Featured Application
This work has applications to protect tooth enamel for orthodontic treatment patients. It can be applied to study prevent enamel demineralization.
Abstract
White spot lesions (WSL) that occur on teeth after orthodontic appliances have been attached are caused by bacterial demineralization of the enamel surface. This study investigated the anti-demineralization effect of orthodontic resins containing mesoporous bioactive glass nanoparticles (MBN) doped with gallium, which has antibacterial activity, as well as MBN with increased calcium and phosphate contents as these ions can remineralize enamel. Resins (CF, CharmFill Flow, Dentkist, Seoul, South Korea) containing 1%, 3%, and 5% Ga-doped MBN (GaMBN) were characterized using scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX), X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, and isothermal tests, and their physical properties were measured in terms of Vickers microhardness, bracket retention force, and adhesive remnant index (ARI). Cell viability in the resins was confirmed by testing human dental pulp stem cells (hDPSCs), and ion release tests were performed after 1, 7, and 14 days to determine whether the resins released Ga3+, Ca2+, and PO43–. After 14 days, antibacterial activity was determined using Streptococcus mutans (S. mutans)—the bacteria that causes tooth decay—and the chemical remineralization effect was investigated using a cycle of acid–base solutions. The microhardness of the resins increased with GaMBN concentration whereas their bracket retention force, ARI, and cell viability remained unchanged. The bacterial activity of the 5%-GaMBN resin decreased after 24 and 48 h; however, the change in activity was not statistically significant. Anti-demineralization testing demonstrated that the degree of enamel demineralization decreased as the GaMBN concentration increased, which indicates that resins containing 5%-GaMBN may be viable orthodontic adhesives for preventing WSLs.
1. Introduction
White spot lesions (WSLs) on enamel around orthodontic brackets is a common side effect of orthodontic treatment because cariogenic bacteria such as Streptococcus mutans (S. mutans) colonize plaque deposited around the brackets and produce acids such as lactic acid, which demineralize the enamel surface [1,2,3]. The bacteria responsible for WSLs, S. mutans, is a facultatively anaerobic, Gram-positive coccus (round bacterium) that is commonly found in the human oral cavity and is a cause of caries [4]. In an oral cavity with fixed orthodontic appliances, the number of S. mutans increases and thus leads to the demineralization of enamel [5].
White spot legions on anterior teeth are considered unaesthetic as the demineralized enamel is often whiter than the surrounding enamel [6]; hence, clinicians have made several attempts to prevent the formation of WSLs [7]. For example, researchers have attempted to incorporate antibacterial agents in orthodontic adhesives in order to reduce the causative bacteria and thus prevent WSLs caused by orthodontic devices [8,9,10,11]. Gallium (III) has been used an antibacterial agent as it can mimic Fe (III) [12,13]. Iron (III) is reduced to Fe (II) by intracellular processes whereas Ga (III) is not reduced; thus, Ga (III) interferes with biological redox processes when it binds to Fe (III) protein binding sites in bacteria, which results in an antibacterial effect [12]. According to a study by Valappil et al., Ga-doped PO43−-based glasses exhibited the potential to protect enamel surfaces by inhibiting the growth of S. mutans [13].
Another method for preventing WSLs caused by orthodontic devices involves remineralizing the enamel by supplying Ca2+ and PO43− to the demineralized surface as these ions are leached from enamel by acid [14]. Bioactive glass (BAG) is a basic Si–O–Si structure that consists of SiO2, CaO, and P2O5. When BAG is added to resin paste as a filler, the strength of the paste increases [11]. In a liquid environment containing BAG, the calcium phosphate layer on an enamel surface exposed to acid grows into apatite, which results in an antibacterial effect as pH is increased and oral bacteria is reduced [15]. Mesoporous bioactive glass nanoparticles are widely used as drug carriers because of the high surface to volume ratio created by their internal pore structure [16]. In this study, Ga-doped mesoporous bioactive glass nanoparticles (GaMBN)—with wide surface pores and Ca2+ and PO43− available for remineralization—were synthesized and added to resin to form an orthodontic bonding adhesive with the potential to prevent WSLs. The objective of this study was to determine the clinical applicability of the GaMBN resin both for preventing WSLs and as an orthodontic bonding adhesive.
Therefore, the purpose of this study was to determine whether resin containing Gallium doped mesoporous silica nanoparticles could reduce white spot lesions. To confirm the results, 1%, 3%, and 5% of GaMBN was added to the resin (CF, CharmFill Flow, Dentkist, Seoul, South Korea). After that, chemical and physical properties were checked, and anti-demineralization ability was confirmed. ANOVA and regression were used to analyze the experimental data.
2. Materials and Methods
2.1. Synthesis of Ga-doped Mesoporous Bioactive Glass Nanoparticles (GaMBN)
The GaMBN was synthesized using the modified sol-gel process reported in a previous study [17]. First, 10 mL of 2-ethoxyethanol (Sigma-Aldrich, St. Louis, MO, USA), 2 mL of aqueous ammonia (Samchun, Pyeongtaek, South Korea), and 0.788 g of calcium nitrate tetrahydrate (Ca(NO3)2·4H2O; Sigma-Aldrich, St. Louis, MO, USA) were added to 150 mL of distilled water along with 20 mL of ethanol and 1 g of hexadecyltrimethylammonium bromide (CTAB; Sigma-Aldrich, St. Louis, MO, USA). The mixture was stirred at room temperature for 90 min; 5 mL of tetraethyl orthosilicate (TEOS; Sigma-Aldrich, St. Louis, MO, USA) was added, after 30 min and 0.583 g of triethylphosphate (TEP; Sigma-Aldrich, St. Louis, MO, USA) was added after 60 min. After stirring for 90 min, 1.638 g of gallium (III) nitrate hydrate (Sigma-Aldrich, St. Louis, MO, USA) was added and the mixture was stirred for an additional 30 min. The solution was then stirred vigorously for 4 additional h at room temperature. The mixture was then dried in an oven at 60 °C for 24 h and then calcined in a furnace at 600 °C for 5 h. The molar ratio (mol %) of the resultant SiO2:CaO:P2O5:Ga2O3 was 70:15:5:10.
For MBN, the above process used 0.689 g of Ca(NO3)2·4H2O and 0.51g of TEP in order to obtain SiO2:CaO:P2O5 with 80:15:5.
The samples were ground using a mortar and pestle and passed through a sieve (ø: 0.355 mm).
2.2. Characterization of GaMBN
The surface ions and surface morphology of GaMBN were observed via energy-dispersive X-ray spectroscopy (EDX) using a field emission scanning electron microscope (FESEM; MIRA3, TESCAN, Czech Republic). The composition of the sample was analyzed using X-ray diffraction spectroscopy (XRD; Rigaku, Tokyo, JAPAN) at 40 kV and 40 mA with CuKα radiation (2θ = 10–45°) and attenuated total reflectance-Fourier transformation infrared spectroscopy (FT-IR; Spectrum GX, PerkinElmer, Shelton, CT, USA). Surface area and pore size were determined by measuring N2 adsorption–desorption isotherms and Brunauer–Emmett–Teller (BET) surface areas using an adsorption analyzer (Autosorb-iQ MP, Quantachrome instruments, Boynton Beach, FL, USA) at 77.35 K.
2.3. Preparation of Resin Disks
In order to perform the Vickers hardness and ion release tests, disks with a diameter of 5 mm and a thickness of 1.0 mm were fabricated and analyzed. To prevent light permeation during disk fabrication, GaMBN (1, 3, and 5 wt%) was mixed with resin (CF, CharmFill Flow, Dentkist, Seoul, South Korea) in 2 mL black microcentrifuge tube and the mixtures were mixed twice for 10 s using a mixer (TORNADO SHM-ALM00, Shinhung, Seoul, South Korea). Each homogeneous sample was injected into a brass mold and covered with a 0.2 mm thick glass slide prior to photopolymerization, which was performed by exposing the sample disk to a VALO curing light (Ultradent Products, South Jordan, UT, USA) for 20 s. Through the above process, samples of GaMBN0 (CF 100%), GaMBN1 (GaMBN 1%, CF 99%), GaMBN3 (GaMBN3%, CF 97%), and GaMBN5 (GaMBN 5%, CF 95%) were made.
