Amelogenin Exon 5 Peptide Promotes Cell Proliferation and Osteogenic Differentiation in Human Dental Pulp Stem Cells
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Proliferation Assay
2.3. Alkaline Phosphatase (ALP) Activity and Osteocalcin (OCN)
2.4. Extracellular Matrix Mineralization
2.5. Gene Expression of Osteogenic Differentiation
2.6. Western Blot Analyses
2.7. Statistical Analysis
3. Results
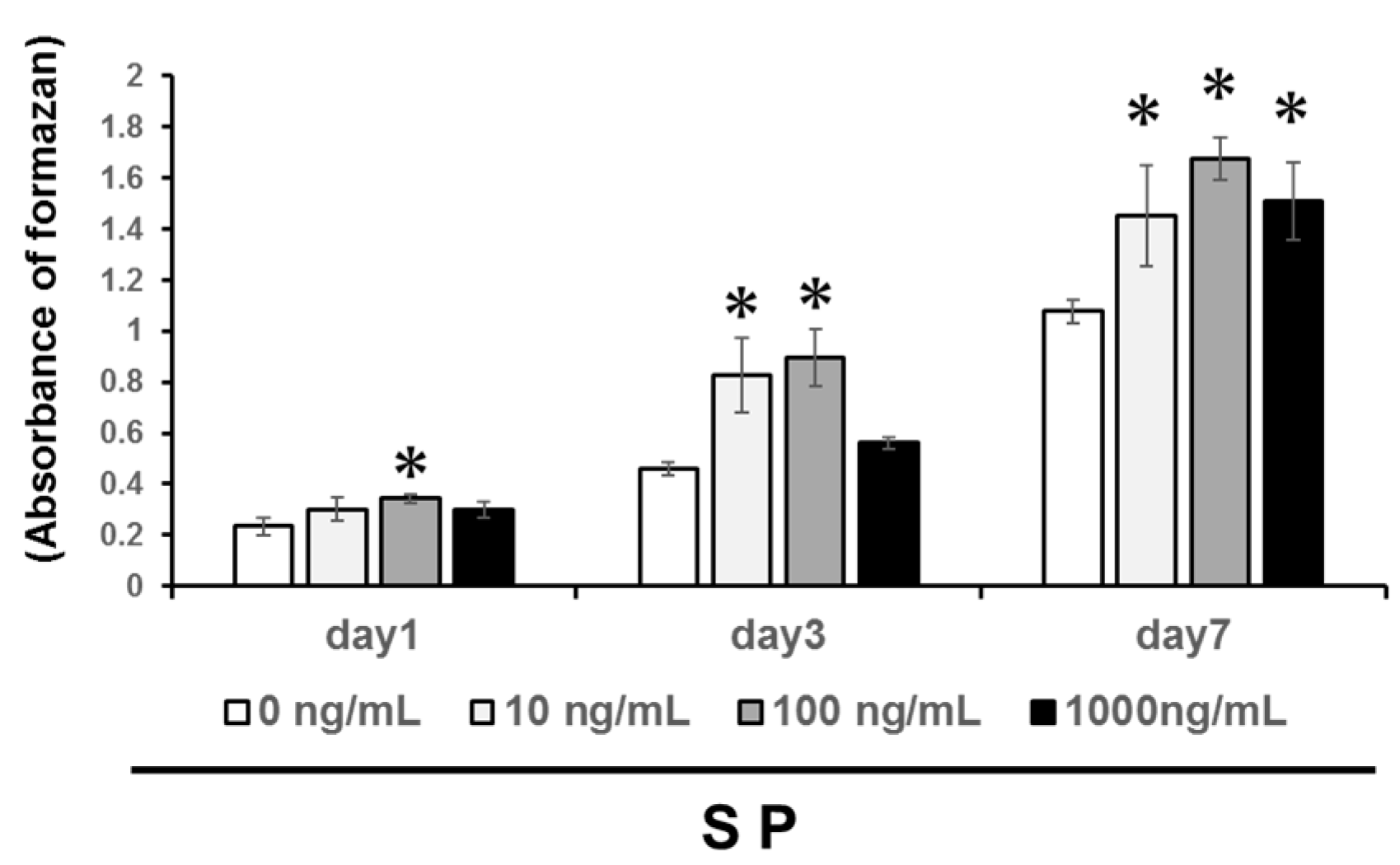
3.1. Cell Proliferation
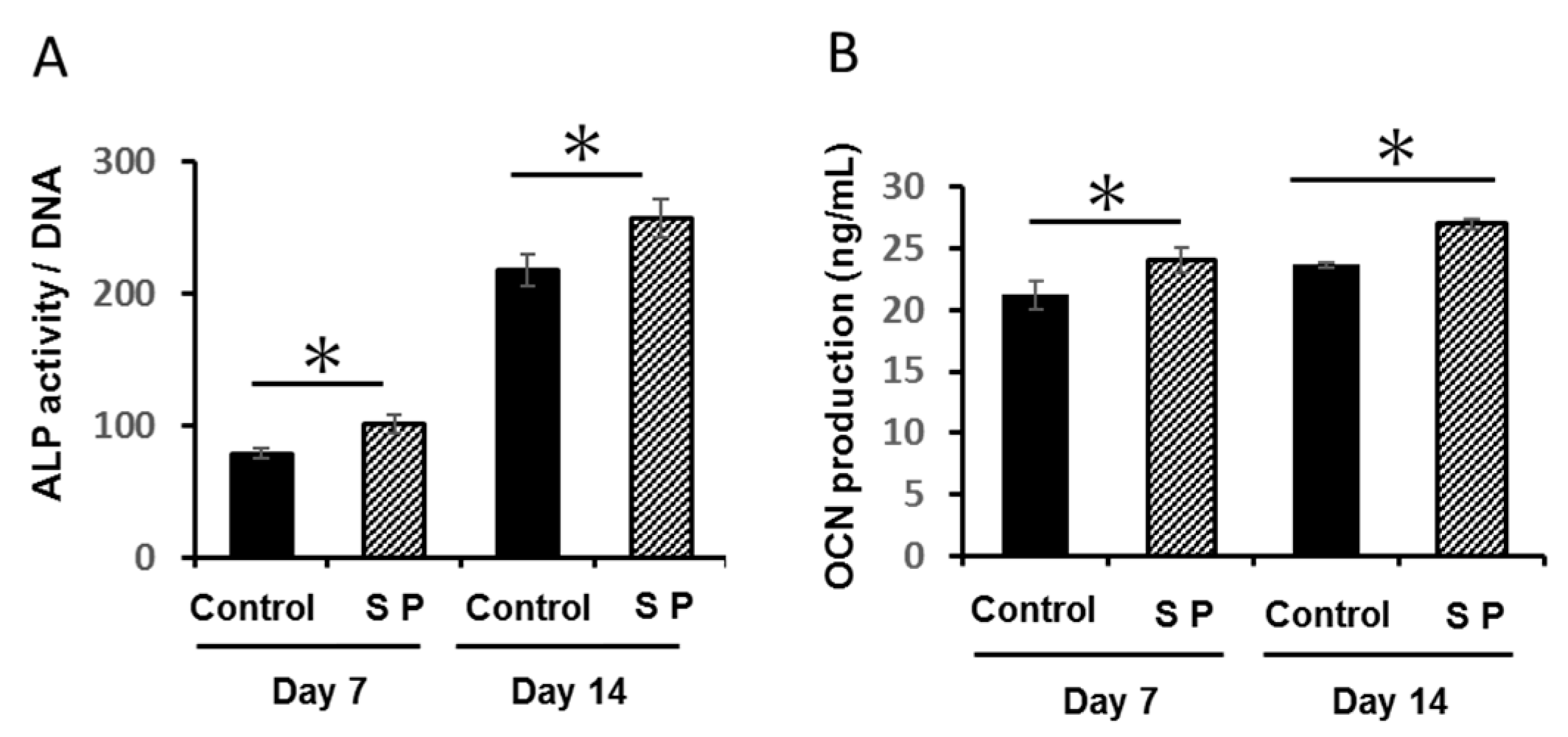
3.2. ALP Activity and OCN Production
3.3. Osteogenic Gene Expression
3.4. Extracellular Matrix Mineralization
3.5. Phosphorylation of the MAPK Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lyngstadaas, S.P.; Risnes, S.; Nordbo, H.; Flones, A.G. Amelogenin gene similarity in vertebrates: DNA sequences encoding amelogenin seem to be conserved during evolution. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 1990, 160, 469–472. [Google Scholar] [CrossRef]
- Fincham, A.G.; Belcourt, A.B.; Termine, J.D.; Butler, W.T.; Cothran, W.C. Amelogenins. Sequence homologies in enamel-matrix proteins from three mammalian species. Biochem. J. 1983, 211, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, K.; Alvares, K.; George, A.; Veis, A. Two related low molecular mass polypeptide isoforms of amelogenin have distinct activities in mouse tooth germ differentiation in vitro. J. Bone Miner. Res. 2005, 20, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.D.; Olsen, I.; Knowles, J.C.; Donos, N. Differential effect of amelogenin peptides on osteogenic differentiation in vitro: Identification of possible new drugs for bone repair and regeneration. Tissue Eng. A 2012, 18, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Hammarström, L.; Matsumoto, K.; Lyngstadaas, S.P. The induction of reparative dentine by enamel proteins. Int. Endod. J. 2002, 35, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Slaby, I.; Matsumoto, K.; Ritchie, H.H.; Lyngstadaas, S.P. Immunohistochemical characterization of rapid dentin formation induced by enamel matrix derivative. Calcif. Tissue Int. 2004, 75, 243–252. [Google Scholar] [CrossRef]
