Stabilization of a Residual Soil Using Calcium and Magnesium Hydroxide Nanoparticles: A Quick Precipitation Method
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Residual Soil
2.2. Soil Sample Preparation
2.3. Nanoparticles Preparation
2.4. Geotechnical Tests
2.4.1. Particle Size Distribution, Atterberg Limits, and Specific Gravity
2.4.2. Standard Proctor Compaction
2.4.3. Hydraulic Conductivity
2.4.4. Unconfined Compressive Strength (UCS)
2.5. Microstructural Tests
2.5.1. X-ray Diffraction (XRD)
2.5.2. Variable-Pressure Scanning Electron Microscope (VP-SEM) and Energy-Dispersive X-ray Spectroscopy (EDX)
2.6. Statistical Analysis
3. Results and Discussions
3.1. Effect of Nanoparticles on Atterberg Limits
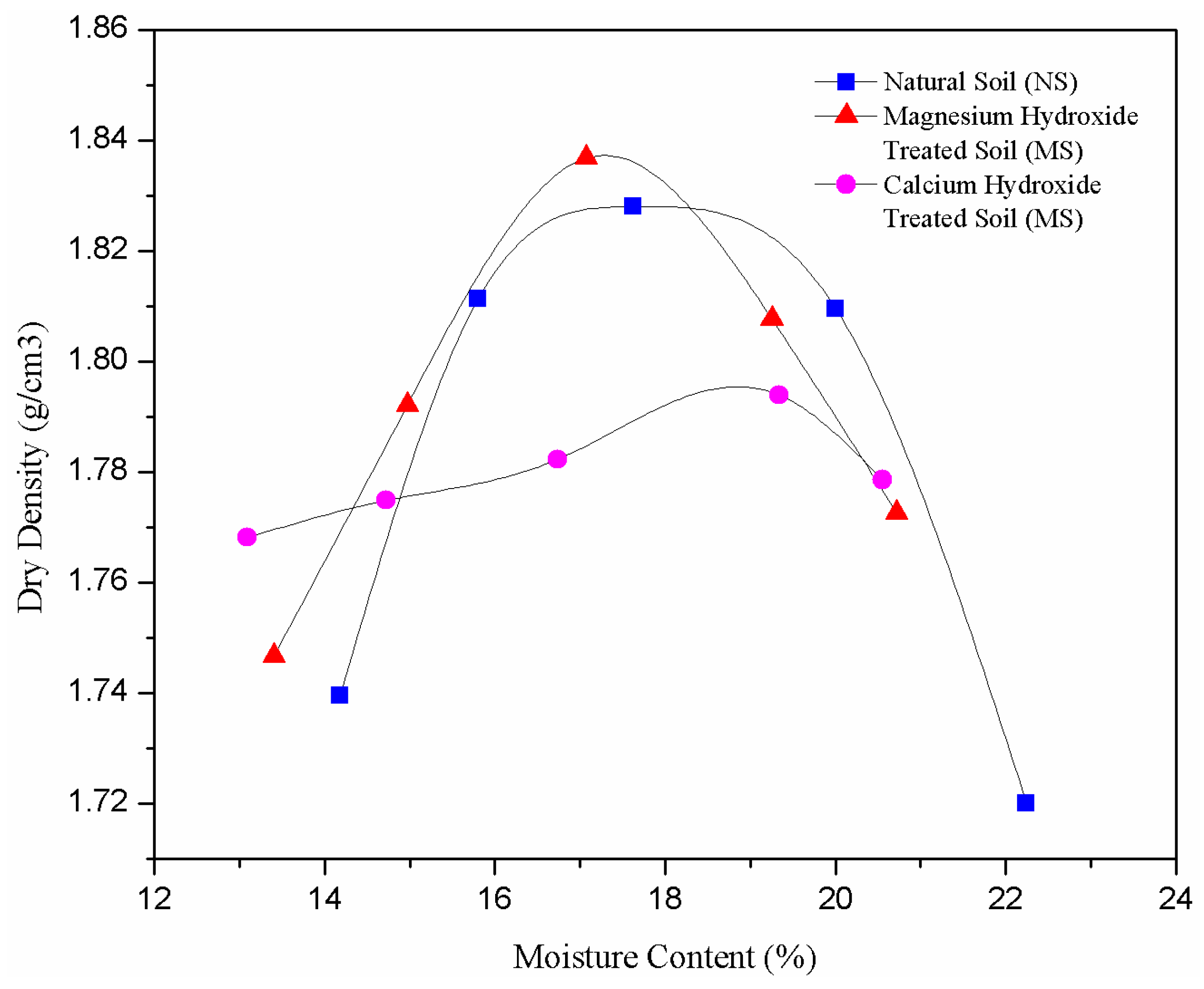
