Assessment of an MnCe-GAC Treatment Process for Tetramethylammonium-Contaminated Wastewater from Optoelectronic Industries
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characteristics of Wastewater Composition from Photoelectric and Semiconductor Factories
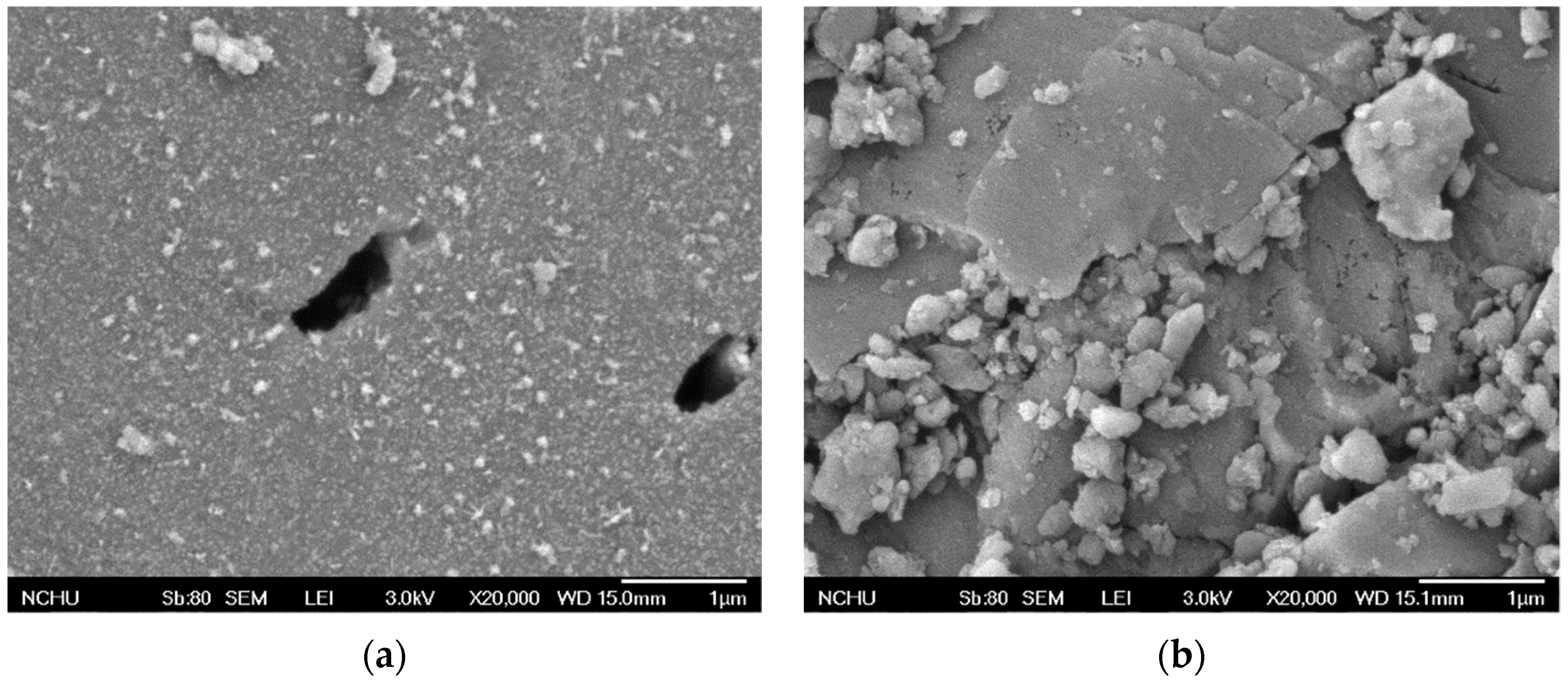
3.2. Characteristics of MnCe-GAC
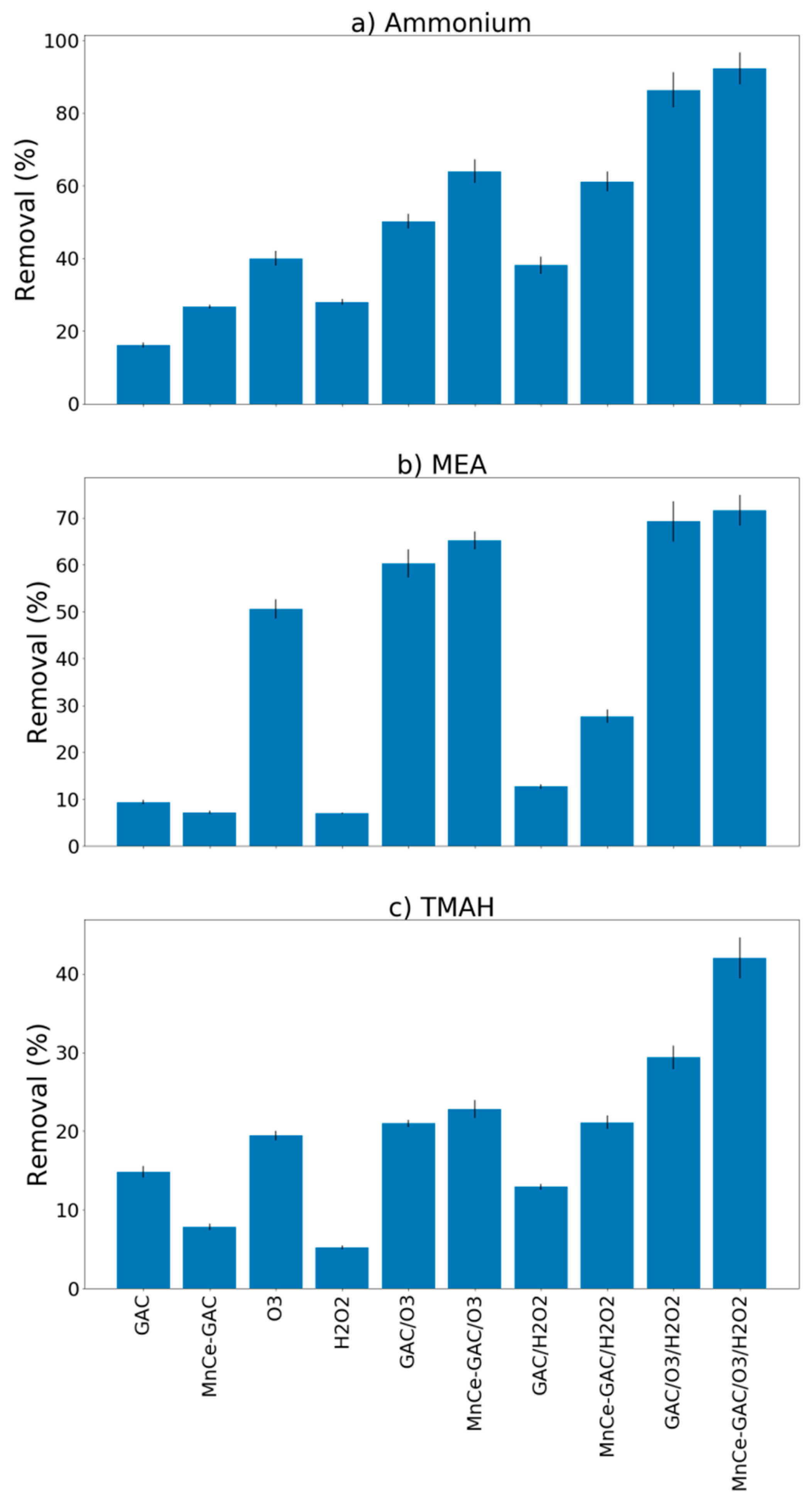
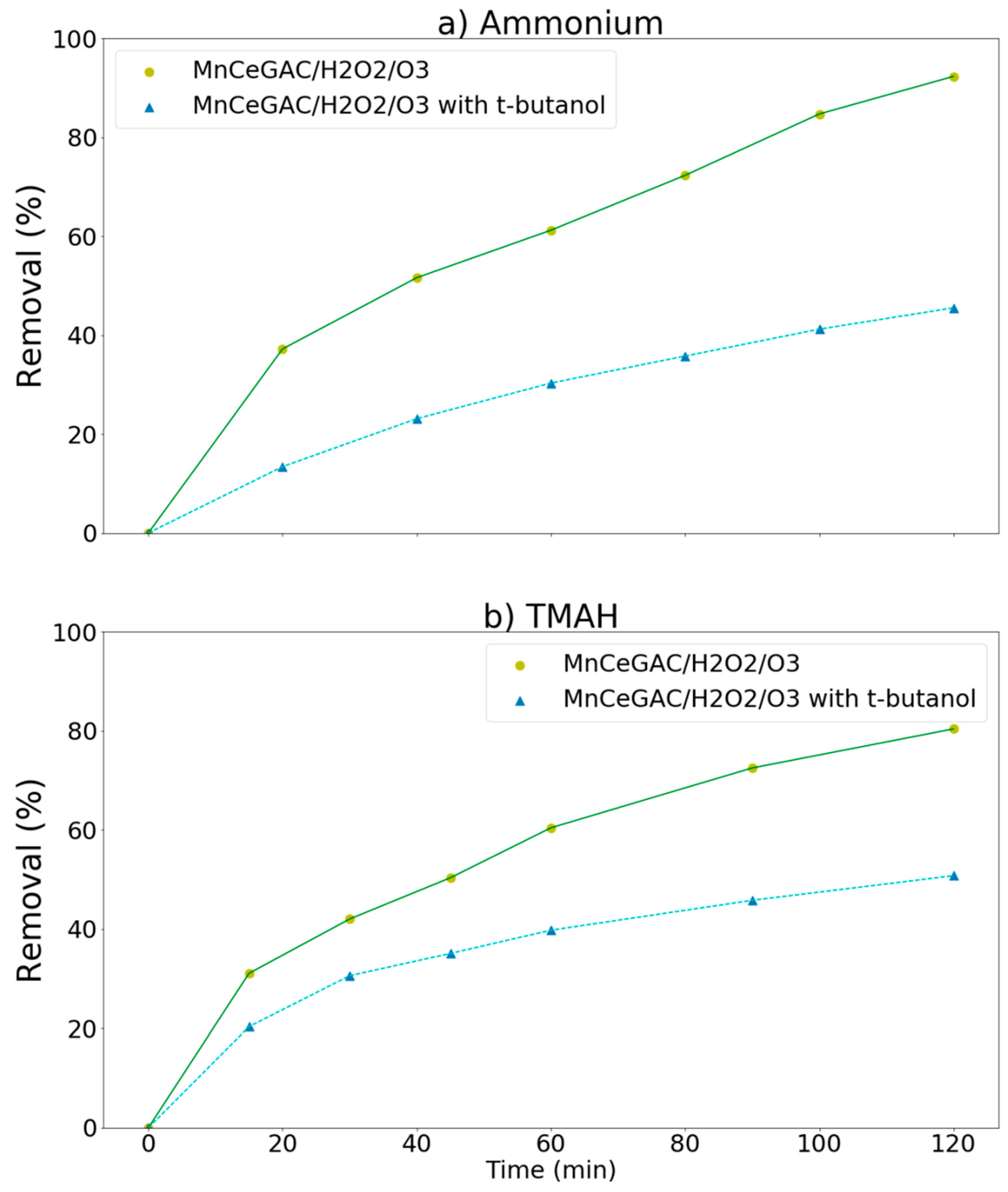
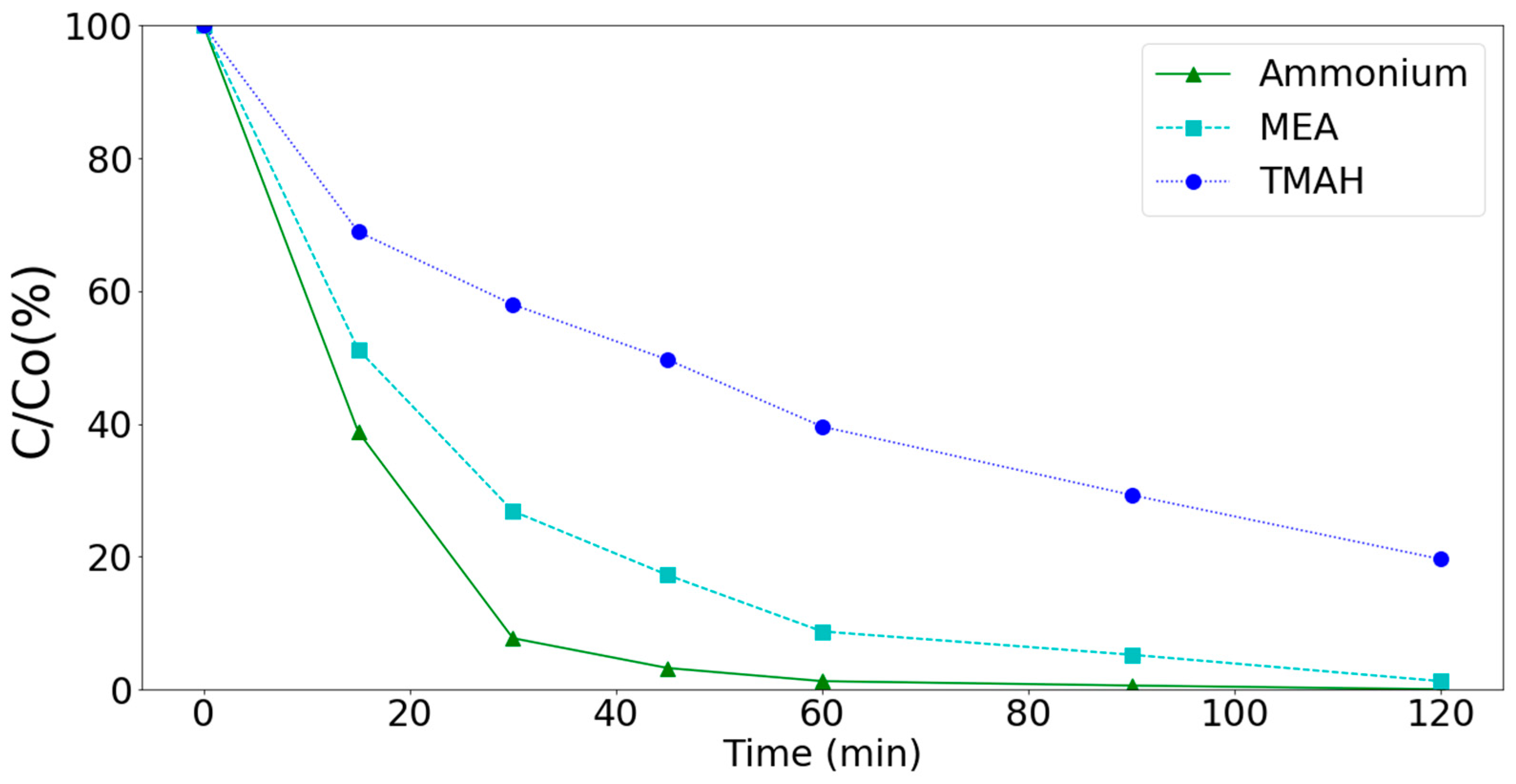
3.3. Removal of Ammonium, MEA, and TMAH by Various Processes (Synthetic Wastewater)
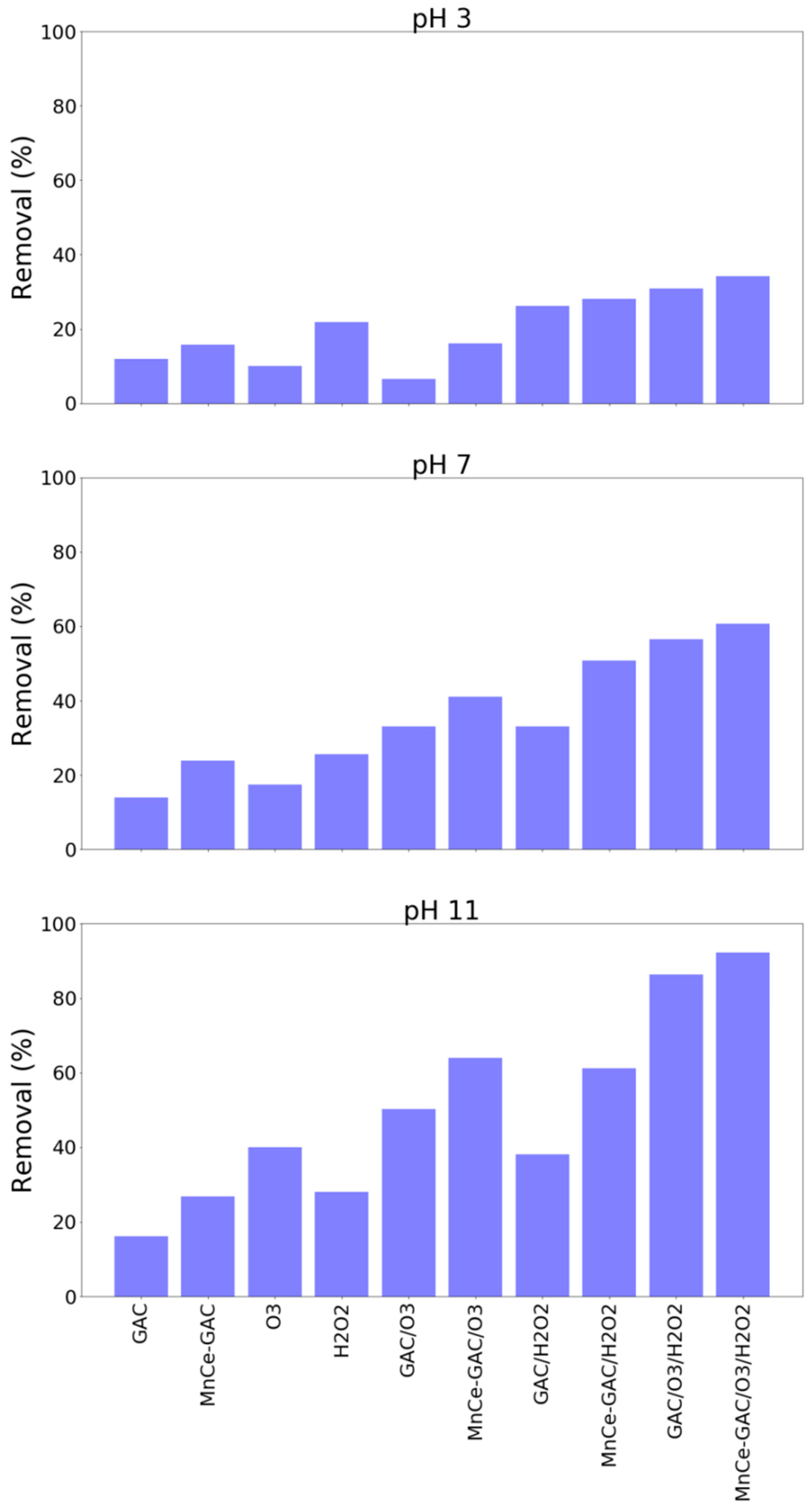
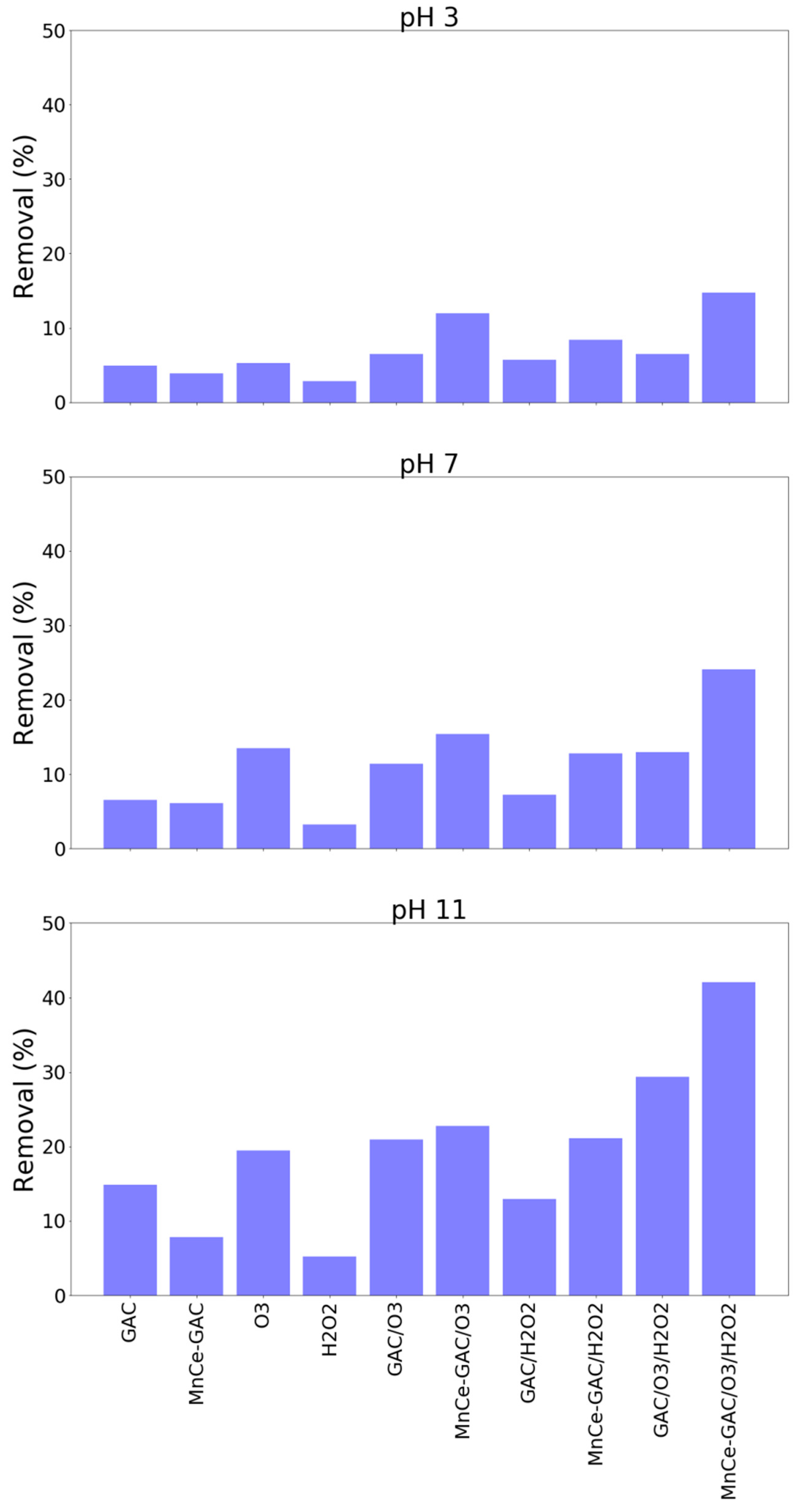
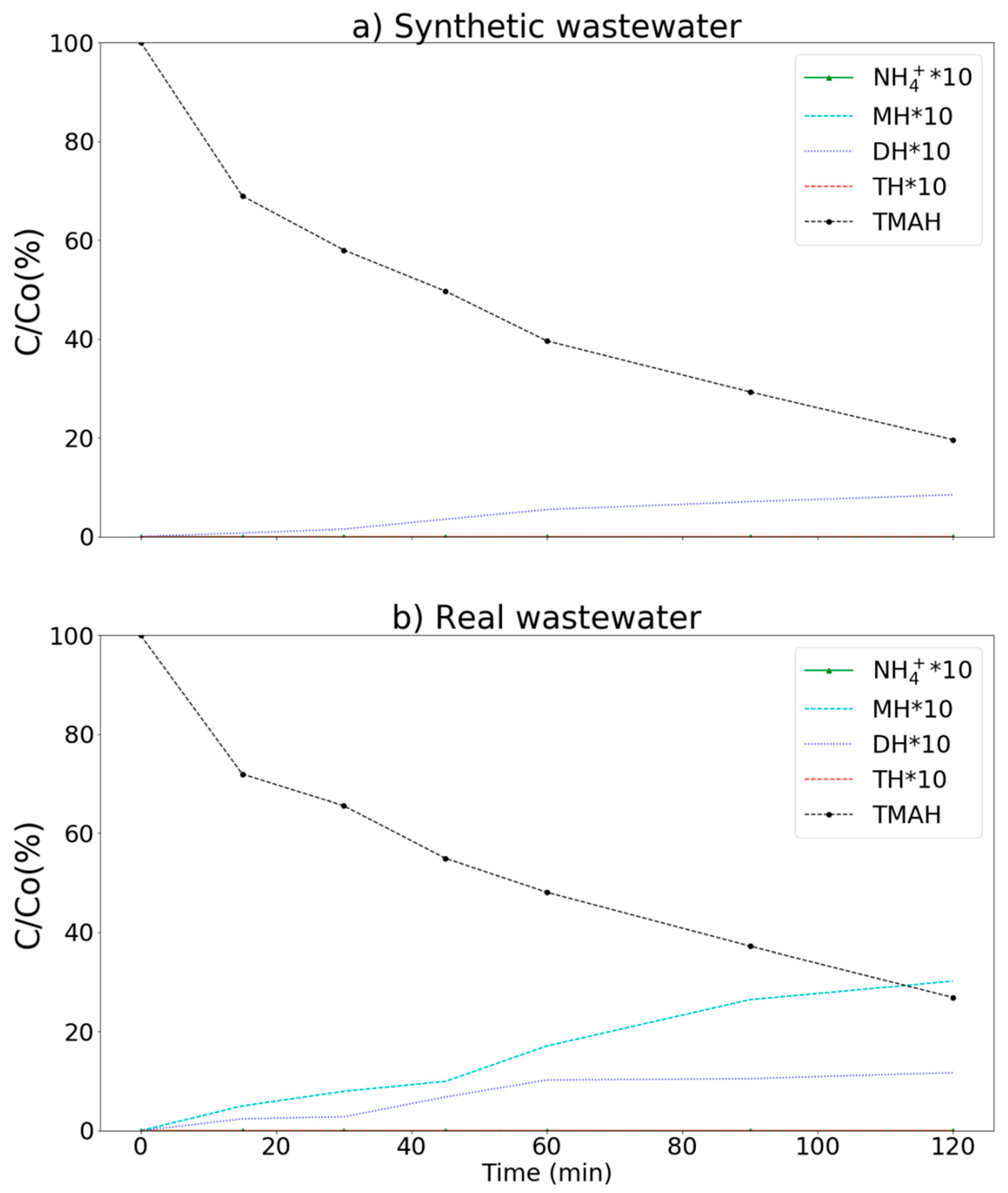
3.4. Real Wastewater
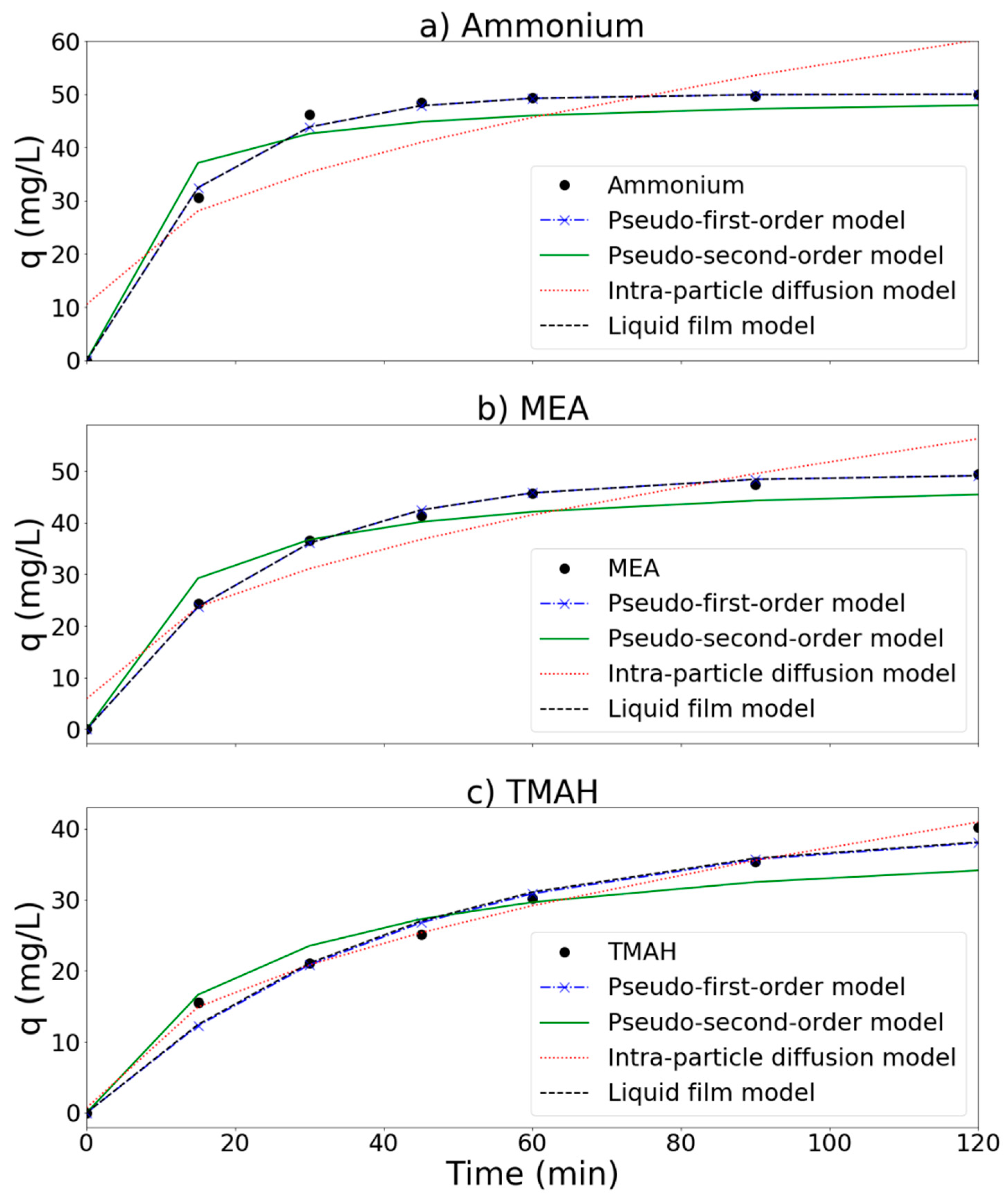
3.5. Reaction Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Su, C.-C.; Chen, C.-M.; Anotai, J.; Lu, M.-C. Removal of monoethanolamine and phosphate from thin-film transistor liquid crystal display (tft-lcd) wastewater by the fluidized-bed fenton process. Chem. Eng. J. 2013, 222, 128–135. [Google Scholar] [CrossRef]
- Ligaray, M.; Futalan, C.M.; de Luna, M.D.; Wan, M.-W. Removal of chemical oxygen demand from thin-film transistor liquid-crystal display wastewater using chitosan-coated bentonite: Isotherm, kinetics and optimization studies. J. Clean. Prod. 2018, 175, 145–154. [Google Scholar] [CrossRef]
- Zubel, I.; Kramkowska, M.; Rola, K. Silicon anisotropic etching in tmah solutions containing alcohol and surfactant additives. Sens. Actuators A Phys. 2012, 178, 126–135. [Google Scholar] [CrossRef]