2.4. In Vitro Ion Release Test
The resin disk was submerged in distilled water in accordance with ISO 10993-12(3 cm2/mL) Sample Preparation and Reference Materials, shaken at 200 rpm for 1, 7, and 14 days using a shaking machine at room temperature, and analyzed by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES, Optima 8300, PerkinElmer, Waltham, MA, USA). The ions of interest were Ca2+ and PO43–, which are related to remineralization, and Ga3+, which is related to antibacterial activity.
2.5. Microhardness
Five resin disks containing GaMBN were prepared, and five measurements were performed per disk. For microhardness testing (MVK-H1, Akashi, Tokyo, Japan), 200 gf was loaded on the top of the prepared resin disks.
2.6. Bracket Retention Force
The bracket retention force of the GaMBN-containing resins was measured using a universal testing machine (Instron Corporation, Canton, MA, USA). Eight extracted premolars were used for orthodontic testing; teeth with prosthetic treatments, preservation treatments, cracks, WSLs, caries, or enamel defects were excluded. Bonding was performed according to the protocol recommended by Transbond™ XT Primer, and the brackets were tested using the following method. Each tooth was cleaned using fluorine-free pumice from a prophylaxis cup, which was applied to the side of the tooth that would be attached to the bracket, washed with distilled water for 10 s, and then gently air-dried. Each tooth was then exposed to 15 s of etching and suction with 35% phosphoric acid gel (Ultra Etch, Ultradent, USA), and subsequently washed with distilled water and gently air-dried. The dried tooth surface was coated with Transbond™ XT Light Cure Adhesive Primer (3M, Nonrovia, CA, USA), gently air-dried for 2 s, and then a premolar bracket (Orthos AP Metal Bracket, Ormco, Glendora, CA, USA) was applied along the facial axis of the clinical crown with sample resin. The excess sample resin around the bracket was removed and the sample resin securing the bracket was photopolymerized along a mesial and distal angle for 5 s.
Bracket-bonded teeth were stored in distilled water for 24 h prior to testing with the Instron universal testing machine. The steel rod of the machine was placed perpendicular to the bracket and the maximum load was measured at a crosshead speed of 1 mm/min. The measured load (N) value was divided by the bracket base area (12.98 mm2) and converted to bond strength (MPa). The adhesive remaining on teeth that were separated from their bracket was assessed using the adhesive remnant index (ARI): (1) All of the adhesive remained on the tooth; (2) more than 90% of the adhesive remained on the tooth; (3) 10–90% of the adhesive remained on the tooth; (4) less than 10% of the adhesive remained on the tooth; and (5) no adhesive remained on the tooth.
This bracket retention testing was reviewed and approved by the Institutional Review Board of Pusan National University Dental Hospital (PNUDH-2018046).
2.7. Cell Viability Assays
The resin disks (n = 6) were disinfected using low-temperature plasma (LOWTEM Crystal 50, Gunpo-si, South Korea). A suspension of human dental pulp stem cells (hDPSCs; PT-5025, Lonza, Basel, Switzerland), 10% fetal bovine serum (FBS; Gibco, NY, USA), and 1% antibiotics (Penicillin-streptomycin; Gibco, NY, USA) was added to Dulbecco’s Modified Eagle Medium (DMEM; Gibco, NY, USA) and incubated in a cell incubator held at 37 ℃ and supplied with 5% CO2; the cultured cells were used in the assays. Thiazolyl blue tetrazolium bromide (M2128, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in phosphate buffered saline (PBS) to obtain 5 mg/mL and then a tenth of the total medium volume treated with MTT reagent was added to the assay solution and incubated at 37 ℃ for 3 h. After 3 h, the media were removed and washed in dimethyl sulfoxide (DMSO) on a shaking machine for 5 min (in vitro). Aliquots of 100 μL were pipetted into a 96-well plate and analyzed at 620 nm using an ELISA instrument (SunriseTM, TECAN, Mendendorf, Switzerland).
2.8. Antibacterial Properties
The resin disks (n = 4) were photopolymerized onto the bottom surface of the 96-well plate using the control resin after the 96-well plate was disinfected using low-temperature plasma (LOWTEM Crystal 50, Gunpo-si, South Korea). Prior to inoculation with S. mutans (KFCC, Kyeonggi-do, South Korea), PBS was added to the plate and subjected to 24 h of pre-incubation. The S. mutans was added to a brain heart infusion (BHI) medium to obtain 1.0 × 105 CFU/mL, applied to the resin disks, and incubated at 37 °C. After incubating for 24 and 48 h, the absorbance at 620 nm was measured (SunriseTM, TECAN, Mendendorf, Switzerland).
2.9. Anti-Demineralization Properties
The anti-demineralization tests used to assess the remineralization effect of the GaMBN-containing resin (CF, CharmFill Flow, Dentkist, Seoul, South Korea) were based on methods previously reported in the literature, and were reviewed and approved by the Institutional Review Board of Pusan National University Dental Hospital (PNUDH-2018046) [8,9,10,11]. Each group of experiments used ten healthy premolar teeth—untreated enamel with no WSLs or other enamel defects—that had been extracted for orthodontic purposes.
Each tooth was embedded in acrylic resin (Caulk Orthodontic Resin, Dentsply Caulk, York, PA) using a mold, and etching and adhesive application were conducted following the same method as the bracket retention tests. GaMBN (1, 3, and 5 wt%) was mixed with resin (CF, CharmFill Flow, Dentkist, Seoul, South Korea) in 2 mL black microcentrifuge tube and the mixtures were mixed twice for 10 s using a mixer (TORNADO SHM-ALM00, Shinhung, Seoul, South Korea).
Photopolymerization was performed for 5 s after applying resin (CF, CharmFill Flow, Dentkist, Seoul, South Korea) containing 0, 1, 3, and 5 wt% GaMBN to the tooth surface and the teeth were stored in distilled water for 24 h. The following precipitation cycle was repeated for 14 days: 6 h in 500 mL demineralizing solution (Biosesang, Kyeonggi-do, South Korea) followed by 18 h in 500 mL remineralizing solution (Biosesang, South Korea). The solutions were replaced with fresh ones every week and the teeth were washed—1 min in distilled water and gently air-dried—before they were moved between solutions.