3.2. Effect of Nanoparticles on Compaction Characteristics
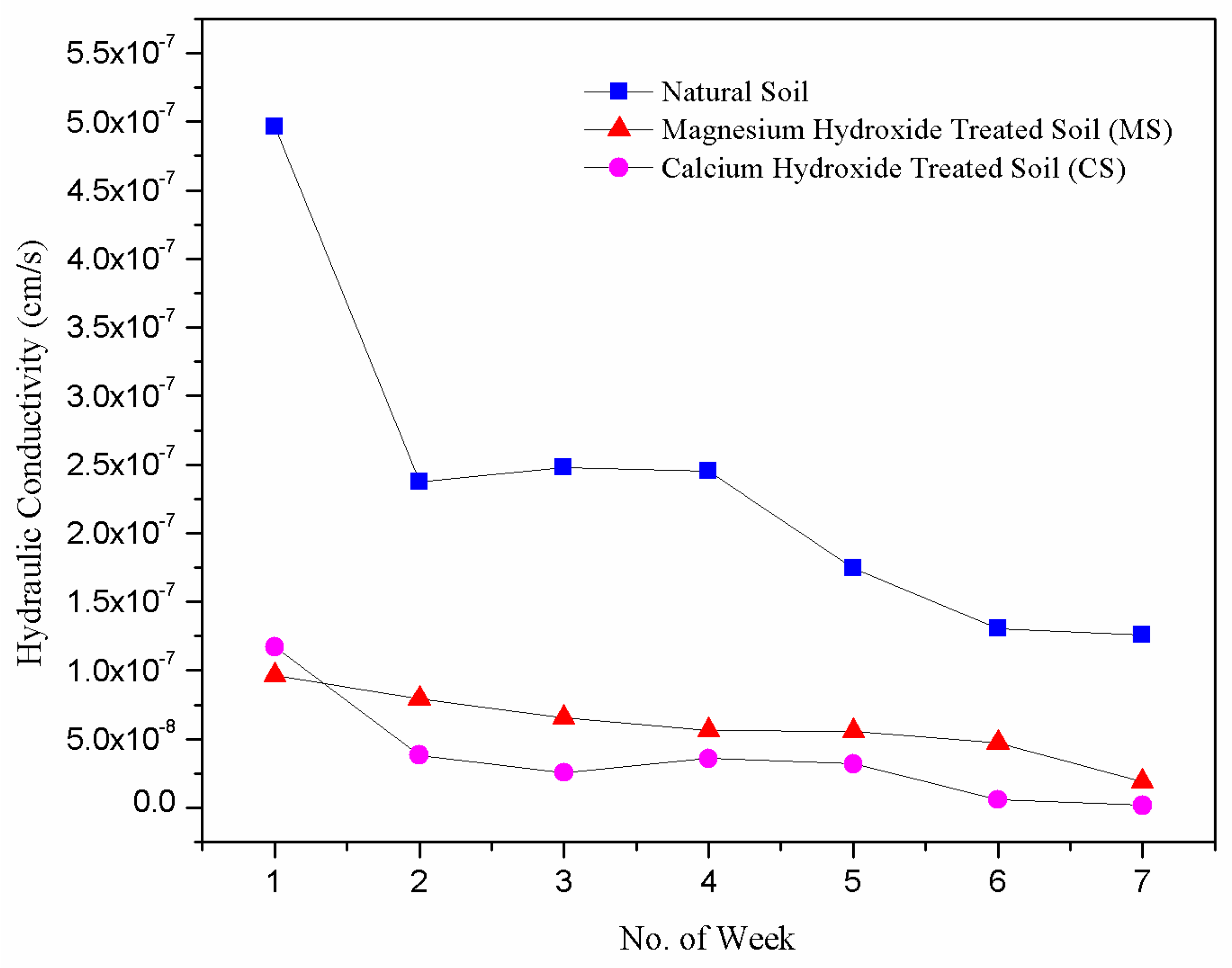
3.3. Effect of Nanoparticles on Hydraulic Conductivity
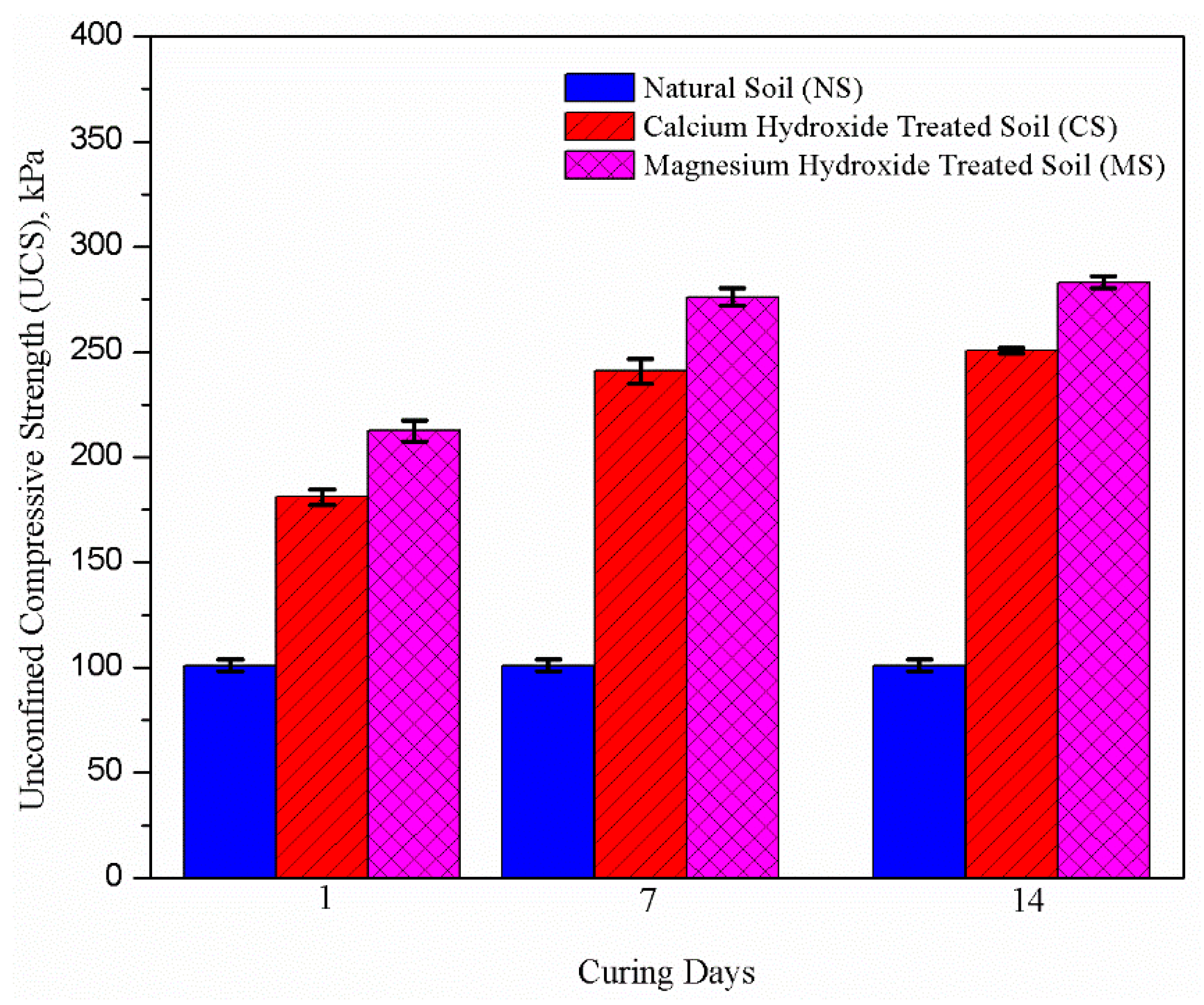
3.4. Effect of Nanoparticles on Unconfined Compressive Strength (UCS)
3.5. Effect of Nanoparticles on Microstructural Properties
3.5.1. X-ray Diffraction (XRD)
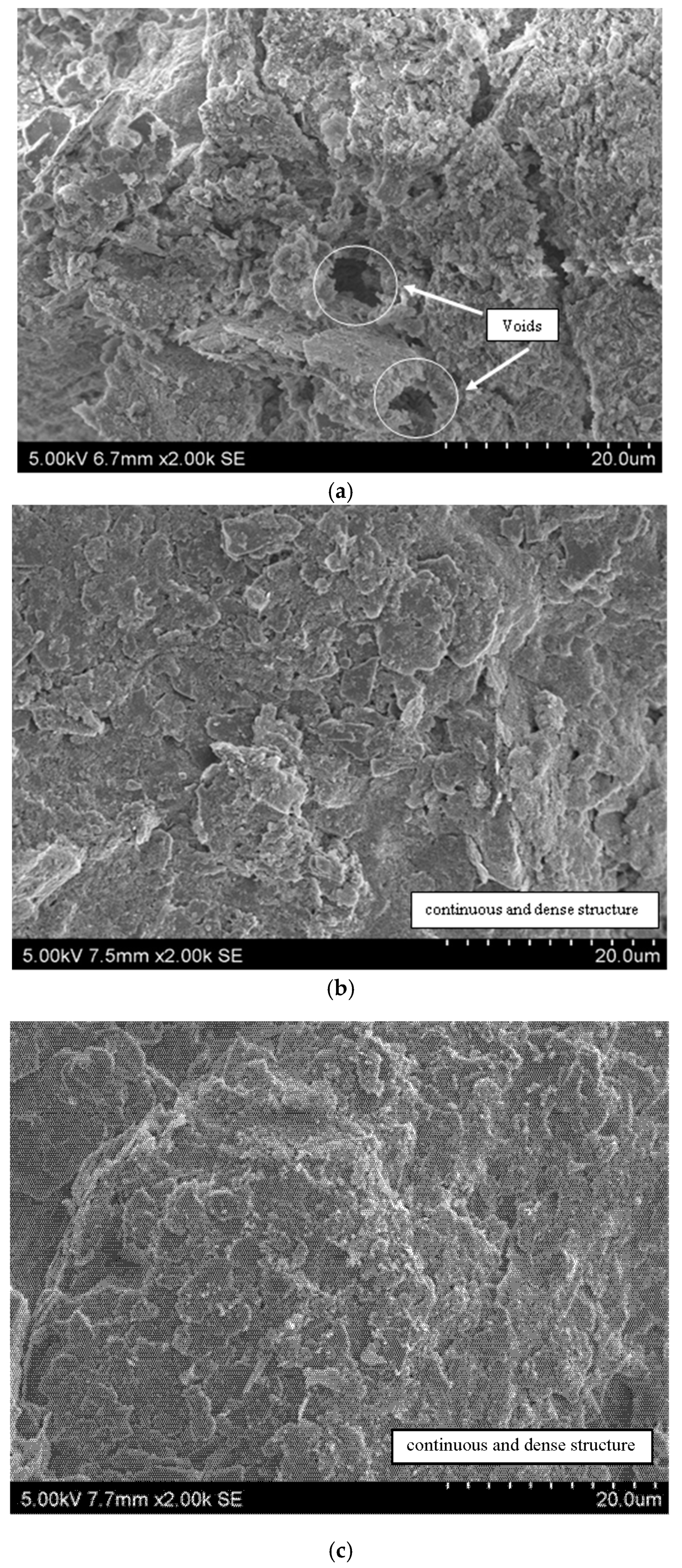
3.5.2. Variable Pressure Scanning Electron Microscope (VP-SEM)
3.5.3. Energy-Dispersive X-ray Spectroscopy (EDX)
4. Conclusions
- The Atterberg limits of both treated soil samples reduced as a result of decreased DDL thickness. The change in the Atterberg limits caused a transition in soil plasticity classification from high plasticity clay (CH) to intermediate plasticity clay (CI).
