Development and Evaluation of a Prototype Scratch Apparatus for Wound Assays Adjustable to Different Forces and Substrates
Abstract
:1. Introduction
2. Experimental Section
2.1. Characterization of Manually and Automatically Performed Scratch Assays
2.2. Evaluation of the Harvesting Capabilities of the Scratch Device
3. Results and Discussion
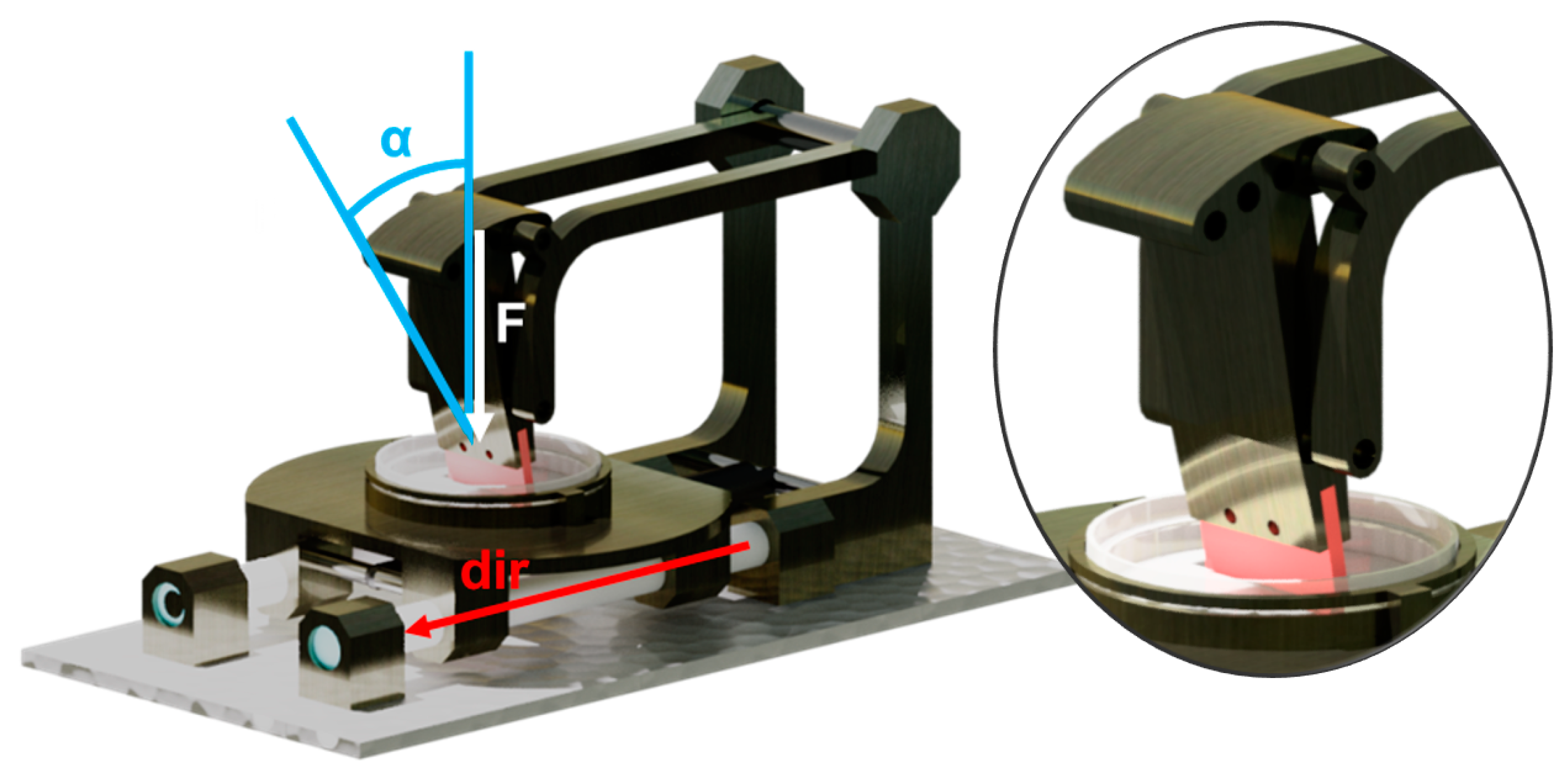
3.1. Conception and Construction of the Apparatus
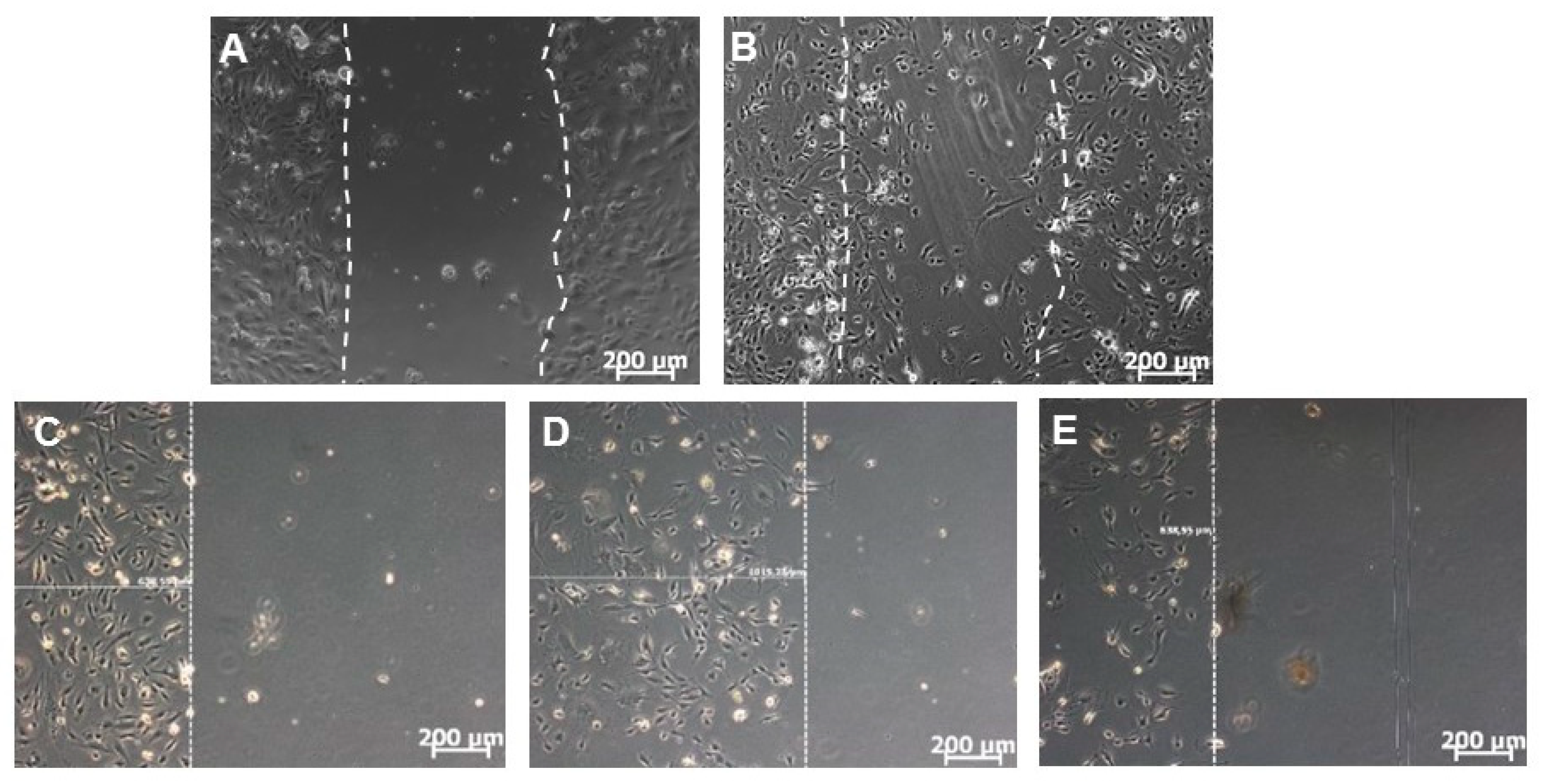
3.2. Comparison of Manually and Automatically Performed Scratching
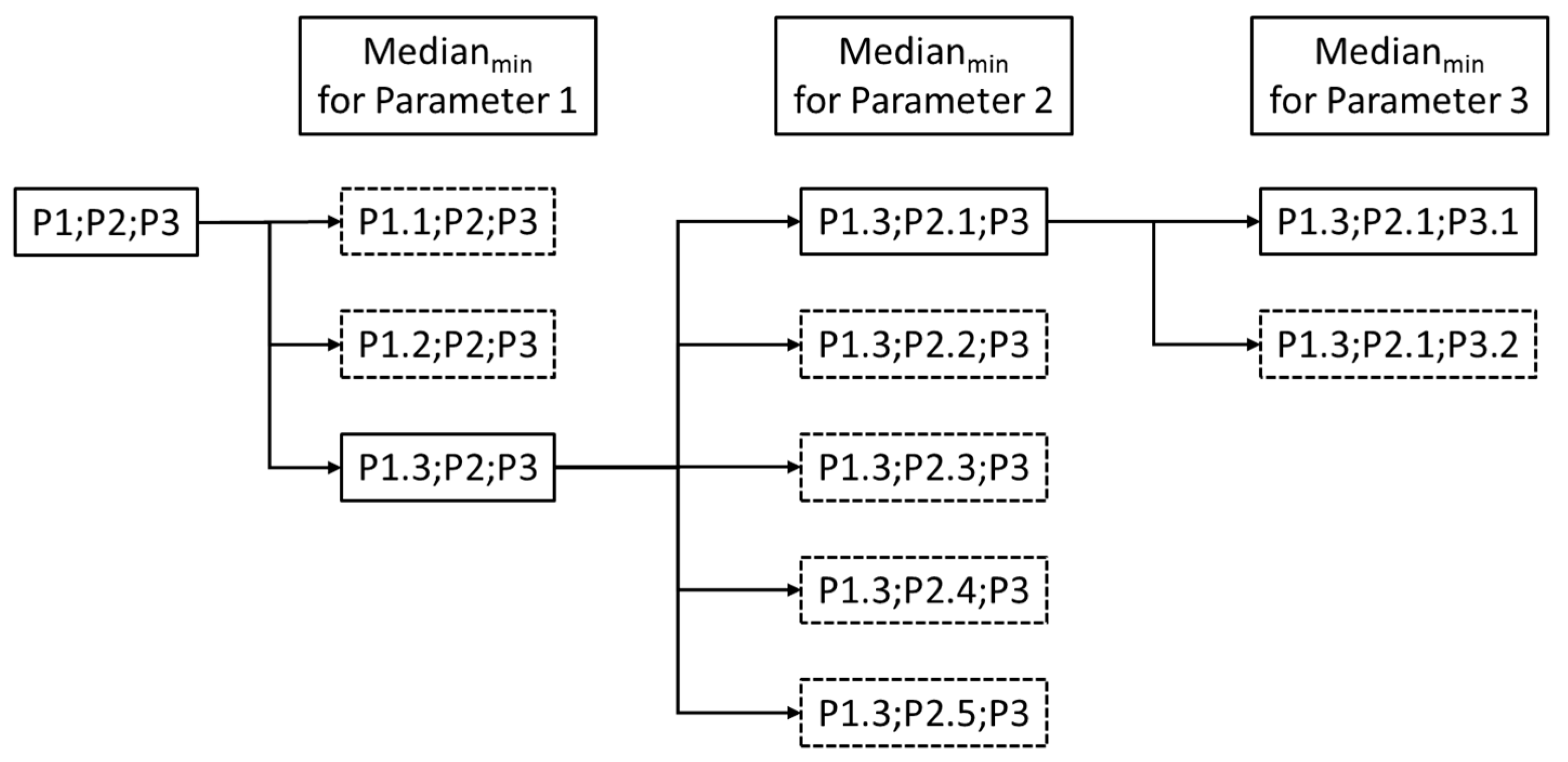
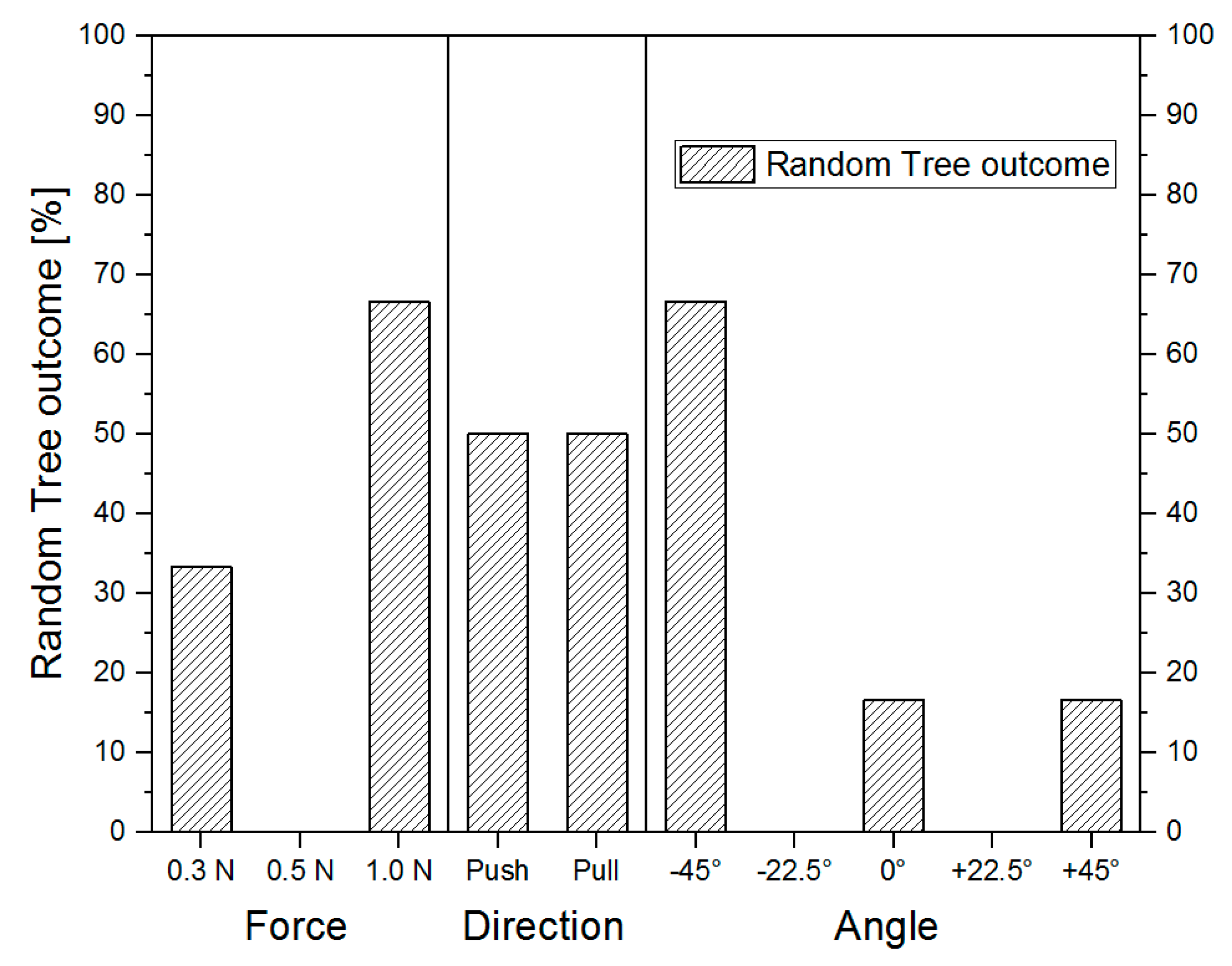
3.3. Comparison of Different Operational Settings Using the Scratch Device
3.4. Perfomance of the Automated Scratch Assays on the Harvesting Capabilities
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kanitakis, J. Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002, 12, 390–401. [Google Scholar] [PubMed]
- Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [PubMed]
- Sorg, J.M.R.H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar]
- Lampugnani, M.G. Cell Migration into a Wounded Area In Vitro. In Adhesion Protein Protocols; Humana Press: Totowa, NJ, USA, 1999; pp. 177–182. [Google Scholar]
- Rodriguez, L.G.; Wu, X.; Guan, J.-L. Wound-Healing Assay. In Cell Migration: Developmental Methods and Protocols; Guan, J.-L., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 23–29. ISBN 978-1-59259-860-1. [Google Scholar]
- Stamm, A.; Reimers, K.; Strauß, S.; Vogt, P.; Scheper, T.; Pepelanova, I. In vitro wound healing assays - State of the art. BioNanoMaterials 2016, 17, 79–87. [Google Scholar] [CrossRef]
- Pijuan, J.; Barceló, C.; Moreno, D.F.; Maiques, O.; Sisó, P.; Marti, R.M.; Macià, A.; Panosa, A. In vitro Cell Migration, Invasion, and Adhesion Assays: From Cell Imaging to Data Analysis. Front. Cell Dev. Biol. 2019, 7, 1–16. [Google Scholar] [CrossRef]
