Nobutaka Mukae, Jumpei Arata, Koji Iihara, and Makoto Hashizume. 2019. "Comparison of Feature Vector Compositions to Enhance the Performance of NIRS-BCI-Triggered Robotic Hand Orthosis for Post-Stroke Motor Recovery" Applied Sciences 9, no. 18: 3845. https://doi.org/10.3390/app9183845