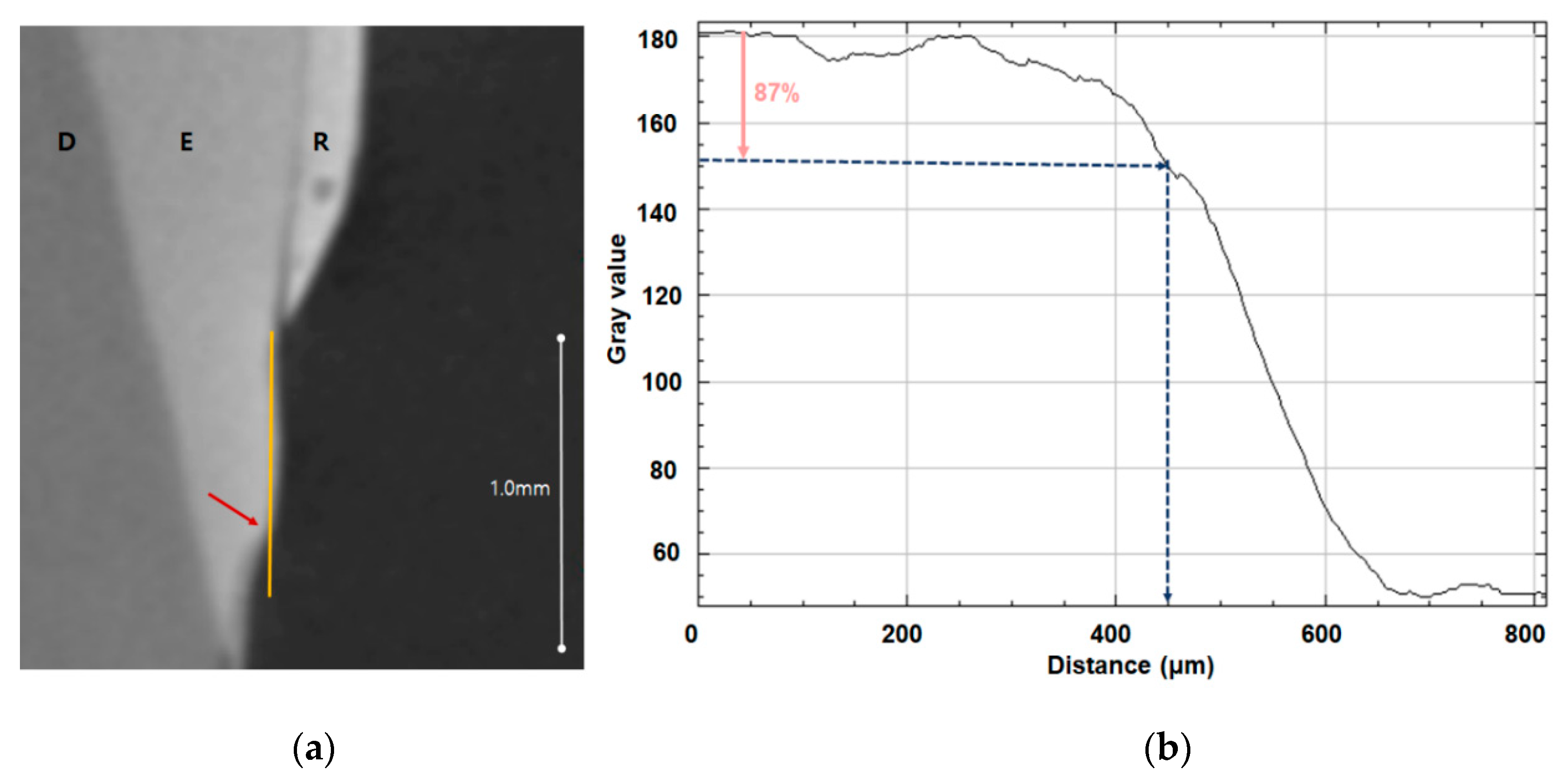
The teeth were monitored using micro-CT (InspeXio SMX-90CT Plus Benchtop Micro Focus X-ray, Shimadzu, Japan) at 90 KV and 109 μA, and the measured micro-CT data was analyzed using ImageJ (National Institutes of Health, Bethesda, MD, USA) (Figure 1). Brightness was used to determine whether enamel had been remineralized, with 87% brightness relative to control enamel set as the threshold; when brightness dropped below this threshold, the distance from the end of the orthodontic bonding primer coating was the remineralization length (Figure 1, Table 1).
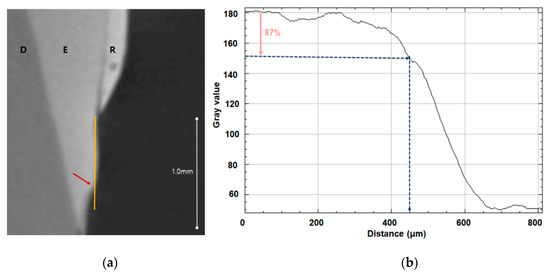
Figure 1.
Remineralization length analysis. (a) Micro-CT slice of the region of interest at the center of the lesion perpendicular to the enamel surface; the remineralization length is indicated by the yellow line, D indicates dentin, E indicates enamel, and R indicates the resin sample. (b) Plot of brightness versus distance from the resin prepared using ImageJ; the pink arrow indicates 87% brightness relative to the reference point and the blue arrow indicates the remineralization length.

Table 1.
Composition of the demineralizing and remineralizing solutions.
2.10. Statistical Analysis
The data are expressed as mean ± standard deviation. The Shapiro–Wilk test used to test normality, and Levene’s test was used to assess the equality of variances. For the Vickers hardness and anti-demineralization tests, the Pearson correlation coefficient was used to assess the regression of the data on GaMBN concentration. The differences in the results of the different GaMBN