- Liang, C.; Park, A.Y.; Guan, J. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Silva Nunes, J.P.; Martins Dias, A.A. ImageJ macros for the user-friendly analysis of soft-agar and wound-healing assays. Biotechniques 2017, 62, 175–179. [Google Scholar] [CrossRef] [Green Version]
- Vargas, A.; Angeli, M.; Pastrello, C.; Mcquaid, R.; Li, H.; Jurisicova, A.; Jurisica, I. Robust quantitative scratch assay. Bioinformatics 2016, 32, 1439–1440. [Google Scholar] [CrossRef]
- Lan, R.; Geng, H.; Hwang, Y.; Mishra, P.; Skloss, W.L. A novel wounding device suitable for quantitative biochemical analysis of wound healing and regeneration of cultured epithelium. Wound Repair Regen. 2010, 18, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Fong, E.; Tzlil, S.; Tirrell, D.A. Boundary crossing in epithelial wound healing. Proc. Natl. Acad. Sci. USA 2010, 107, 19302–19307. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, D.L.; Boettiger, A.N.; Bar-Sagi, D.; Carbeck, J.D.; Shvartsman, S.Y. Role of boundary conditions in an experimental model of epithelial wound healing. Am. J. Physiol. Cell Physiol. 2006, 291, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Hettler, A.; Werner, S.; Eick, S.; Laufer, S.; Weise, F. A New In Vitro Model to Study Cellular Responses after Thermomechanical Damage in Monolayer Cultures. PLoS ONE 2013, 8, e82635. [Google Scholar] [CrossRef] [PubMed]
- Giaever, I.; Keese, C.R. Micromotion of mammalian cells measured electrically. Proc. Natl. Acad. Sci. USA 1991, 88, 7896–7900. [Google Scholar] [CrossRef] [PubMed]
- Zordan, M.D.; Mill, C.P.; Ii, D.J.R.; Leary, J.F. A High Throughput, Interactive Imaging, Bright-Field Wound Healing Assay. Cytom. Part A 2011, 79, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Uygun, B.; Banerjee, I.; Nahmias, Y.; Zhang, Q.; Berthiaume, F.; Latina, M.; Yarmush, M.L. Low power laser irradiation stimulates the proliferation of adult human retinal pigment epithelial cells in culture. Cell. Mol. Bioeng. 2009, 2, 87–103. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Invest. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef] [Green Version]