- Calcium hydroxide and magnesium hydroxide nanoparticles showed different effects on the compaction characteristics of the treated soil samples. The MDD of CS decreased with increased OMC due to the pozzolanic reactions between the soil and calcium ions. The compaction characteristics of MS portrayed a different trend whereby their MDD decreased with increased OMC as a result of reduced DDL thickness.
- The hydraulic conductivity of calcium and magnesium hydroxide nanoparticles-treated samples reduced significantly with permeation time. This is probably due to the clogging of soil pores by the cementing gels formed. These phenomena retarded the movement of water along the compacted soil matrix and resulted in lower hydraulic conductivity.
- The UCS of nanoparticles-treated samples increased with increasing curing time due to enhanced interlocking between the soil particles. It is quite likely that the flocculation of soil particles and the formation of cementing gels enhanced the bonding of the soil particles and gave rise to a denser soil structure.
- The appearance of denser and compacted soil structure as shown in the VP-SEM images of CS and MS justified the explanation of the reduced hydraulic conductivity and increased UCS of treated soil samples. In addition, the formation of new cementing gels such as calcium silicate hydrate, calcium aluminate hydrate, magnesium silicate hydrate, and magnesium aluminate hydrate, which act as soil binders, were observed from the XRD analysis of treated soil samples.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| C-A-H | calcium aluminate hydrates |
| CS | calcium hydroxide treated soil |
| C-S-H | calcium silicate hydrates |
| DDL | diffuse double layer |
| EDX | energy-dispersive x-ray spectroscopy |
| M-A-H | magnesium aluminate hydrate |
| MS | magnesium hydroxide treated soil |
| M-S-H | magnesium silicate hydrate |
| MDD | maximum dry density |
| NS | natural soil |
| OMC | optimum moisture content |
| UCS | unconfined compressive strength |
| VP-SEM | variable-pressure scanning electron microscope |
| XRD | X-ray diffraction |
References
- Tan, B.K. Country case study: Engineering geology of tropical residual soils in Malaysia. In Tropical Residual Soils Engineering; Huat, B.B.K., Gue, S.S., Ali, F.H., Eds.; Taylor & Francis Group: London, UK, 2004; pp. 237–243. [Google Scholar]
- Taha, M.R.; Kabir, M.H. Tropical residual soil as compacted soil liners. Environ. Geol. 2004, 47, 375–381. [Google Scholar] [CrossRef]
- Singh, H.; Jotisankasa, A.; Huat, B.B.K. Residual soils of southeast asia. In Handbook of Tropical Residual Soils Engineering; Huat, B.B.K., Toll, D.G., Prasad, A., Eds.; CRC Press: New York, NY, USA, 2013; pp. 491–532. [Google Scholar]
- Latifi, N.; Eisazadeh, A.; Marto, A.; Meehan, C.L. Tropical residual soil stabilization: A powder form material for increasing soil strength. Constr. Build. Mater. 2017, 147, 827–836. [Google Scholar] [CrossRef]