- Hammarström, L. Enamel matrix, cementum development and regeneration. J. Clin. Periodontol. 1997, 24, 658–668. [Google Scholar] [CrossRef]
- Hammarström, L.; Heijl, L.; Gestrelius, S. Periodontal regeneration in a buccal dehiscence model in monkeys after application of enamel matrix proteins. J. Clin. Periodontol. 1997, 24, 669–677. [Google Scholar] [CrossRef]
- Kim, N.H.; Tominaga, K.; Tanaka, A. Analysis of eosinophilic round bodies formed after injection of enamel matrix derivative into the backs of rats. J. Periodontol. 2005, 76, 1934–1941. [Google Scholar] [CrossRef]
- Hida, T.; Tominaga, K.; Tanaka, A. Tissue Reaction to synthetic oligopeptide derived from enamel matrix derivative in rats. Oral Sci. Int. 2010, 7, 26–33. [Google Scholar] [CrossRef]
- Noguchi, M.; Tominaga, K.; Tanaka, A.; Ueda, M. Hard tissue formation induced by synthetic oligopeptide derived from an enamel matrix derivative. Oral. Med. Pathol. 2012, 16, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, Y.; Takahashi, S.; Tominaga, K.; Komasa, S.; Shida, M.; Hayashi, H.; Tanaka, A.; Umeda, M. Effect of oligopeptide derived from enamel matrix derivatives on the proliferation, adhesion migration of human periodontal ligament cells. J. Conserv. Dent. 2012, 55, 227–235. (In Japanese) [Google Scholar]
- Katayama, N.; Kato, H.; Taguchi, Y.; Tanaka, A.; Umeda, M. The effects of synthetic oligopeptide derived from enamel matrix derivative on cell proliferation and osteoblastic differentiation of human mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 14026–14043. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Katayama, N.; Taguchi, Y.; Tominaga, K.; Umeda, M.; Tanaka, A. A synthetic oligopeptide derived from enamel matrix derivative promotes the differentiation of human periodontal ligament stem cells into osteoblast-like cells with increased mineralization. J. Periodontol. 