- Menger, B.; Vogt, P.M.; Allmeling, C.; Radtke, C. AmbLOXe—An Epidermal Lipoxygenase of the Mexican Axolotl in the Context of Amphibian Regeneration and Its Impact on Human Wound Closure In Vitro. Ann. Surg. 2011, 253, 410–418. [Google Scholar] [CrossRef]
- Bennett, J.; Cassidy, H.; Slattery, C.; Ryan, M.; McMorrow, T. Tacrolimus Modulates TGF-β Signaling to Induce Epithelial-Mesenchymal Transition in Human Renal Proximal Tubule Epithelial Cells. J. Clin. Med. 2016, 5, 50. [Google Scholar] [CrossRef]
- Klettner, A.; Tahmaz, N.; Dithmer, M.; Richert, E.; Roider, J. Effects of aflibercept on primary RPE cells: Toxicity, wound healing, uptake and phagocytosis. Br. J. Ophthalmol. 2014, 98, 1448–1452. [Google Scholar] [CrossRef]
- Topman, G.; Sharabani-yosef, O.; Gefen, A. Medical Engineering & Physics A standardized objective method for continuously measuring the kinematics of cultures covering a mechanically damaged site. Med. Eng. Phys. 2012, 34, 225–232. [Google Scholar]
- Baudin, B.; Bruneel, A.; Bosselut, N.; Vaubourdolle, M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat. Protoc. 2007, 2, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Molladavoodi, S.; Wulff, D.; Robichaud, M.; Gorbet, M. Corneal epithelial cells exposed to shear stress show altered cytoskeleton and migratory behaviour. PLoS ONE 2017, 12, e0178981. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Cui, Y.; Li, Z.; Jiao, Z.; Zhang, Y.; He, Y.; Chen, G.; Cheng, G.; Zhou, Q.; Wang, W.; et al. Radiation-induced miR-208a increases the proliferation and radioresistance by targeting p21 in human lung cancer cells. J. Exp. Clin. Cancer Res. 2016, 35, 7. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschläger, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat. Res. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Goetsch, K.P.; Niesler, C.U. Optimization of the scratch assay for in vitro skeletal muscle wound healing analysis. Anal. Biochem. 2011, 411, 158–160. [Google Scholar] [CrossRef]