- Sindhuri, V.; Son, D.-H.; Lee, D.-G.; Sakong, S.; Jeong, Y.-H.; Cho, I.-T.; Lee, J.-H.; Kim, Y.-T.; Cristoloveanu, S.; Bae, Y.; et al. 1/f noise characteristics of algan/gan finfets with and without tmah surface treatment. Microelectron. Eng. 2015, 147, 134–136. [Google Scholar] [CrossRef]
- Kuan-Foo, C.; Show-Ying, Y.; Huey-Song, Y.; Pan, J.R. Anaerobic treatment of tetra-methyl ammonium hydroxide (tmah) containing wastewater. IEEE Trans. Semicond. Manuf. 2008, 21, 486–491. [Google Scholar] [CrossRef]
- Shibata, J.; Murayama, N.; Matsumoto, S. Recovery of tetra-methyl ammonium hydroxide from waste solution by ion exchange resin. Resour. Process. 2006, 53, 199–203. [Google Scholar] [CrossRef]
- Hori, H.; Wachi, S.; Iwamura, K.; Sano, T. Visible light-induced decomposition of monoethanolamine in water using graphitic carbon nitride as a photocatalyst. J. Photochem. Photobiol. A Chem. 2018, 351, 162–169. [Google Scholar] [CrossRef]
- Wu, C.L.; Su, S.B.; Chen, J.L.; Chang, C.P.; Guo, H.R. Tetramethylammonium ion causes respiratory failure related mortality in a rat model. Resuscitation 2012, 83, 119–124. [Google Scholar] [CrossRef]
- Liu, B.; Yoshinaga, K.; Wu, J.-H.; Chen, W.-Y.; Terashima, M.; Goel, R.; Pangallo, D.; Yasui, H. Kinetic analysis of biological degradation for tetramethylammonium hydroxide (tmah) in the anaerobic activated sludge system at ambient temperature. Biochem. Eng. J. 2016, 114, 42–49. [Google Scholar] [CrossRef]
- Lin, C.C.; Yang, C.C.; Ger, J.; Deng, J.F.; Hung, D.Z. Tetramethylammonium hydroxide poisoning. Clin. Toxicol. (Phila) 2010, 48, 213–217. [Google Scholar] [CrossRef]