concentrations (bracket retention test, cell viability test, antibacterial test) were subject to a one-way analysis of variance (ANOVA) test followed by Duncan’s post-hoc test. The Kruskal–Wallis test was used to analyze the nonparametric ARI; the significance level was set to 5%. All statistical analyses were performed using R (Version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Characterization of GaMBN
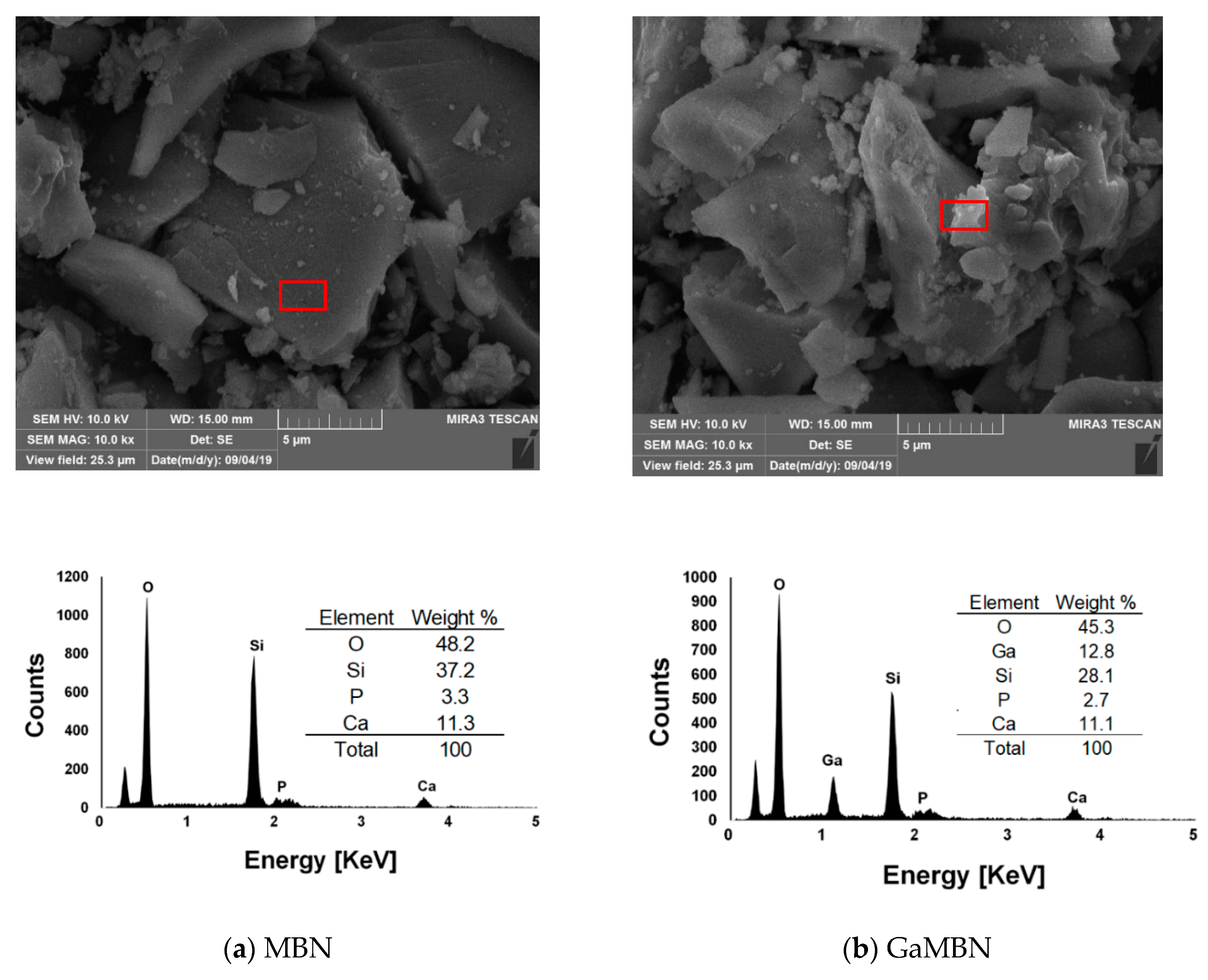
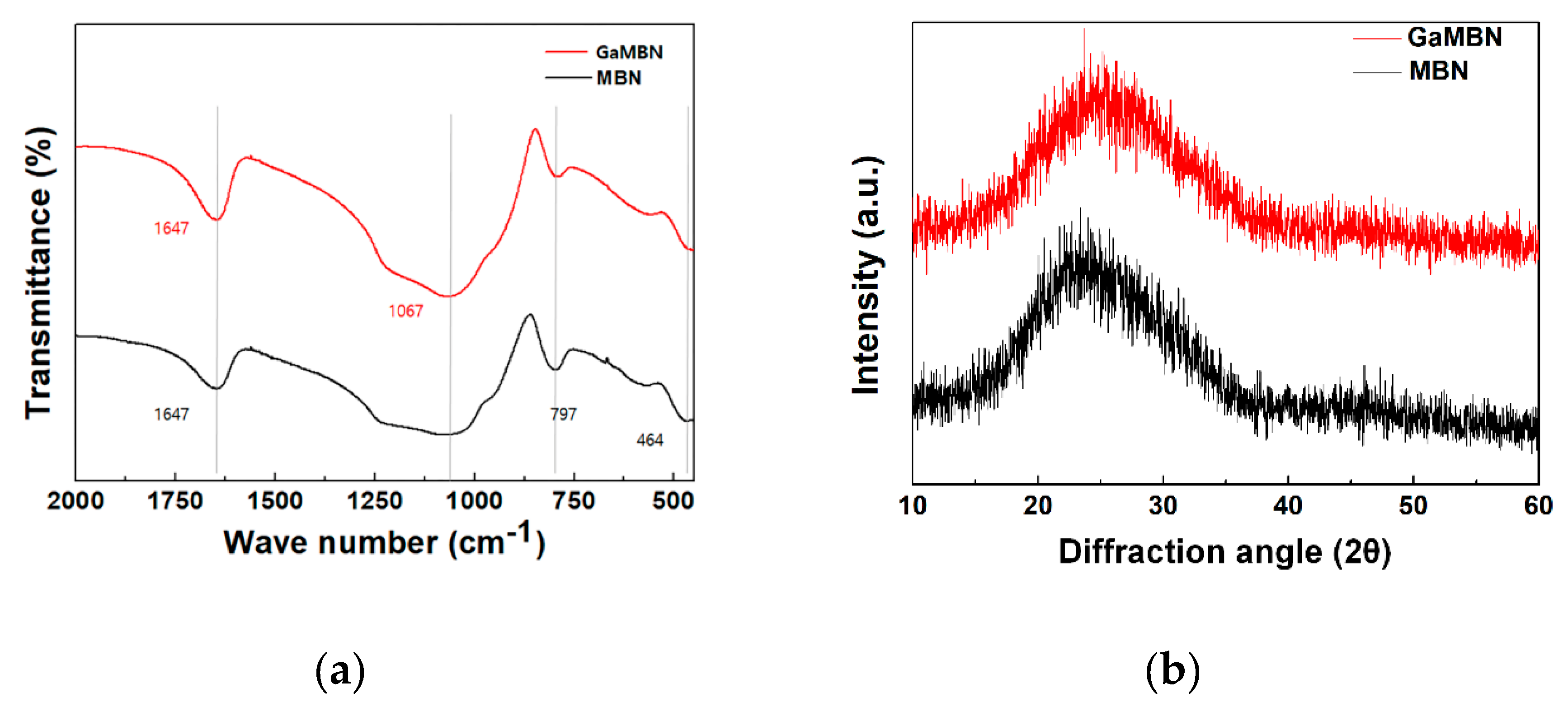
The morphology of the sol-gel samples was observed like a typical heterogeneous glass surface. Based on the EDX results for MBN, O and Si were the predominant elements, followed by P and Ca. For GaMBN, O and Si still made up the largest fraction, followed by Ga, then P and Ca ions. These results confirm that each of the expected ions were present in the glass matrix. MBN and GaMBN molecules were 7–15 μm in diameter (Figure 2). In the FTIR spectra of MBN and GaMBN each had a PO4 peak at 464 cm−1, Si–O–Si asymmetric stretching peaks at 797 and 1067 cm−1, and a peak near 1647 cm−1 attributed to the absorption of water by the Si-OH surface. The XRD spectra of MBN and GaMBN were similar (Figure 3); however, MBN had an amorphous state, which indicates the presence of a glass.
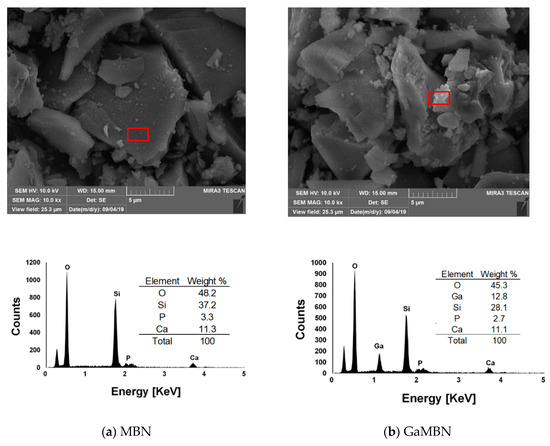
Figure 2.
SEM images and EDX results of the (a) mesoporous bioactive glass nanoparticles (MBN) and (b) Ga-doped mesoporous bioactive glass nanoparticles (GaMBN). Red box indicates the region of interest.
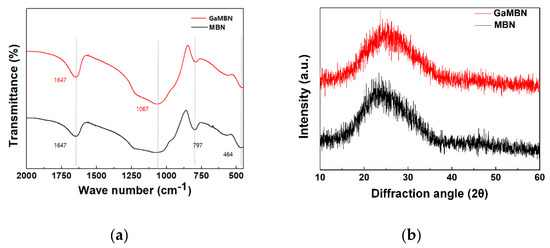
Figure 3.
(a) FTIR and (b) XRD spectra of MBN and GaMBN.
