Preparation of Steel Slag Ceramics with Different MgO/Al2O3 Ratios
Abstract
:1. Introduction
2. Experiment
2.1. Raw Materials and Composition Design
2.2. Preparation of Ceramic Samples
2.3. Characterization of Ceramic Samples
3. Results and Discussion
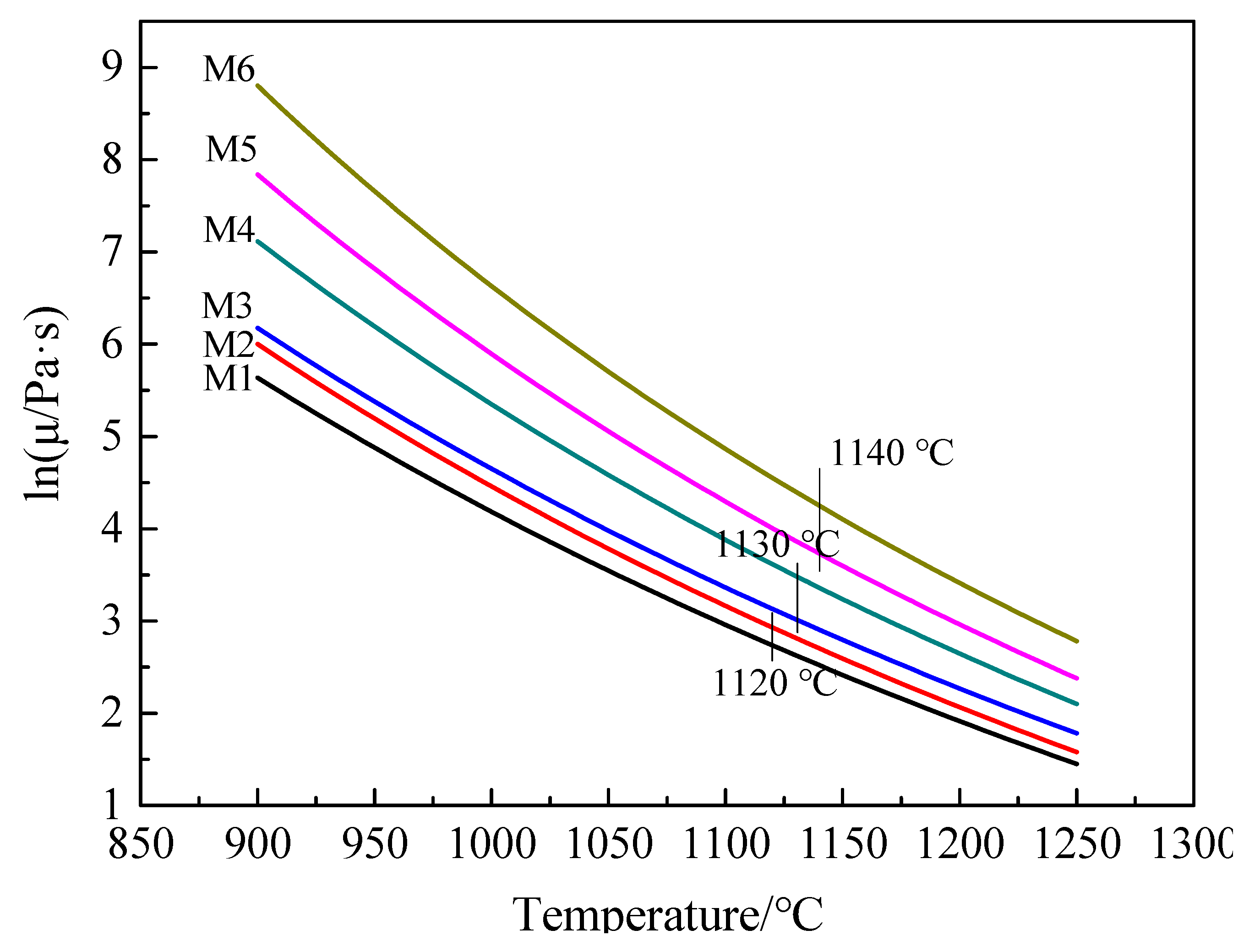
3.1. Calculation of Viscosity by FactSage Software
3.2. XRD Analysis of the Ceramic Samples
3.3. FTIR Analysis of the Ceramic Samples
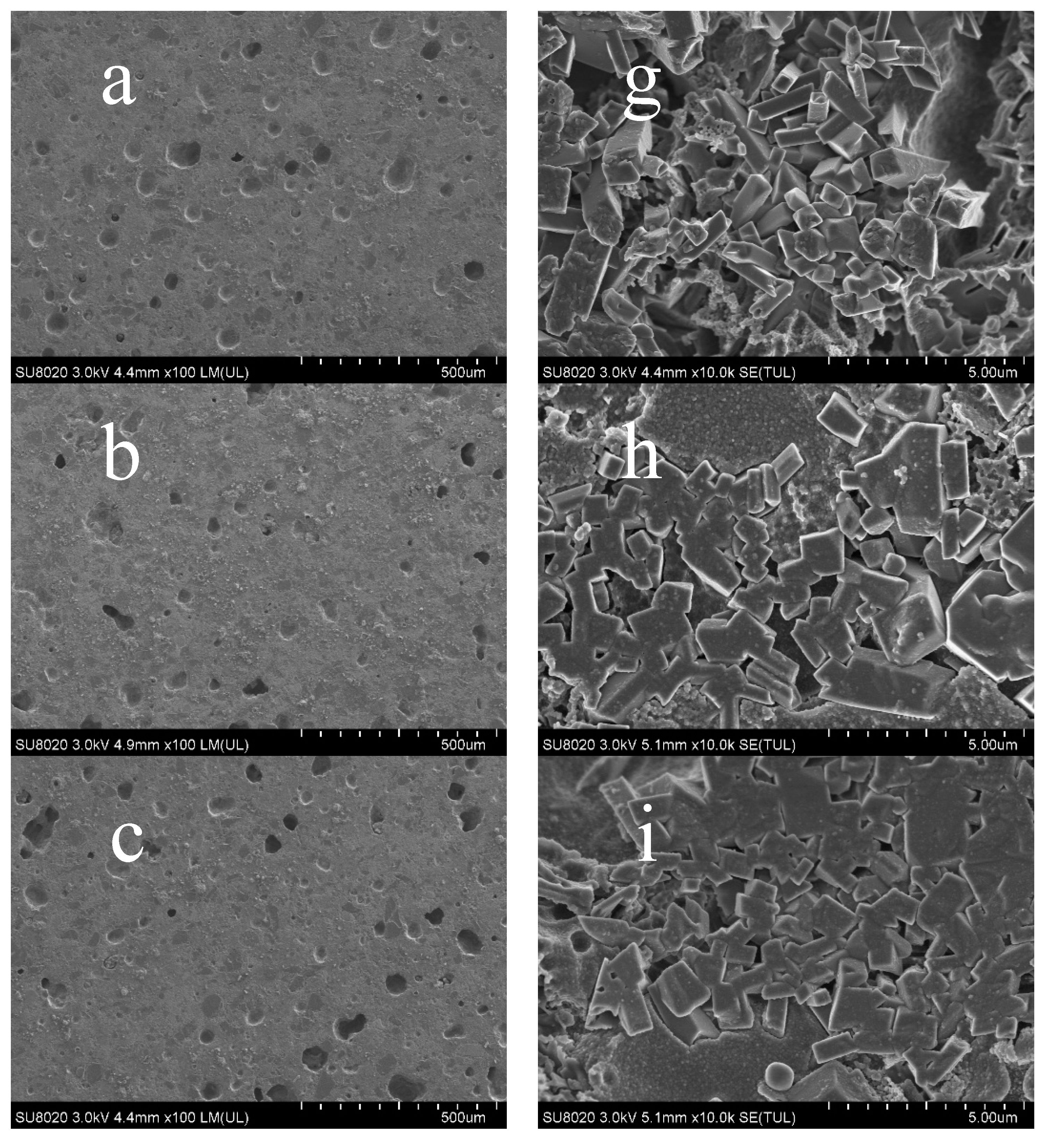
3.4. SEM Analysis of the Ceramic Samples
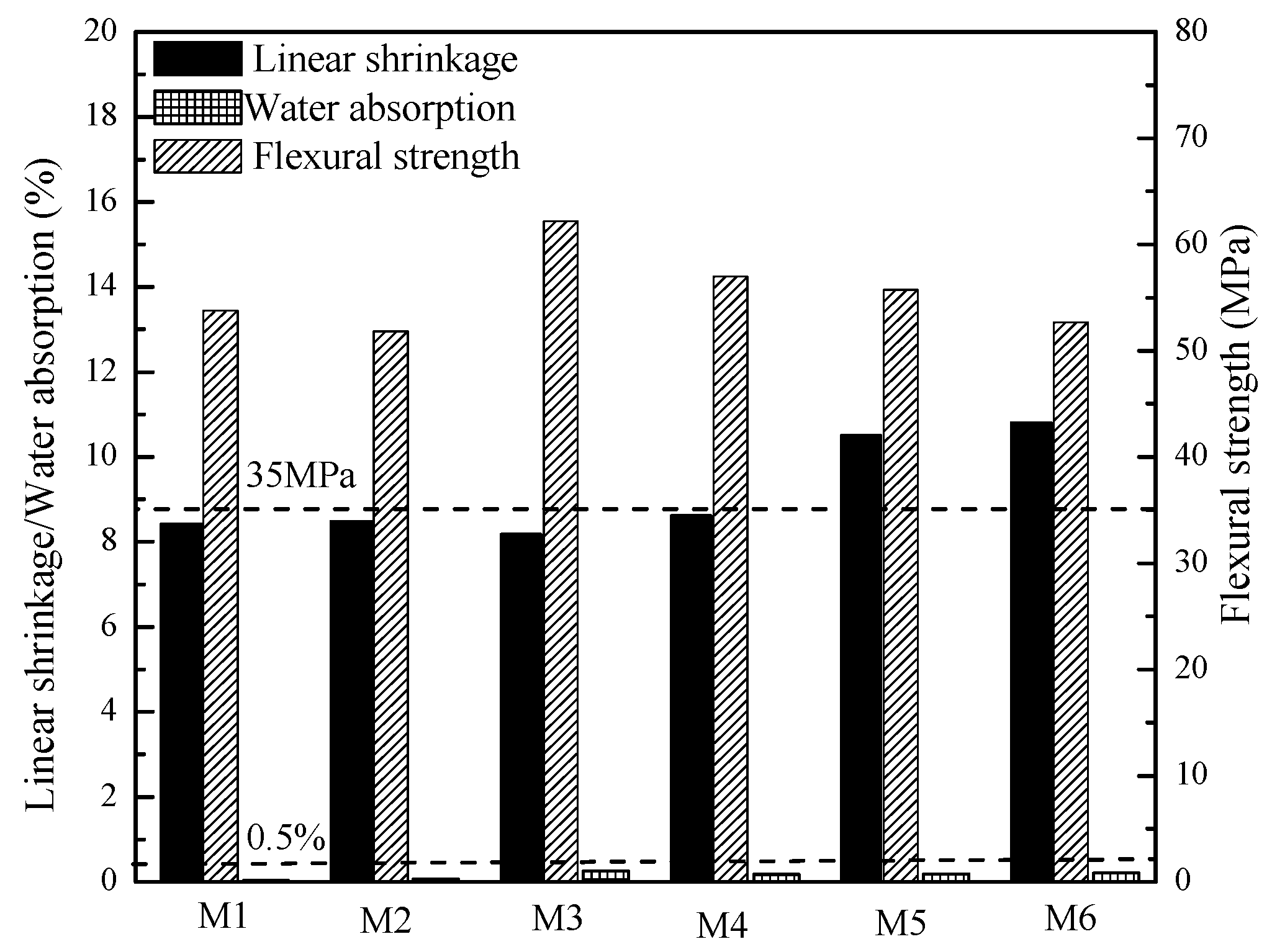
3.5. Physical-Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Han, F.; Zhang, Z. Properties of 5-year-old concrete containing steel slag powder. Powder Technol. 2018, 334, 27–35. [Google Scholar] [CrossRef]
- Guzel, G.; Deveci, H. Properties of polymer composites based on bisphenol A epoxy resins with original/modified steel slag. Polym. Compos. 2018, 39, 513–521. [Google Scholar] [CrossRef]
- Guo, R.X.; Wang, C.; Niu, Z.-L.; Yan, F. Effect of water erosion on the dynamic modulus of steel slag asphalt mixture. Bull. Chin. Ceram. Soc. 2018, 1, 166–172. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, D.; Yan, P. Design and experimental study of a ternary blended cement containing high volume steel slag and blast-furnace slag based on Fuller distribution model. Constr. Build. Mater. 2017, 140, 248–256. [Google Scholar] [CrossRef]
- Sung, C.; Huang, R.; Tsai, C.; Wu, Y.-H.; Lin, W.-T.; Weng, T.-L. Application on cementitious materials to promote durability of alkali-activated concrete containing co-fired fly ash and water-quenched slag. Mon. Chem. Chem. Mon. 2017, 148, 1349–1354. [Google Scholar] [CrossRef]