- Chen, S.Y.; Lu, L.A.; Lin, J.G. Biodegradation of tetramethylammonium hydroxide (tmah) in completely autotrophic nitrogen removal over nitrite (canon) process. Bioresour. Technol. 2016, 210, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.N.; Whang, L.M.; Chen, P.C. Biological treatment of thin-film transistor liquid crystal display (tft-lcd) wastewater using aerobic and anoxic/oxic sequencing batch reactors. Chemosphere 2010, 81, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Prahas, D.; Liu, J.C.; Ismadji, S.; Wang, M.-J. Adsorption of tetramethylammonium hydroxide on activated carbon. J. Environ. Eng. 2012, 138, 232–238. [Google Scholar] [CrossRef]
- Chang, S.; Lin, K.-Y.A.; Lu, C. Efficient adsorptive removal of tetramethylammonium hydroxide (tmah) from water using graphene oxide. Sep. Purif. Technol. 2014, 133, 99–107. [Google Scholar] [CrossRef]
- Chang, S.; Lu, C.; Lin, K.-Y.A. Comparisons of kinetics, thermodynamics and regeneration of tetramethylammonium hydroxide adsorption in aqueous solution with graphene oxide, zeolite and activated carbon. Appl. Surf. Sci. 2015, 326, 187–194. [Google Scholar] [CrossRef]
- Chiou, C.-S.; Chuang, K.-J.; Lin, Y.-F.; Chen, H.-W.; Ma, C.-M. Application of ozone related processes to mineralize tetramethyl ammonium hydroxide in aqueous solution. Int. J. Photoenergy 2013, 2013, 191742. [Google Scholar] [CrossRef]
- Hirano, K.; Okamura, J.; Taira, T.; Sano, K.; Toyoda, A.; Ikeda, M. An efficient treatment technique for tmah wastewater by catalytic oxidation. IEEE Trans. Semicond. Manuf. 2001, 14, 202–206. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Jiang, C.; Xu, T. Electrodialysis process for the recycling and concentrating of tetramethylammonium hydroxide (tmah) from photoresist developer wastewater. Ind. Eng. Chem. Res. 2013, 52, 18356–18361. [Google Scholar] [CrossRef]