- Büth, H.; Luigi Buttigieg, P.; Ostafe, R.; Rehders, M.; Dannenmann, S.R.; Schaschke, N.; Stark, H.J.; Boukamp, P.; Brix, K. Cathepsin B is essential for regeneration of scratch-wounded normal human epidermal keratinocytes. Eur. J. Cell Biol. 2007, 86, 747–761. [Google Scholar] [CrossRef]
- Wiegand, C.; Hipler, U.-C. Methods for the measurement of cell and tissue compatibility including tissue regeneration processes. GMS Krankenhaushygiene Interdiszip. 2008, 3, 1–9. [Google Scholar]
- Penick, K.J.; Solchaga, L.A.; Berilla, J.A.; Welter, J.F. Performance of polyoxymethylene plastic (POM) as a component of a tissue engineering bioreactor. J. Biomed. Mater. Res. Part A 2005, 75, 168–174. [Google Scholar] [CrossRef]
- Klepeis, V.E.; Weinger, I.; Kaczmarek, E.; Trinkaus-Randall, V. P2Y receptors play a critical role in epithelial cell communication and migration. J. Cell. Biochem. 2004, 93, 1115–1133. [Google Scholar] [CrossRef]
- Chmielewski, S.; Olejnik, A.; Sikorski, K.; Pelisek, J.; Błaszczyk, K.; Aoqui, C.; Nowicka, H.; Zernecke, A.; Heemann, U.; Wesoly, J.; et al. STAT1-dependent signal integration between IFNγ and TLR4 in vascular cells reflect pro-atherogenic responses in human atherosclerosis. PLoS ONE 2014, 9, 1–26. [Google Scholar] [CrossRef]
- Horckmans, M.; Robaye, B.; Léon-Gómez, E.; Lantz, N.; Unger, P.; Dol-Gleizes, F.; Clouet, S.; Cammarata, D.; Schaeffer, P.; Savi, P.; et al. P2Y4 nucleotide receptor: A novel actor in post-natal cardiac development. Angiogenesis 2012, 15, 349–360. [Google Scholar] [CrossRef] [PubMed]