2013, 84, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura, S.; Ito, K.; Sugito, T.; Yoshimi, R.; Nagasaka, T.; Ueda, M. A feasibility of useful cell-based therapy by bone regeneration with deciduous tooth stem cells, dental pulp stem cells, or bone-marrow-derived mesenchymal stem cells for clinical study using tissue engineering technology. Tissue Eng. Part A 2010, 16, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Saghiri, M.A.; Asatourian, A.; Sorenson, C.M.; Sheibani, N. Role of angiogenesis in endodontics: Contributions of stem cells and proangiogenic and antiangiogenic factors to dental pulp regeneration. J. Endod. 2015, 41, 797–803. [Google Scholar] [CrossRef]
- Moonesi Rad, R.; Atila, D.; Akgün, E.E.; Evis, Z.; Keskin, D.; Tezcaner, A. Evaluation of human dental pulp stem cells behavior on a novel nanobiocomposite scaffold prepared for regenerative endodontics. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 928–948. [Google Scholar] [CrossRef]
- Noda, S.; Kawashima, N.; Yamamoto MHashimoto, K.; Nara, K.; Sekiya, I.; Okiji, T. Effect of cell culture density on dental pulp-derived mesenchymal stem cells with reference to osteogenic differentiation. Sci. Rep. 2019, 9, 5430. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Ge, L. Effects of the enamel matrix derivative on the proliferation and odontogenic differentiation of human dental pulp cells. J. Dent. 2014, 42, 53–59. [Google Scholar] [CrossRef]
- Taguchi, Y.; Yasui, N.; Takahashi, S.; Tominaga, K.; Kato, H.; Komasa, S.; Shida, M.; Hayashi, H.; Tanaka, A.; Umeda, M. Hard tissue formation by human periodontal ligament fibroblast Cells treated with an emdogain-derived oligopeptide in vitro. J. Hard Tissue Biol. 2012, 21, 375–384. [Google Scholar] [CrossRef]
- Weinreb, M.; Shinar, D.; Rodan, G.A. Different pattern of alkaline phosphatase, osteopontin, and osteocalcin expression in developing rat bone visualized by in situ hybridization. J. Bone Miner. Res. 1990, 5, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Nomura, S.; Yamaguchi, A.; Suda, T.; Yoshiki, S. In situ hybridization of bone matrix proteins in undecalcified adult rat bone sections. J. Histochem. Cytochem. 1992, 40, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, R.; Takagi, M.; Igarashi, M.; Ito, K. Gene expression and immunohistochemical localization of osteonectin in association with early bone formation in the developing mandible. Histochem. J. 2002, 34, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Aubin, J.E.; Liu, F.; Malaval, L.; Gupta, A.K. Osteoblast and chondroblast differentiation. Bone 1995, 17, 77S–83S. [Google Scholar] [CrossRef]