- Wang, C.-W.; Liang, C. Oxidative degradation of tmah solution with uv persulfate activation. Chem. Eng. J. 2014, 254, 472–478. [Google Scholar] [CrossRef]
- Huang, J.; Wang, K.S.; Liang, C. Oxidative degradation of tetramethylammonium hydroxide (tmah) by uv/persulfate and associated acute toxicity assessment. J. Environ. Sci. Health Part A 2017, 52, 930–937. [Google Scholar] [CrossRef]
- Federation, W.E.; American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Kishimoto, N.; Nakamura, E. Bromate formation characteristics of uv irradiation, hydrogen peroxide addition, ozonation, and their combination processes. Int. J. Photoenergy 2012, 2012, 107293. [Google Scholar] [CrossRef]
- Afzal, A.; Chelme-Ayala, P.; Drzewicz, P.; Martin, J.W.; Gamal El-Din, M. Effects of ozone and ozone/hydrogen peroxide on the degradation of model and real oil-sands-process-affected-water naphthenic acids. Ozone Sci. Eng. 2015, 37, 45–54. [Google Scholar] [CrossRef]
- Hey, G.; Vega, S.R.; Fick, J.; Tysklind, M.; Ledin, A.; La Cour Jansen, J.; Andersen, H.R. Removal of pharmaceuticals in wwtp effluents by ozone and hydrogen peroxide. Water SA 2014, 40, 165–174. [Google Scholar] [CrossRef]
- Gottschalk, C.; Libra, J.; Saupe, A. Ozonation of Water and Waste Water: A Practical Guide to Understanding Ozone and its Application; John Wiley & Sons: Hoboken, NJ, USA, 2000; p. 189. [Google Scholar]