- Rashid, A.S.A.; Latifi, N.; Meehan, C.L.; Manahiloh, K.N. Sustainable Improvement of Tropical Residual Soil Using an Environmentally Friendly Additive. Geotech. Geol. Eng. 2017, 35, 2613–2623. [Google Scholar] [CrossRef]
- Behnood, A. Soil and clay stabilization with calcium- and non-calcium-based additives: A state-of-the-art review of challenges, approaches and techniques. Transp. Geotech. 2018, 17, 14–32. [Google Scholar] [CrossRef]
- Gu, K.; Jin, F.; Al-Tabbaa, A.; Shi, B.; Liu, C.; Gao, L. Incorporation of reactive magnesia and quicklime in sustainable binders for soil stabilisation. Eng. Geol. 2015, 195, 53–62. [Google Scholar] [CrossRef]
- Kafodya, I.; Okonta, F. Effects of natural fiber inclusions and pre-compression on the strength properties of Lime-Fly ash stabilised soil. Constr. Build. Mater. 2018, 170, 737–746. [Google Scholar] [CrossRef]
- Ayeldeen, M.; Kitazume, M. Using fiber and liquid polymer to improve the behaviour of Cement-Stabilized soft clay. Geotext. Geomembr. 2017, 45, 592–602. [Google Scholar] [CrossRef]
- Consoli, N.C.; Arcari Bassani, M.A.; Festugato, L. Effect of Fiber-Reinforcement on the strength of cemented soils. Geotext. Geomembr. 2010, 28, 344–351. [Google Scholar] [CrossRef]
- Iranpour, B.; Haddad, A. The influence of nanomaterials on collapsible soil treatment. Eng. Geol. 2016, 205, 40–53. [Google Scholar] [CrossRef]
- Emmanuel, E.; Lau, C.C.; Anggraini, V.; Pasbakhsh, P. Stabilization of a soft marine clay using halloysite nanotubes: A Multi-Scale approach. Appl. Clay Sci. 2019, 173, 65–78. [Google Scholar] [CrossRef]
- Changizi, F.; Haddad, A. Strength properties of soft clay treated with mixture of nano-SiO2 and recycled polyester fiber. J. Rock Mech. Geotech. Eng. 2015, 7, 367–378. [Google Scholar] [CrossRef]
- Coo, J.L.; So, Z.P.S.; Ng, C.W.W. Effect of nanoparticles on the shrinkage properties of clay. Eng. Geol. 2016, 213, 84–88. [Google Scholar] [CrossRef]
- Alsharef, J.M.A.; Taha, M.R.; Firoozi, A.A.; Govindasamy, P. Potential of using nanocarbons to stabilize weak soils. Appl. Environ. Soil Sci. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Naval, S.; Chandan, K.; Sharma, D. Stabilization of expansive soil using nanomaterials. In Proceedings of the International Interdisciplinary Conference on Science Technology Engineering Management Pharmacy and Humanities, Singapore, 22–23 April 2017. [Google Scholar]
- Ealias, A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 1–15. [Google Scholar]
- Anggraini, V.; Asadi, A.; Farzadnia, N.; Jahangirian, H.; Huat, B.B.K. Effects of coir fibres modified with Ca(OH)2 and Mg(OH)2 nanoparticles on mechanical properties of lime-treated marine clay. Geosynth. Int. 2016, 23, 206–218. [Google Scholar] [CrossRef]
- Shahmiri, M.; Ibrahim, N.A.; Shayesteh, F.; Asim, N.; Motallebi, N. Preparation of PVP-Coated copper oxide nanosheets as antibacterial and antifungal agents. J. Mater. Res. 2013, 28, 3109–3118. [Google Scholar] [CrossRef]