- Ohyama, M.; Suzuki, N.; Yamaguchi, Y.; Maeno, M.; Otsuka, K.; Ito, K. Effect of enamel matrix derivative on the differentiation of C2C12 cells. J. Periodontol. 2002, 73, 543–550. [Google Scholar] [CrossRef]
- Wiesmann, H.P.; Meyer, U.; Plate, U.; Höhling, H.J. Aspects of collagen mineralization in hard tissue formation. Int. Rev. Cytol. 2005, 242, 121–156. [Google Scholar]
- Karanxha, L.; Park, S.J.; Son, W.J.; Nör, J.E.; Min, K.S. Combined effects of simvastatin and enamel matrix derivative on odontoblastic differentiation of human dental pulp cells. J. Endod. 2013, 39, 76–82. [Google Scholar] [CrossRef]
- Tanimoto, K.; Huang, Y.C.; Tanne, Y.; Kunimatsu, R.; Michida, M.; Yoshioka, M.; Ozaki, N.; Sasamoto, T.; Yoshimi, Y.; Kato, Y.; et al. Amelogenin Enhances the Osteogenic Differentiation of Mesenchymal Stem Cells Derived from Bone Marrow. Cells Tissues Organs. 2012, 196, 411–419. [Google Scholar] [CrossRef]
- Wang, W.; Yi, X.; Ren, Y.; Xie, Q. Effects of adenosine triphosphate on proliferation and odontoblastic differentiation of human dental pulp cells. J. Endod. 2016, 42, 1483–1489. [Google Scholar] [CrossRef]
- Junttila, M.R.; Li, S.P.; Westermarck, J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008, 22, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Pullikuth, A.K.; Catling, A.D. Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics: A perspective. Cell Signal. 2007, 19, 1621–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, N.; Horikawa, M.; Watanabe, M.; Kitagawa, S.; Kudo, Y.; Takata, T. Possible involvement of extracellular signal-regulated kinases 1/2 in mitogenic response of periodontal ligament cells to enamel matrix derivative. Eur. J. Oral Sci. 2002, 110, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Taguchi, Y.; Imai, K.; Ruan, Y.; Tsai, Y.W.; Chen, Y.C.; Shida, M.; Taguchi, R.; Tominaga, K.; Umeda, M. The Enhancing Effects of Amelogenin Exon 5-Encoded Peptide from Enamel Matrix Derivative on Odontoblast-Like KN-3 Cells. Appl. Sci. 2018, 8, 1890. [Google Scholar] [CrossRef]