- Lu, L.-W.; Peng, Y.-P.; Chang, C.-N. Applying an activated carbon/silver catalyst to the decomposition of the aqueous solutions of tetramethyl ammonium hydroxide. J. Taiwan Inst. Chem. Eng. 2018, 88, 130–136. [Google Scholar] [CrossRef]
- Tong, S.-P.; Xie, D.-M.; Wei, H.; Liu, W.-P. Degradation of sulfosalicylic acid by o3/uv o3/tio2/uv, and o3/v-o/tio2: A comparative study. Ozone Sci. Eng. 2005, 27, 233–238. [Google Scholar] [CrossRef]
- Rischbieter, E.; Stein, H.; Schumpe, A. Ozone solubilities in water and aqueous salt solutions. J. Chem. Eng. Data 2000, 45, 338–340. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Beltrán, F.J.; González, M.; Domínguez, J. Effect of high salt concentrations on ozone decomposition in water. J. Environ. Sci. Health Part A 2008, 24, 823–842. [Google Scholar] [CrossRef]
- Christenson, H.K.; Yaminsky, V.V. Solute effects on bubble coalescence. J. Phys. Chem. 1995, 99, 10420. [Google Scholar] [CrossRef]
- Walker, A.B.; Tsouris, C.; DePaoli, D.W.; Thomas Klasson, K. Ozonation of soluble organics in aqueous solutions using microbubbles. Ozone Sci. Eng. 2001, 23, 77–87. [Google Scholar] [CrossRef]
- Boncz, M.A.; Bruning, H.; Rulkens, W.H.; Zuilhof, H.; Sudhölter, E.J.R. The effect of salts on ozone oxidation processes. Ozone Sci. Eng. 2005, 27, 287–292. [Google Scholar] [CrossRef]
- Hoigné, J. Chemistry of aqueous ozone and transformation of pollutants by ozonation and advanced oxidation processes. In Quality and Treatment of Drinking Water II; Hrubec, J., Ed.; Springer: Berlin, Germany, 1998; pp. 83–141. [Google Scholar]