- McClendon, J.; Jansing, N.L.; Redente, E.F.; Gandjeva, A.; Ito, Y.; Colgan, S.P.; Ahmad, A.; Riches, D.W.H.; Chapman, H.A.; Mason, R.J.; et al. Hypoxia-Inducible Factor 1α Signaling Promotes Repair of the Alveolar Epithelium after Acute Lung Injury. Am. J. Pathol. 2017, 187, 1772–1786. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Yasuma, T.; Mizutani, T.; Fowler, B.J.; Tarallo, V.; Yasuma, R.; Kim, Y.; Bastos-Carvalho, A.; Kerur, N.; Gelfand, B.D.; et al. IL-18 is not therapeutic for neovascular age-related macular degeneration. Nat. Med. 2014, 20, 1372–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaebisch, C.; Schipper, D.; Babczyk, P.; Tobiasch, E. The role of purinergic receptors in stem cell differentiation. Comput. Struct. Biotechnol. J. 2015, 13, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Babczyk, P.; Conzendorf, C.; Klose, J.; Schulze, M.; Harre, K.; Tobiasch, E. Stem Cells on Biomaterials for Synthetic Grafts to Promote Vascular Healing. J. Clin. Med. 2014, 3, 39–87. [Google Scholar] [CrossRef]
- Zippel, N.; Limbach, C.A.; Ratajski, N.; Urban, C.; Luparello, C.; Pansky, A.; Kassack, M.U.; Tobiasch, E. Purinergic Receptors Influence the Differentiation of Human Mesenchymal Stem Cells. Stem Cells Dev. 2011, 21, 884–900. [Google Scholar] [CrossRef]
- Hielscher, D.; Kaebisch, C.; Braun, B.J.V.; Gray, K.; Tobiasch, E. Stem Cell Sources and Graft Material for Vascular Tissue Engineering. Stem Cell Rev. Reports 2018, 14, 642–667. [Google Scholar] [CrossRef]
- Zhang, Y.; Khan, D.; Delling, J.; Tobiasch, E. Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells. Sci. World J. 2012, 2012, 793823. [Google Scholar] [CrossRef]
- Seifert, A.; Werheid, D.F.; Knapp, S.M.; Tobiasch, E. Role of Hox genes in stem cell differentiation. World J. Stem Cells 2015, 7, 583. [Google Scholar] [CrossRef]
- Tobiasch, E. Differentiation Potential of Adult Human Mesenchymal Stem Cells. In Stem Cell Engineering; Springer: Berlin/Heidelberg, Germany, 2011; pp. 61–77. [Google Scholar]