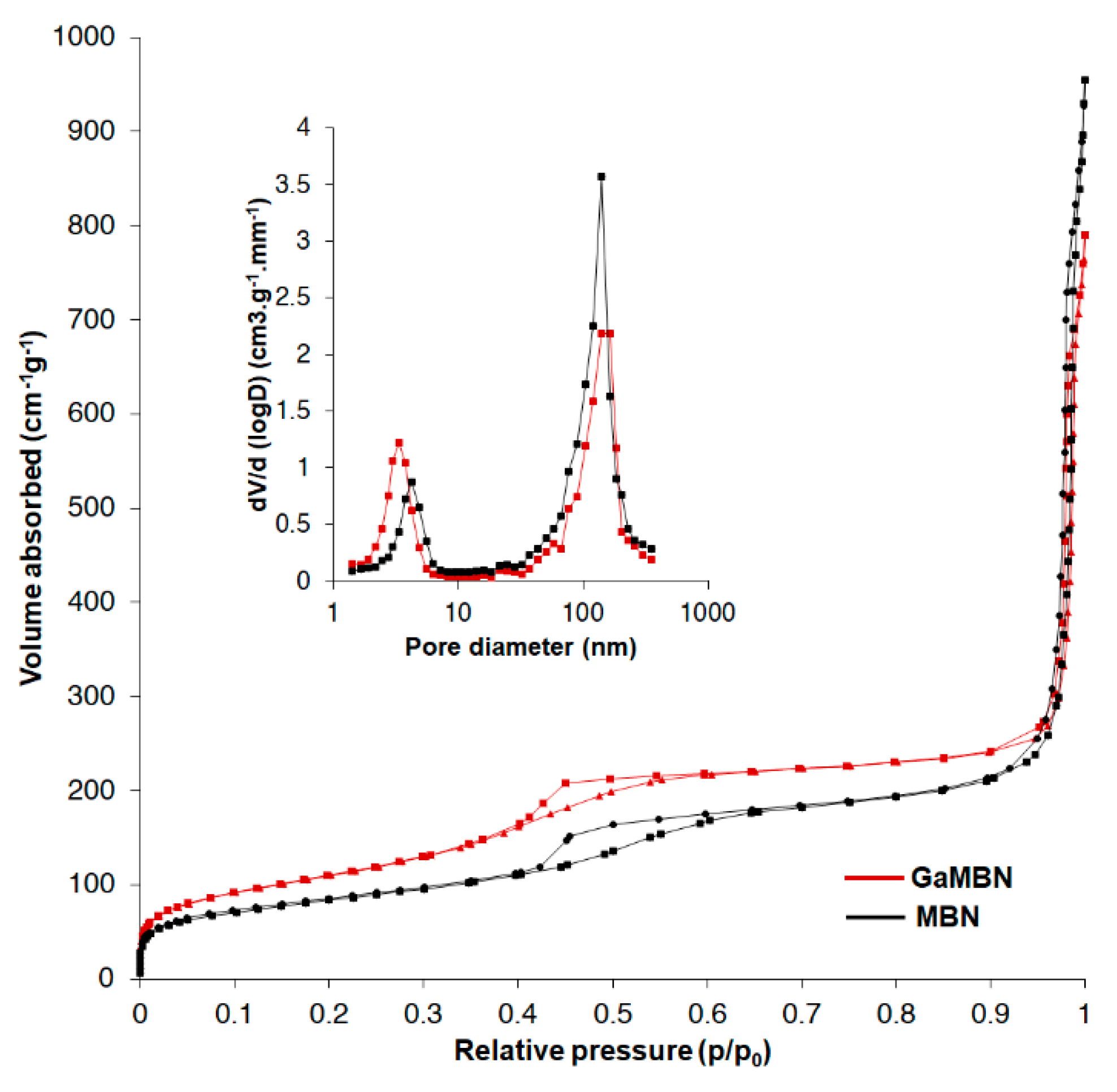
The N2 adsorption–desorption isotherms revealed that MBN and GaMBN both exhibited the type IV isotherm of mesoporous materials (IUPAC classification). The type IV isotherm is the result of capillary condensation caused by the pore structure, and exhibits characteristics of a type-H1 hysteresis loop. The results of specific surface area (SBET), pore volume (Vp), and pore diameter (Dp) measurements demonstrated that when the MBN was doped with Ga, the SBET increased, Vp decreased, and the Dp was relatively unchanged (Figure 4, Table 2).
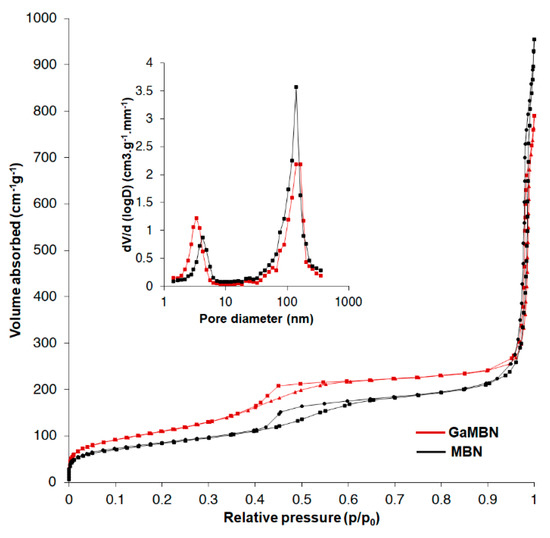
Figure 4.
N2 adsorption–desorption isotherms and pore size distribution of MBN and GaMBN.

Table 2.
N2 adsorption–desorption results.
3.2. In Vitro Ion Release Test
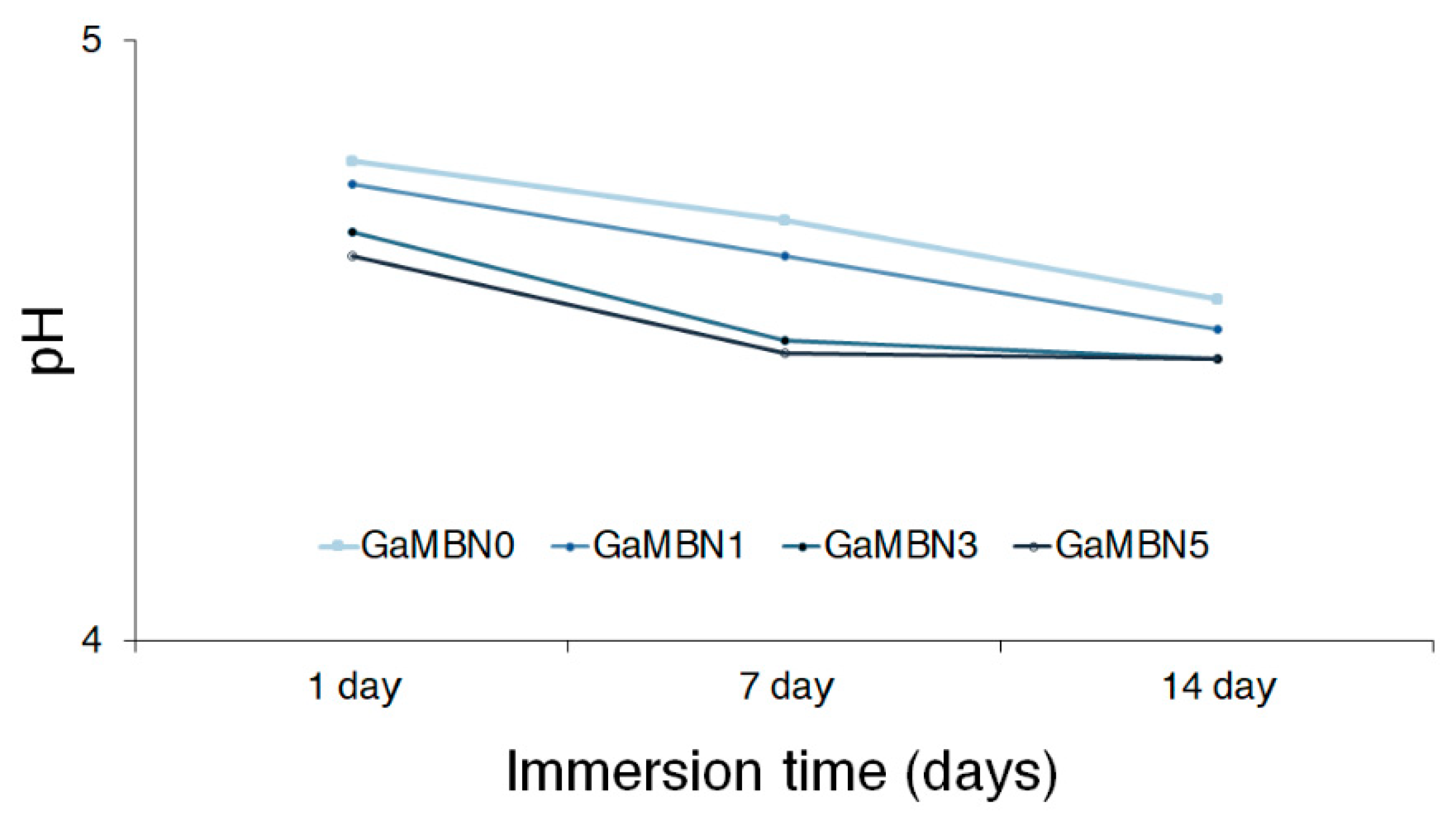
Figure 5 illustrates the relationship between pH and immersion time for the resin disks containing 0, 1, 3, and 5 wt% GaMBN: GaMBN0, GaMBN1, GaMBN3, and GaMBN5, respectively. The pH decreased with increasing GaMBN content, and generally decreased with immersion time for each disk.