- Rosales, J.; Cabrera, M.; Agrela, F. Effect of stainless steel slag waste as a replacement for cement in mortars: Mechanical and statistical study. Constr. Build. Mater. 2017, 142, 444–458. [Google Scholar] [CrossRef]
- Hu, J. Comparison between the effects of superfine steel slag and superfine phosphorus slag on the long-term performances and durability of concrete. J. Therm. Anal. Calorim. 2017, 128, 1251–1263. [Google Scholar] [CrossRef]
- Moon, K.H.; Falchetto, A.C.; Wang, D.; Riccardi, C.; Wistuba, M.P. Mechanical performance of asphalt mortar containing hydrated lime and EAFSS at low and high temperatures. Materials 2017, 10, 743. [Google Scholar] [CrossRef]
- Badiee, H.; Maghsoudipour, A.; Raissi Dehkordi, B. Use of Iranian steel slag for production of ceramic floor tiles. Adv. Appl. Ceram. 2008, 107, 111–115. [Google Scholar] [CrossRef]
- Dana, K.; Dey, J.; Das, S.K. Synergistic effect of fly ash and blast furnace slag on the mechanical strength of traditional porcelain tiles. Ceram. Int. 2005, 31, 147–152. [Google Scholar] [CrossRef]
- Goli, H.; Hesami, S.; Ameri, M. Laboratory evaluation of damage behavior of warm mix asphalt containing steel slag aggregates. J. Mater. Civ. Eng. 2017, 29, 4017009. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Papadimitriou, G.D.; Tsivilis, S.; Koroneos, C. Utilization of steel slag for Portland cement clinker production. J. Hazard. Mater. 