- Wu, R.; Ma, Z.; Gu, Z.; Yang, Y. Preparation and characterization of CuO nanoparticles with different morphology through a simple quick-precipitation method in DMAC–Water mixed solvent. J. Alloy. Compd. 2010, 504, 45–49. [Google Scholar] [CrossRef]
- Zhu, J.; Li, D.; Chen, H.C.; Yang, X.; Lu, L.; Wang, X. Highly dispersed CuO nanoparticles prepared by a novel Quick-Precipitation method. Mater. Lett. 2004, 58, 3324–3327. [Google Scholar] [CrossRef]
- Jahangirian, H.; Ismail, M.H.S.; Haron, M.J.; Rafiee-Moghaddam, R.; Shameli, K.; Hosseini, S.; Kalantari, K.; Khandanlou, R.; Gharibshahi, E.; Soltaninejad, S. Synthesis and characterization of zeolite/Fe3O4 nanocomposite by green quick precipitation method. Dig. J. Nanomater. Biostruct. 2013, 8, 1405–1413. [Google Scholar]
- Khandanlou, R.; Ahmad, M.; Shameli, K.; Kalantari, K. Synthesis and characterization of rice straw/Fe3O4 nanocomposites by a quick precipitation method. Molecules 2013, 18, 6597–6607. [Google Scholar] [CrossRef]
- Anggraini, V.; Asadi, A.; Farzadnia, N.; Jahangirian, H.; Huat, B.B.K. 2016b Reinforcement benefits of nanomodified coir fiber in Lime-Treated marine clay. J. Mater. Civ. Eng. 2016, 28, 1–8. [Google Scholar] [CrossRef]
- Malaysia Ministry of Natural Resources and Environment. Geological Map, Map. Scale 1: 63360; Director- General Minerals and Geoscience Department: Kuala Lumpur, Malaysia, 2010.
- BS 1377:1990. Methods of Test for Soils for Civil Engineering Purposes; British Standards Institution: London, UK, 1990. [Google Scholar]
- ASTM D5856-15. Standard Test Method for Measurement of Hydraulic Conductivity of Porous Material Using a Rigid-Wall, Compaction-Mold Permeameter; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- ASTM D2166. Standard Test Method for Unconfined Compressive Strength of Cohesive Soil; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Amadi, A.A.; Okeiyi, A. Use of quick and hydrated lime in stabilization of lateritic soil: Comparative analysis of laboratory data. Int. J. Geo-Eng. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Di Sante, M.; Fratalocchi, E.; Mazzieri, F. Effects of variation in compaction water content on geotechnical properties of Lime-Treated clayey soil. Procedia Eng. 2016, 158, 63–68. [Google Scholar] [CrossRef]
- Jha, A.K.; Sivapullaiah, P.V. Mechanism of improvement in the strength and volume change behavior of lime stabilized soil. Eng. Geol. 2015, 198, 53–64. [Google Scholar] [CrossRef]
- Daniel, D.E. (Ed.) Clay liners. In Geotechnical Practice for Waste Disposal; Chapman & Hall: London, UK, 1993; pp. 137–163. [Google Scholar]
- Rowe, R.K.; Quigley, R.M.; Brachman, R.W.I. Barrier Systems for Waste Disposal, 2nd ed.; Spon Press: New York, NY, USA, 2004. [Google Scholar]
- USEPA. Requirements for Hazardous Waste Landfill Design, Construction and Closure; USEPA: Cincinnati, OH, USA, 1989.