- Ando, K.; Kunimatsu, R.; Awada, T.; Yoshimi, Y.; Tsuka, Y.; Sumi, K.; Horie, K.; Abe, T.; Nakajima, K.; Tanimoto, K. Effects of Human Full-length Amelogenin and C-terminal Amelogenin Peptide on the Proliferation of Human Mesenchymal Stem Cells Derived from Adipose Tissue. Curr. Pharm. Des. 2018, 24, 2993–3001. [Google Scholar] [CrossRef]
- Hakki, S.S.; Bozkurt, S.B.; Türkay, E.; Dard, M.; Purali, N.; Götz, W. Recombinant amelogenin regulates the bioactivity of mouse cementoblasts in vitro. Int. J. Oral Sci. 2018, 10, 15. [Google Scholar] [CrossRef]
- Huang, Y.C.; Tanimoto, K.; Tanne, Y.; Kamiya, T.; Kunimatsu, R.; Michida, M.; Yoshioka, M.; Yoshimi, Y.; Kato, Y.; Tanne, K. Effects of human full-length amelogenin on the proliferation of human mesenchymal stem cells derived from bone marrow. Cell Tissue Res. 2010, 342, 205–212. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Tanimoto, K.; Tanne, Y.; Kamiya, T.; Ohkuma, S.; Huang, Y.C.; Yoshimi, Y.; Miyauchi, M.; Takata, T.; Tanne, K. Amelogenin enhances the proliferation of cementoblast lineage cells. J. Periodontol. 2011, 82, 1632–1638. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, H.; Taguchi, Y.; Yamawaki, I.; Ruan, Y.; Wu, Q.; Nakano, Y.; Tsumori, N.; Nakata, T.; Noguchi, M.; Umeda, M. Amelogenin Exon 5 Peptide Promotes Cell Proliferation and Osteogenic Differentiation in Human Dental Pulp Stem Cells. Appl. Sci. 2019, 9, 4425. https://doi.org/10.3390/app9204425
Kato H, Taguchi Y, Yamawaki I, Ruan Y, Wu Q, Nakano Y, Tsumori N, Nakata T, Noguchi M, Umeda M. Amelogenin Exon 5 Peptide Promotes Cell Proliferation and Osteogenic Differentiation in Human Dental Pulp Stem Cells. Applied Sciences. 2019; 9(20):4425. https://doi.org/10.3390/app9204425
Chicago/Turabian StyleKato, Hirohito, Yoichiro Taguchi, Isao Yamawaki, Yaru Ruan, Qingchao Wu, Yuji Nakano, Norimasa Tsumori, Takaya Nakata, Masahiro Noguchi, and Makoto Umeda. 2019. "Amelogenin Exon 5 Peptide Promotes Cell Proliferation and Osteogenic Differentiation in Human Dental Pulp Stem Cells" Applied Sciences 9, no. 20: 4425. https://doi.org/10.3390/app9204425