2008, 152, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.; Liang, Y.; Liu, Z.; Xiao, J.; Duan, A. Impacts of steel-slag-based silicate fertilizer on soil acidity and silicon availability and metals-immobilization in a paddy soil. PLoS ONE 2016, 11, e168163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yan, P.; Wang, D. Research on mineral characteristics of converter steel slag and its comprehensive utilization of internal and external recycle. J. Clean. Prod. 2017, 156, 50–61. [Google Scholar] [CrossRef]
- Qiu, H.; Zhang, H.; Zhao, B.; Zhu, J.; Liu, D. Dynamics study on vanadium extraction technology from chloride leaching steel slag. Rare Met. Mater. Eng. 2013, 42, 696–699. [Google Scholar] [CrossRef]
- Guo, A.; Liu, J.; Xu, R.; Xu, H.; Wang, C. Preparation of mullite from desilication-flyash. Fuel 2010, 89, 3630–3636. [Google Scholar] [CrossRef]
- Chukwudi, B.; Ademusuru, P.O.; Okorie, B.A. Characterization of sintered ceramic tiles produced from steel slag. J. Miner. Mater. Charact. Eng. 2012, 11, 863–868. [Google Scholar] [CrossRef]
- Ozturk, Z.B.; Gultekin, E.E. Preparation of ceramic wall tiling derived from blast furnace slag. Ceram. Int. 2015, 41, 12020–12026. [Google Scholar] [CrossRef]
- Jiang, F.; Li, Y.; Zhao, L.; Cang, D. Novel ceramics prepared from inferior clay rich in CaO and Fe2O3: Properties, crystalline phases evolution and densification process. Appl. Clay Sci. 2017, 143, 199–204. [Google Scholar] [CrossRef]
- Karamanova, E.; Avdeev, G.; Karamanov, A. Ceramics from blast furnace slag, kaolin and quartz. J. Eur. Ceram. Soc. 2011, 31, 989–998. [Google Scholar] [CrossRef]