- Setiawan, M.; Tan, X.-W.; Goh, T.-W.; Hin-Fai Yam, G.; Mehta, J.S. Inhibiting glycogen synthase kinase-3 and transforming growth factor-β signaling to promote epithelial transition of human adipose mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2017, 490, 1381–1388. [Google Scholar] [CrossRef]
- Ramenzoni, L.; Weber, F.; Attin, T.; Schmidlin, P. Cerium Chloride Application Promotes Wound Healing and Cell Proliferation in Human Foreskin Fibroblasts. Materials (Basel) 2017, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Salem, M.; Semlali, A.; Leung, K.P.; Rouabhia, M. Antimicrobial peptide KSL-W promotes gingival fibroblast healing properties in vitro. Peptides 2017, 93, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Scheiblich, H.; Roloff, F.; Singh, V.; Stangel, M.; Stern, M.; Bicker, G. Nitric oxide/cyclic GMP signaling regulates motility of a microglial cell line and primary microglia in vitro. Brain Res. 2014, 1564, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Mavi, M.F.; Ji, J.Y. Endothelial wound recovery is influenced by treatment with shear stress, wound direction, and substrate. Cell. Mol. Bioeng. 2013, 6, 310–325. [Google Scholar] [CrossRef]
- Lin, X.; Helmke, B.P. Cell structure controls endothelial cell migration under fluid shear stress. Cell. Mol. Bioeng. 2009, 2, 231–243. [Google Scholar] [CrossRef]
- Kang, Z.; Zhu, H.; Jiang, W.; Zhang, S. Protocatechuic acid induces angiogenesis through PI3K-Akt-eNOS-VEGF signalling pathway. Basic Clin. Pharmacol. Toxicol. 2013, 113, 221–227. [Google Scholar] [CrossRef]
- De Jong, E.K.; Breukink, M.B.; Schellevis, R.L.; Bakker, B.; Mohr, J.K.; Fauser, S.; Keunen, J.E.E.; Hoyng, C.B.; Den Hollander, A.I.; Boon, C.J.F. Chronic central serous chorioretinopathy is associated with genetic variants implicated in age-related macular degeneration. Ophthalmology 2015, 122, 562–570. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Li, J.; Wang, D.; Li, Q. Effect of microRNA-135a on Cell Proliferation, Migration, Invasion, Apoptosis and Tumor Angiogenesis Through the IGF-1/PI3K/Akt Signaling Pathway in Non-Small Cell Lung Cancer. Cell. Physiol. Biochem. 