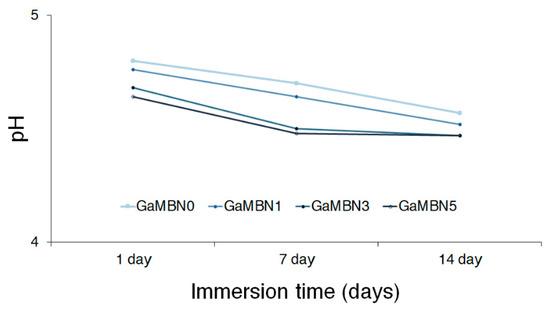
Figure 5.
Relationship between pH and immersion time for the resin disks containing 0, 1, 3, and 5 wt% GaMBN: GaMBN0, GaMBN1, GaMBN3, and GaMBN5, respectively.
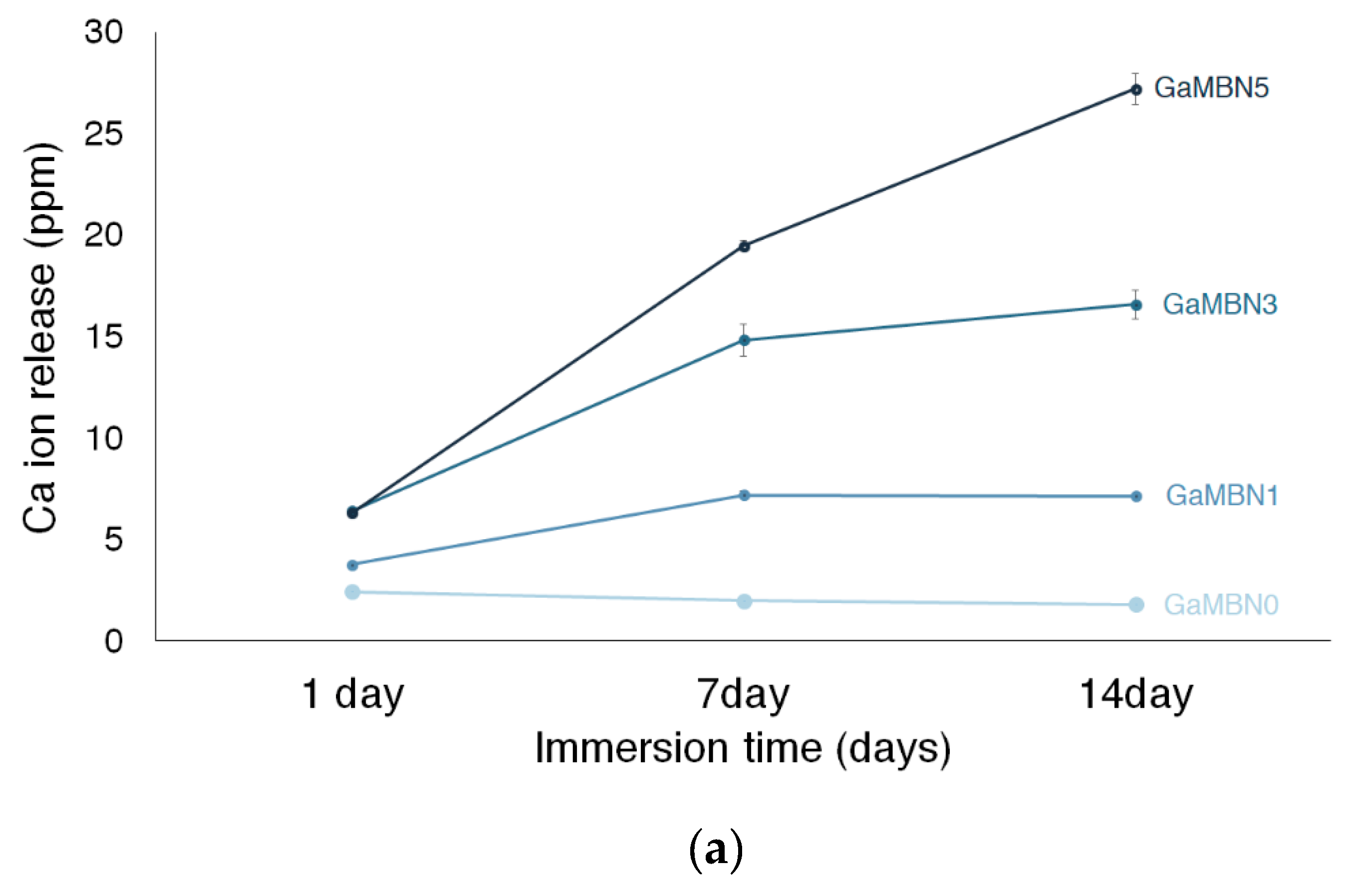
Figure 6 shows the Ca, P, and Ga release of each GaMBN resin. In terms of Ca ion release, more ions were released as the GaMBN concentration of the resin increased. For GaMBN0, the amount of Ca ions released after 1 day (2.4 ± 0.1 ppm), 7 days (2.0 ± 0.1 pp), and 14 days (1.8 ± 0.0 ppm) were similar. The Ca released by the GaMBN1 resin increased over the first 7 days and remained constant over the second 7-day period: 1 day (3.8 ± 0.1 ppm), 7 days (7.2 ± 0.2 ppm), and 14 days (7.1 ± 0.1 ppm). The GaMBN3 resin exhibited a similar trend to GaMBN1 with a higher magnitude of Ca release: 1 day (6.4 ± 0.1 ppm), 7 days (17.8 ± 0.8 ppm), and 14 days (16.6 ± 0.1 ppm). Unlike the other resins, the Ca released by the GaMBN5 resin increased with time: 1 day (6.3 ± 0.2 ppm), 7 days (19.5 ±0.3 ppm), and 14 days (27.2 ± 0.8 ppm).