- Zhao, L.H.; Li, Y.; Cang, D.-Q.; Wei, R.F. Effect of Al2O3 on the sintering properties of pyroxene ceramics. J. Synth. Cryst. 2015, 11, 3346–3349. [Google Scholar] [CrossRef]
- Ai, X.B.; Li, Y.; Gu, X.M.; Cang, D.-Q. Development of ceramic based on steel slag with different magnesium content. Adv. Appl. Ceram. 2013, 4, 213–218. [Google Scholar] [CrossRef]
- Belmonte, D.; Ottonello, G.; Zuccolini, M.V.; Attene, M. The system MgO-Al2O3-SiO2 under pressure: A computational study of melting relations and phase diagrams. Chem. Geol. 2016, 461, 54–64. [Google Scholar] [CrossRef]
- Verein Deutscher Eisenhuttenleute. Slag Atlas, 2nd ed.; Verlag Stahleisen mbH: Dusseldorf, Germany, 1995. [Google Scholar]
- Jung, I.H.; Decterov, S.A.; Pelton, A.D. Critical thermodynamic evaluation and optimization of the MgO-AlO, CaO-MgO-AlO, and MgO-AlO-SiO Systems. J. Phase Equilib. 2004, 25, 329–345. [Google Scholar] [CrossRef]
- Gheribi, A.E.; Robelin, C.; Digabel, S.L.; Audet, C.; Pelton, A.D. Calculating all local minima on liquidus surfaces using the FactSage software and databases and the Mesh Adaptive Direct Search algorithm. J. Chem. Thermodyn. 2011, 43, 1323–1330. [Google Scholar] [CrossRef]
- Belmonte, D.; Ottonello, G.; Zuccolini, M.V. Ab initio-assisted assessment of the CaO-SiO2 system under pressure. Calphad 2017, 59, 12–30. [Google Scholar] [CrossRef]
- Li, Y.; Zong, Y.B.; Cang, D.-Q. Effect of phase separation structure on the crystallization property. Adv. Mater. Res. 2010, 105, 787–790. [Google Scholar] [CrossRef]
- Liu, Z.; Zong, Y.; Hou, J. Preparation of slag glass ceramic from electric arc furnace slag, quartz sand and talc under various MgO/Al2O3 ratios. Br. Ceram. Trans. 2015, 3, 144–151. [Google Scholar] [CrossRef]