2017, 42, 1431–1446. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Sung, P.-H.; Chung, S.-Y.; Hsu, S.-L.; Chung, W.-J.; Sheu, J.-J.; Hsueh, S.-K.; Chen, K.-H.; Wu, R.-W.; Yip, H.-K. Hyperbaric Oxygen Therapy Enhanced Circulating Levels of Endothelial Progenitor Cells and Angiogenesis Biomarkers, Blood Flow, in Ischemic Areas in Patients with Peripheral Arterial Occlusive Disease. J. Clin. Med. 2018, 7, 548. [Google Scholar] [CrossRef]
- Stoeckius, M.; Erat, A.; Fujikawa, T.; Hiromura, M.; Koulova, A.; Otterbein, L.; Bianchi, C.; Tobiasch, E.; Dagon, Y.; Sellke, F.W.; et al. Essential roles of Raf/extracellular signal-regulated kinase/mitogen- activated protein kinase pathway, YY1, and Ca2+ influx in growth arrest of human vascular smooth muscle cells by bilirubin. J. Biol. Chem. 2012, 287, 15418–15426. [Google Scholar] [CrossRef]
- Liebert, M.A.; Soares, M.P.; Usheva, A.; Brouard, S.; Berberat, P.O.; Gunther, L.; Tobiasch, E.; Bach, F.H. Heme Oxygenase-1-Derived Carbon Monoxide. J. Biol. Chem. 2002, 277, 17950–17961. [Google Scholar]
- Brouard, S.; Berberat, P.O.; Tobiasch, E.; Seldon, M.P.; Bach, F.H.; Soares, M.P. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-κB to protect endothelial cells from tumor necrosis factor-α-mediated apoptosis. J. Biol. Chem. 2002, 277, 17950–17961. [Google Scholar] [CrossRef] [PubMed]
- Brouard, S.; Otterbein, L.E.; Anrather, J.; Tobiasch, E.; Bach, F.H.; Choi, A.M.; Soares, M.P. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis [In Process Citation]. J. Exp. Med. 2000, 192, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.I.; Cruz, N.D.; Lujan, O.R.; Propper, C.R.; Kellar, R.S. In Vitro Scratch Assay to Demonstrate Effects of Arsenic on Skin Cell Migration. J. Vis. Exp. 2019, 144, e58838. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.J.; Brown, J.M.; John, M. Introduction to Biomedical Equipment Technology; Prentice Hall: Upper Saddle River, NJ, USA, 2001; ISBN 0130104922. [Google Scholar]