- Tizaoui, C.; Bouselmi, L.; Mansouri, L.; Ghrabi, A. Landfill leachate treatment with ozone and ozone/hydrogen peroxide systems. J. Hazard. Mater. 2007, 140, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.L.C.; Silva, M.B.; Izário Filho, H.J. Leachate treatment process at a municipal stabilized landfill by catalytic ozonation: An exploratory study from taguchi orthogonal array. Braz. J. Chem. Eng. 2009, 26, 481–492. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, H.M.; Sayed, M.; Cooper, W.J. Advanced oxidation for the treatment of chlorpyrifos in aqueous solution. Chemosphere 2013, 93, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-M.; Lu, C.-S.; Chen, Y.-Y.; Chang, D.T.; Fan, H.-J. Landfill leachate treatment with mn and ce oxides impregnated gac–ozone treatment process. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 536–543. [Google Scholar] [CrossRef]
- Kim, S.; Choi, W. Kinetics and mechanisms of photocatalytic degradation of (ch3)nnh4-n+ (0 ≤ n ≤ 4) in tio2 suspension: The role of oh radicals. Environ. Sci. Technol. 2002, 36, 2019–2025. [Google Scholar] [CrossRef]
- Chen, H.; Sayari, A.; Adnot, A.; Larachi, F. Composition-activity effects of mn–ce–o composites on phenol catalytic wet oxidation. Appl. Catal. B Environ. 2001, 32, 195–204. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1979. [Google Scholar]
- Wilczak, A.; Keinath, T.M. Kinetics of sorption and desorption of copper (ii) and lead (ii) on activated carbon. Water Environ. Res. 1993, 65, 238–244. [Google Scholar] [CrossRef]