- Liu, Z.; Zong, Y.; Ma, H.; Dai, W.; Cang, D. Influence of Al2O3 content on microstructure and properties of different binary basicity slag glass ceramics. Adv. Appl. Ceram. 2014, 7, 394–403. [Google Scholar] [CrossRef]
- Agathopoulos, S.; Tulyaganova, D.U.; Ventura, J.M.G.; Saranti, S.K.A.; Karakassides, M.A.; Ferreira, J.M.F. Structural analysis and devitrification of glasses based on the CaO–MgO–SiO2, system with B2O3, Na2O, CaF2, and P2O5 additives. J. Non-Cryst. Solids 2006, 352, 322–328. [Google Scholar] [CrossRef]
- Nogami, M.; Ogawa, S.; Nagasaka, K. Preparation of cordierite glass by the sol-gel process. J. Mater. Sci. 1989, 12, 4339–4342. [Google Scholar] [CrossRef]
- Agathopoulos, S.; Tulyaganova, D.U.; Ventura, J.M.G.; Saranti, S.K.A.; Karakassides, M.A.; Ferreira, J.M.F. Formation of hydroxyapatite onto glasses of the CaO-MgO-SiO2 system with B2O3, Na2O, CaF2, and P2O5 additives. Biomaterials 2006, 27, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.B.; Zhang, X.D.; Mukiza, E. Effect of Fly Ash on the Properties of Ceramics Prepared from Steel Slag. Appl. Sci. 2018, 8, 1187. [Google Scholar] [CrossRef]



| Content (wt%) | SiO2 | CaO | Al2O3 | Fe2O3 | MgO | K2O | Na2O | TiO2 | MnO2 |
|---|---|---|---|---|---|---|---|---|---|
| Steel slag | 11.65 | 52.69 | 2.07 | 21.89 | 4.18 | 0.10 | 0.14 | 1.05 | 2.78 |
| Clay | 64.28 | 0.98 | 20.38 | 8.99 | 0.71 | 3.25 | 0.16 | 0.96 | - |
| Quartz | 96.5 | 0.09 | 1.85 | 0.83 | 0.11 | 0.51 | - | - | - |