- Yue, P.Y.K.; Leung, E.P.Y.; Mak, N.K.; Wong, R.N.S. A Simplified Method for Quantifying Cell Migration/Wound Healing in 96-Well Plates. J. Biomol. Screen. 2010, 15, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonkman, J.E.N.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing assay using live-cell microscopy. Cell Adhes. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breiman, L. Random Forests. UC Berkeley 1999, TR567, 1–35. [Google Scholar]
- Obulkasim, A.; Meijer, G.A.; van de Wiel, M.A. Stepwise classification of cancer samples using clinical and molecular data. BMC Bioinformatics 2011, 12, 422–434. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grimmig, R.; Babczyk, P.; Gillemot, P.; Schmitz, K.-P.; Schulze, M.; Tobiasch, E. Development and Evaluation of a Prototype Scratch Apparatus for Wound Assays Adjustable to Different Forces and Substrates. Appl. Sci. 2019, 9, 4414. https://doi.org/10.3390/app9204414
Grimmig R, Babczyk P, Gillemot P, Schmitz K-P, Schulze M, Tobiasch E. Development and Evaluation of a Prototype Scratch Apparatus for Wound Assays Adjustable to Different Forces and Substrates. Applied Sciences. 2019; 9(20):4414. https://doi.org/10.3390/app9204414
Chicago/Turabian StyleGrimmig, Roman, Patrick Babczyk, Philipp Gillemot, Klaus-Peter Schmitz, Margit Schulze, and Edda Tobiasch. 2019. "Development and Evaluation of a Prototype Scratch Apparatus for Wound Assays Adjustable to Different Forces and Substrates" Applied Sciences 9, no. 20: 4414. https://doi.org/10.3390/app9204414
APA StyleGrimmig, R., Babczyk, P., Gillemot, P., Schmitz, K.-P., Schulze, M., & Tobiasch, E. (2019). Development and Evaluation of a Prototype Scratch Apparatus for Wound Assays Adjustable to Different Forces and Substrates. Applied Sciences, 9(20), 4414. https://doi.org/10.3390/app9204414