- Fan, H.J.; Shu, H.Y.; Tajima, K. Decolorization of acid black 24 by the fegac/h2o2 process. J. Hazard. Mater. 2006, 128, 192–200. [Google Scholar] [CrossRef]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study. Dye. Pigment. 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Onundi, Y.B.; Mamun, A.A.; Al khatib, M.; Ahmed, Y.M. Adsorption of copper, nickel and lead ions from synthetic semiconductor industrial wastewater by palm shell activated carbon. Int. J. Environ. Sci. Technol. 2010, 7, 751–758. [Google Scholar] [CrossRef] [Green Version]
- Al-Khateeb, L.A.; Almotiry, S.; Salam, M.A. Adsorption of pharmaceutical pollutants onto graphene nanoplatelets. Chem. Eng. J. 2014, 248, 191–199. [Google Scholar] [CrossRef]



| Plant (1) | Product | pH | Cond. (2) | SS | COD | BOD | Nitrate | Sulfate | NH3-N | TMAH | Discharge |
|---|---|---|---|---|---|---|---|---|---|---|---|
| μS/cm | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | CMD | |||
| Plant 1 | LCD-GS | 7.1 | 1351 | 32.7 | 82.2 | 38.44 | 6.84 | 130.46 | 2.12 | 35.03 | 5447 |
| Plant 2 | PV, TFT-LCD | 6.8 | 2255 | 40.4 | 80 | 36.65 | 30.42 | 156.59 | 3.24 | 127.86 | 9892 |
| Plant 3 | TFT-LCD | 6.9 | 3588 | 79.4 | 296.6 | 169.43 | 42.18 | 160.32 | 9.78 | 100.21 | 12,381 |
| Plant 4 | TFT-LCD | 6.7 | 3254 | 82.0 | 205.1 | 70.31 | 15.25 | 309.75 | 1.73 | 64.97 | 8738 |
| Plant 5 | FM, DRAM | 6.9 | 1546 | 16.1 | 28.8 | 14.78 | 8.01 | 281.31 | 6.87 | 39.76 | 5691 |
| Plant 6 | IC | 6.5 | 7390 | 6.4 | 150.1 | 35.70 | 188.16 | 1876.62 | 12.54 | 91.19 | 3565 |
| Plant 7 | IC | 6.5 | 7394 | 4.8 | 148.7 | 31.03 | 274.28 | 1850.13 | 9.75 | 84.41 | 3503 |
| Plant 8 | IC | 6.4 | 10,941 | 6.5 | 137.3 | 37.87 | 96.99 | 3167.68 | 13.96 | 109.43 | 4062 |
| Plant 9 | IC | 6.5 | 10,643 | 25.0 | 335.7 | 139.33 | 166.73 | 1212.35 | ND (3) | 84.38 | 17,778 |