- Emmanuel, E.; Anggraini, V.; Raghunandan, M.E.; Asadi, A.; Bouazza, A. Improving the engineering properties of a soft marine clay with forsteritic olivine. Eur. J. Environ. Civ. Eng. 2019, 1–28. [Google Scholar] [CrossRef]
- Volkov, A.G.; Paula, S.; Deamer, D.W. Two mechanisms of permeation of small neutral molecules and hydrates ions across phospholipid bilayers. Bioelectroch. Bioenerg. 1997, 42, 153–160. [Google Scholar] [CrossRef]
- Xue, Q.; Li, J.; Liu, L. Experimental study on Anti-Seepage grout made of leachate contaminated clay in landfill. Appl. Clay Sci. 2013, 80–81, 438–442. [Google Scholar] [CrossRef]
- Tan, T.; Huat, B.B.K.; Anggraini, V.; Shukla, S.K. Improving the engineering behaviour of residual soil with fly ash and treated natural fibres in alkaline condition. Int. J. Geotech. Eng. 2019, 1–14. [Google Scholar] [CrossRef]
- Basha, E.A.; Hashim, R.; Mahmud, H.B.; Muntohar, A.S. Stabilization of residual soil with rice husk ash and cement. Constr. Build. Mater. 2005, 19, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Firoozi, A.A.; Guney Olgun, C.; Firoozi, A.A.; Baghini, M.S. Fundamentals of soil stabilization. Int. J. Geo-Eng. 2017, 8, 1–16. [Google Scholar] [CrossRef]


| Property | Natural Soil (NS) | Calcium Hydroxide Treated Soil (CS) | Magnesium Hydroxide Treated Soil (MS) |
|---|---|---|---|
| Liquid limit (%) | 50.10 | 46.00 | 41 |
| Plastic limit (%) | 21.80 | 20.35 | 19.65 |
| Plasticity index (%) | 28.30 | 23.85 | 21.35 |
| British Standard (BS) Soil Classification System | CH | CI | CI |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Treatment | 40,656.06 | 2 | 20,328.03 | 20.79 | 0.00201 |
| Error | 5866.42 | 6 | 977.74 | ||
| Total | 46,522.48 | 8 |
| Comparison between Treatment Methods | Mean Difference | Standard Error of Mean | t Value | Alpha | Significant |
|---|---|---|---|---|---|
| Calcium hydroxide treated soil (CS) vs. natural soil (NS) | 156.20 | 25.53 | 6.12 | 0.05 | Yes |
| Magnesium hydroxide treated soil (MS) vs. natural soil (NS) | 123.15 | 25.53 | 4.82 | 0.05 | Yes |
| Elements | Natural Soil, NS (%) | Calcium Hydroxide Treated Soil, CS (%) | Magnesium Hydroxide Treated Soil, MS (%) |
|---|---|---|---|
| C | 10.74 | 11.96 | 10.23 |
| O | 61.91 | 61.20 | 59.67 |
| Al | 10.34 | 9.71 | 10.50 |
| Si | 12.85 | 11.41 | 12.61 |
| Fe | 1.09 | 1.12 | 3.40 |
| Pt | 3.08 | 3.01 | 2.56 |
| Ca | - | 1.01 | - |
| Mg | - | - | 0.46 |
| Na | - | 0.25 | 0.25 |
| Cl | - | 0.32 | 0.32 |
| Al/Si | 0.80 | 0.85 | 0.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, L.L.; Namal Jayasanka Perera, S.V.A.D.; Syamsir, A.; Emmanuel, E.; Paul, S.C.; Anggraini, V. Stabilization of a Residual Soil Using Calcium and Magnesium Hydroxide Nanoparticles: A Quick Precipitation Method. Appl. Sci. 2019, 9, 4325. https://doi.org/10.3390/app9204325
Yong LL, Namal Jayasanka Perera SVAD, Syamsir A, Emmanuel E, Paul SC, Anggraini V. Stabilization of a Residual Soil Using Calcium and Magnesium Hydroxide Nanoparticles: A Quick Precipitation Method. Applied Sciences. 2019; 9(20):4325. https://doi.org/10.3390/app9204325
Chicago/Turabian StyleYong, Lee Li, S.V.A.D. Namal Jayasanka Perera, Agusril Syamsir, Endene Emmanuel, Suvash Chandra Paul, and Vivi Anggraini. 2019. "Stabilization of a Residual Soil Using Calcium and Magnesium Hydroxide Nanoparticles: A Quick Precipitation Method" Applied Sciences 9, no. 20: 4325. https://doi.org/10.3390/app9204325