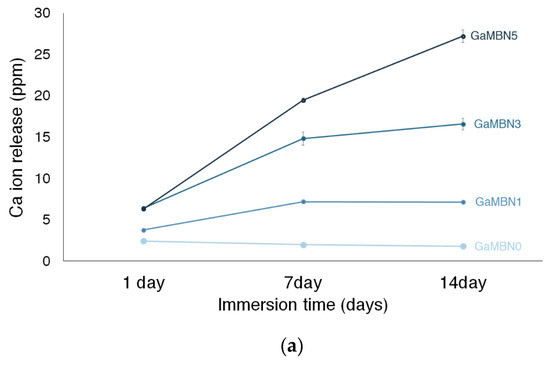
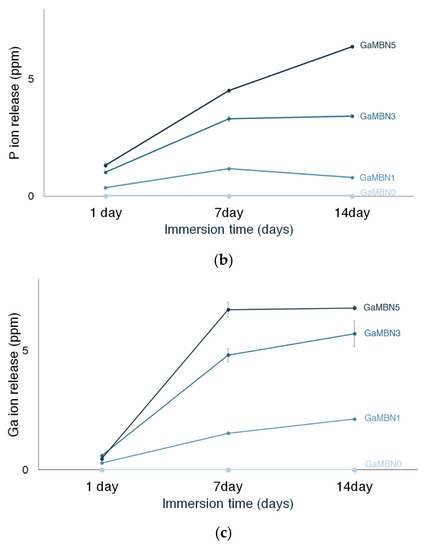
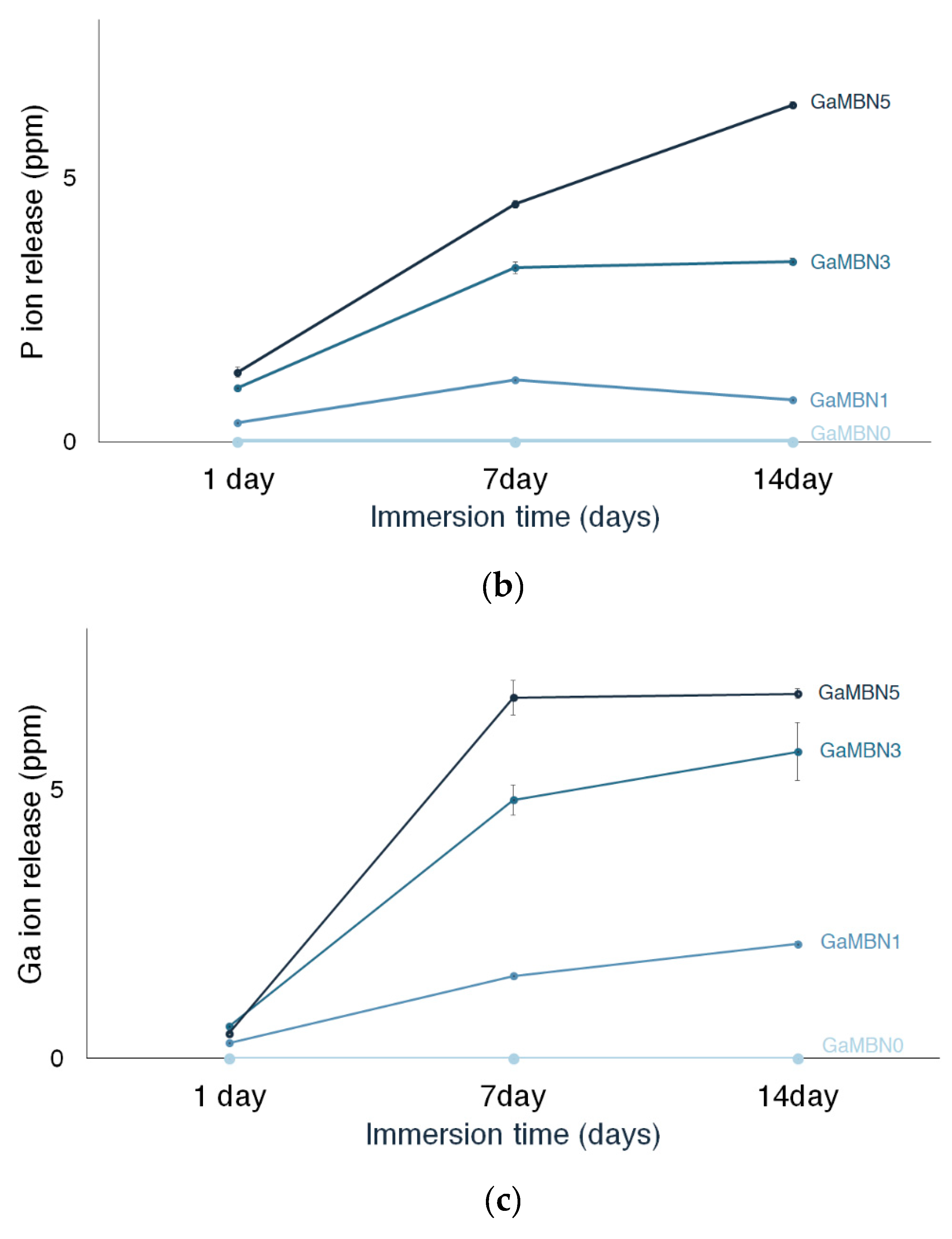
Figure 6.
Concentration of (a) Ca, (b) P, and (c) Ga ions released by the GaMBN resin disks as determined by ICP-OES(Inductively Coupled Plasma Optical Emission Spectrometry) analysis.
The release of P ions also increased with the GaMBN concentration of the resin. No P ions were released by GaMBN0 and the amount of P released by GaMBN1 was relatively consistent: 1 day (0.4 ± 0.0 ppm), 7 days (1.2 ± 0.0 ppm), and 14 days (0.8 ± 0.0 ppm). For GaMBN3, the amount of P released by the resin increased over the first 7 days and remained constant over the second 7-day period: 1 day (1.0 ± 0.0 ppm), 7 days (3.3 ± 0.1 ppm), and 14 days (3.4 ± 0.1 ppm). The P release of GaMBN5 followed the same trend as Ca release and increased with time: 1 day (1.3 ± 0.1 ppm), 7 days (4.5 ± 0.1 pp), and 14 days (6.4 ± 0.1 ppm).
The release of Ga ions also increased with the GaMBN concentration of the resin, and GaMBN0did not release any Ga ions. For GaMBN1, Ga release increased with time: 1 day (0.2 ± 0.0 ppm), 7 days (1.5 ± 0.0 ppm), and 14 days (2.1 ± 0.1 ppm). For GaMBN3, Ga release began to plateau after the first 7 days: 1 day (0.6 ± 0.0 ppm), 7 days (4.8 ± 0.3 pp), and 14 days (5.7 ± 0.5 ppm). The plateau in Ga release was more pronounced for GaMBN5: 1 day (0.5 ± 0.0 ppm), 7 days (6.7 ± 0.3 ppm), and 14 days (6.7 ± 0.1 ppm).
3.3. Vickers Microhardness
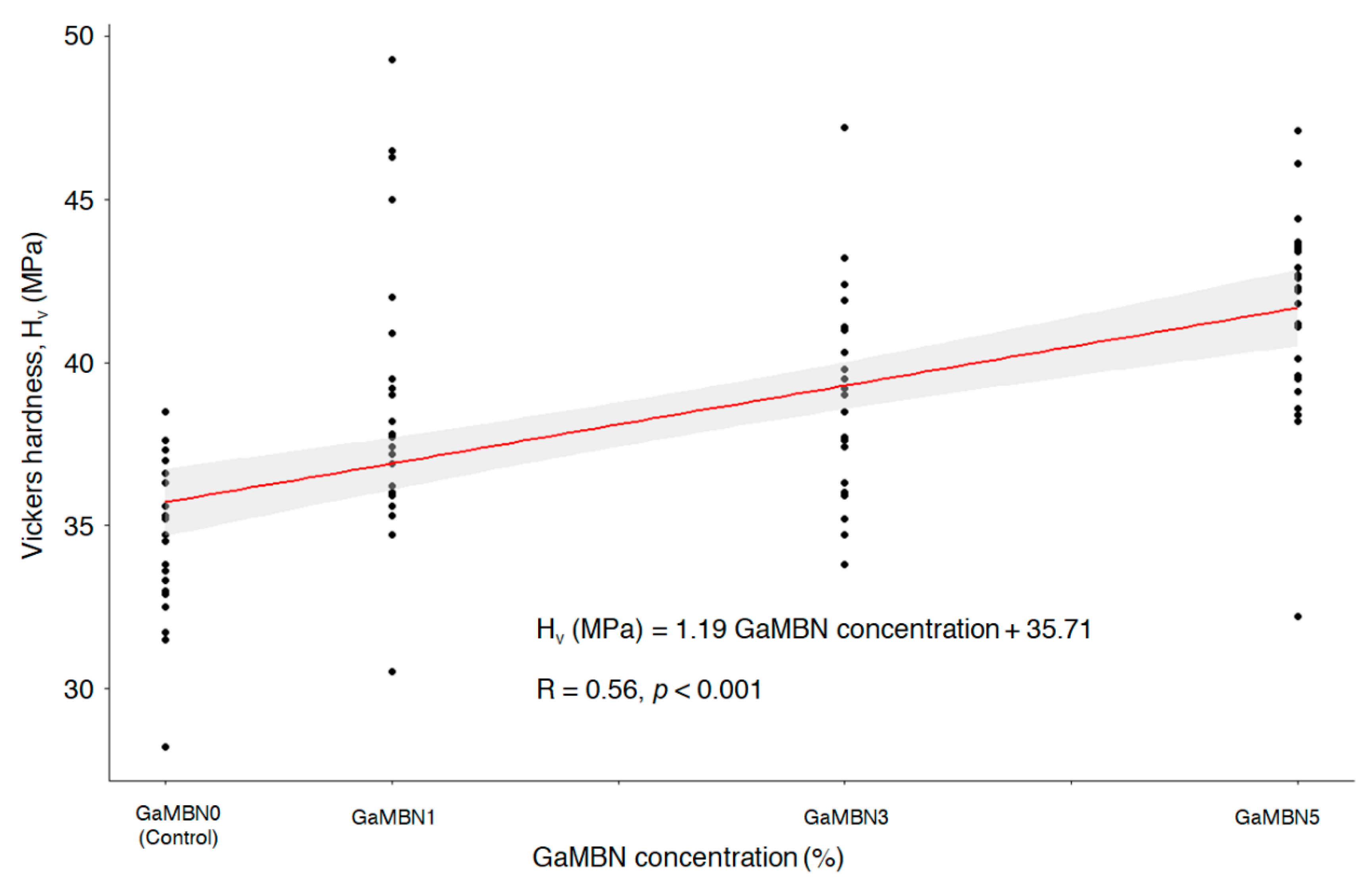
The Vickers microhardness determined for each of GaMBN1, GaMBN3, and GaMBN5 was 38.8 ± 4.2 Hv, 39.0 ± 3.0 Hv, and 41.5 ± 3.1 Hv, respectively. The resins containing Ga exhibited higher microhardness than GaMBN0 (34.3 ± 2.3 Hv) and microhardness increased with GaMBN concentration (R = 0.56, p < 0.001) (Figure 7).