| Plant 10 | IC-P&T | 7.2 | 1568 | 33.3 | 40.3 | 13.65 | 78.31 | 337.31 | ND | 27.33 | 3586 |
| Adsorbate | ||||
|---|---|---|---|---|
| Models | Ammonium | MEA | TMAH | |
| Pseudo-first-order model | K1 | 0.161 | 0.101 | 0.056 |
| RMSE | 1.31 | 0.43 | 2.61 | |
| Pseudo-second-order model | K2 | 0.00383 | 0.00196 | 0.00117 |
| RMSE | 12.81 | 8.89 | 8.15 | |
| Intra-particle diffusion model | Kp | 4.54 | 4.60 | 3.68 |
| Cp | 10.51 | 5.91 | 0.70 | |
| RMSE | 60.25 | 22.28 | 0.38 | |
| Liquid film model | KL | 0.0698 | 0.0437 | 0.0247 |
| RMSE | 1.31 | 0.43 | 2.58 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, D.T.; Park, D.; Zhu, J.-J.; Fan, H.-J. Assessment of an MnCe-GAC Treatment Process for Tetramethylammonium-Contaminated Wastewater from Optoelectronic Industries. Appl. Sci. 2019, 9, 4578. https://doi.org/10.3390/app9214578
Chang DT, Park D, Zhu J-J, Fan H-J. Assessment of an MnCe-GAC Treatment Process for Tetramethylammonium-Contaminated Wastewater from Optoelectronic Industries. Applied Sciences. 2019; 9(21):4578. https://doi.org/10.3390/app9214578
Chicago/Turabian StyleChang, Da Tian, Daeryong Park, Jun-Jie Zhu, and Huan-Jung Fan. 2019. "Assessment of an MnCe-GAC Treatment Process for Tetramethylammonium-Contaminated Wastewater from Optoelectronic Industries" Applied Sciences 9, no. 21: 4578. https://doi.org/10.3390/app9214578
APA StyleChang, D. T., Park, D., Zhu, J.-J., & Fan, H.-J. (2019). Assessment of an MnCe-GAC Treatment Process for Tetramethylammonium-Contaminated Wastewater from Optoelectronic Industries. Applied Sciences, 9(21), 4578. https://doi.org/10.3390/app9214578