| Feldspar | 65.61 | 5.98 | 15.26 | 1.33 | 0.43 | 8.65 | 2.18 | 0.18 | 0.08 |
| Talc | 61.77 | 3.57 | 0.25 | 0.23 | 34.00 | 0.02 | - | - | - |
| M1 (g) | M2 (g) | M3 (g) | M4 (g) | M5 (g) | M6 (g) | |
|---|---|---|---|---|---|---|
| SiO2 | 49.79 | 48.82 | 50.64 | 48.23 | 47.41 | 47.60 |
| CaO | 18.92 | 18.55 | 19.96 | 19.00 | 17.71 | 16.88 |
| Al2O3 | 7.57 | 9.35 | 8.41 | 12.68 | 15.13 | 17.63 |
| Fe2O3 | 9.75 | 9.55 | 10.26 | 9.77 | 9.57 | 8.94 |
| MgO | 9.05 | 8.88 | 5.12 | 4.88 | 4.74 | 3.54 |
| K2O | 1.77 | 1.74 | 2.22 | 2.12 | 2.21 | 2.31 |
| Na2O | 0.31 | 0.30 | 0.42 | 0.40 | 0.39 | 0.42 |
| TiO2 | 0.60 | 0.59 | 0.64 | 0.60 | 0.62 | 0.60 |
| MnO2 | 0.92 | 0.91 | 0.98 | 0.94 | 0.88 | 0.79 |
| MgO/Al2O3 | 1.2 | 0.9 | 0.6 | 0.4 | 0.3 | 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zheng, C.; Liu, S.; Zong, Y.; Zhou, Q.; Qin, S. Preparation of Steel Slag Ceramics with Different MgO/Al2O3 Ratios. Appl. Sci. 2019, 9, 4741. https://doi.org/10.3390/app9224741
Zhang X, Zheng C, Liu S, Zong Y, Zhou Q, Qin S. Preparation of Steel Slag Ceramics with Different MgO/Al2O3 Ratios. Applied Sciences. 2019; 9(22):4741. https://doi.org/10.3390/app9224741
Chicago/Turabian StyleZhang, Xuedong, Chaozhen Zheng, Sanping Liu, Yanbing Zong, Qifan Zhou, and Shuchen Qin. 2019. "Preparation of Steel Slag Ceramics with Different MgO/Al2O3 Ratios" Applied Sciences 9, no. 22: 4741. https://doi.org/10.3390/app9224741
APA StyleZhang, X., Zheng, C., Liu, S., Zong, Y., Zhou, Q., & Qin, S. (2019). Preparation of Steel Slag Ceramics with Different MgO/Al2O3 Ratios. Applied Sciences, 9(22), 4741. https://doi.org/10.3390/app9224741