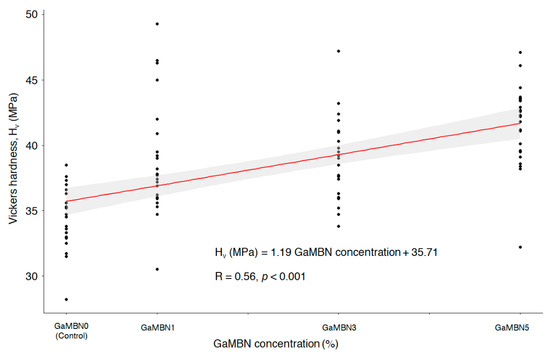
Figure 7.
Correlation between Vickers microhardness and the GaMBN concentration of the resin disk (R = 0.56; p < 0.001).
3.4. Bracket Retention Test
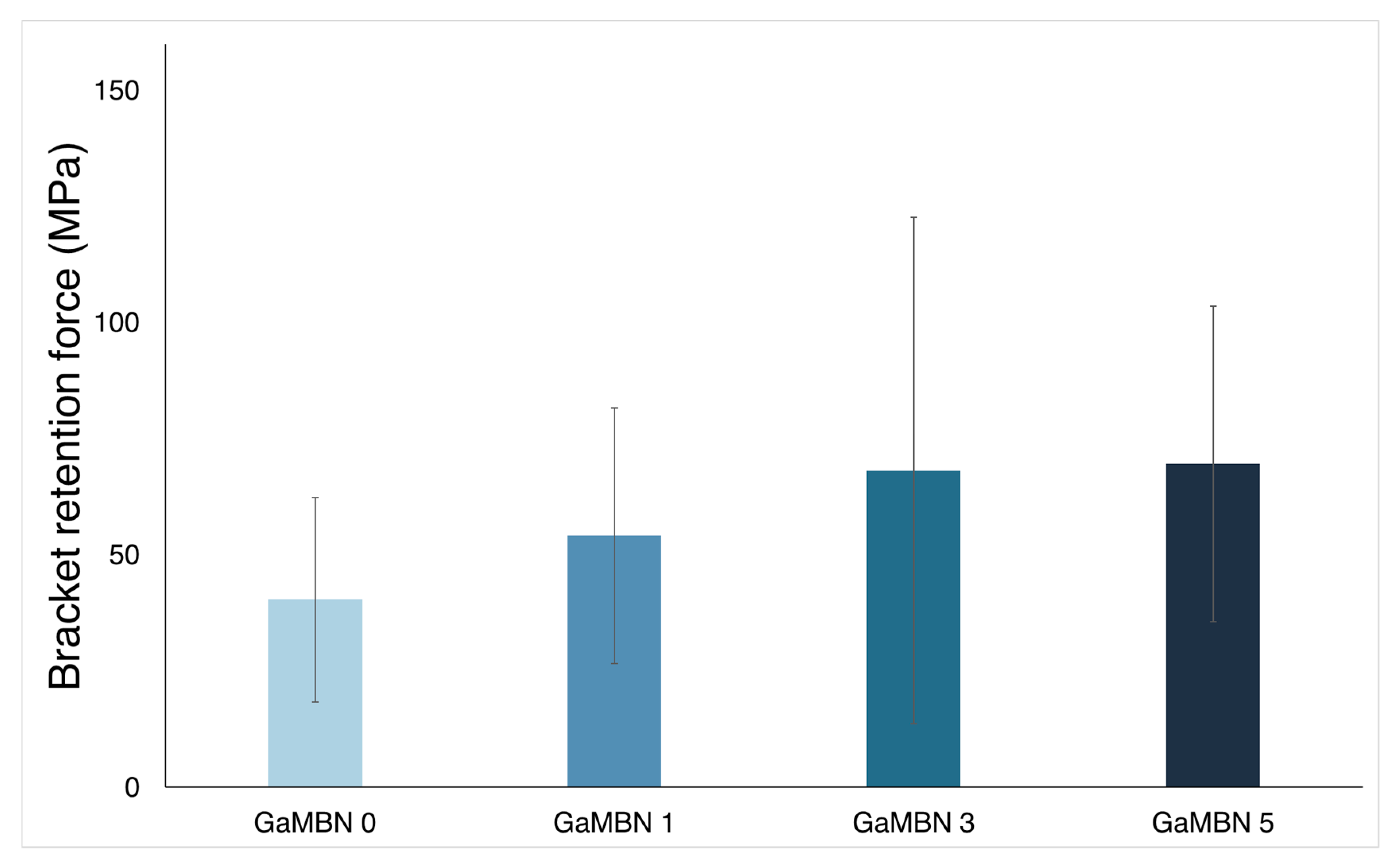
Figure 8 illustrates that the bracket retention test results of GaMBN0 (40.4 ± 22.0 MPa), GaMBN1 (54.1 ± 127.5 MPa), GaMBN3 (68.2 ± 54.4 MPa), and GaMBN55 (69.5 ± 33.9 MPa) were not significantly different (p > 0.05).
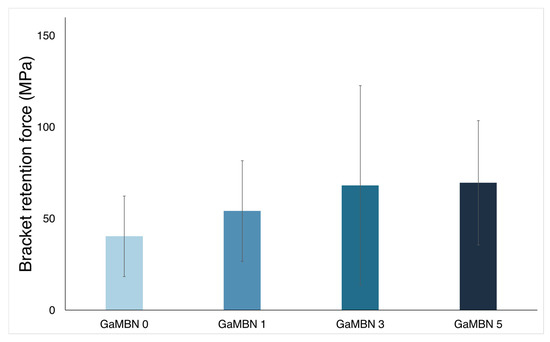
Figure 8.
Bracket retention test results of GaMBN-containing resins. Based on one-way ANOVA (p > 0.01), the results of the four GaMBN resins were not significantly different. Error bars represent the standard error (n = 7).
3.5. Adhesive Remnant Index (ARI)
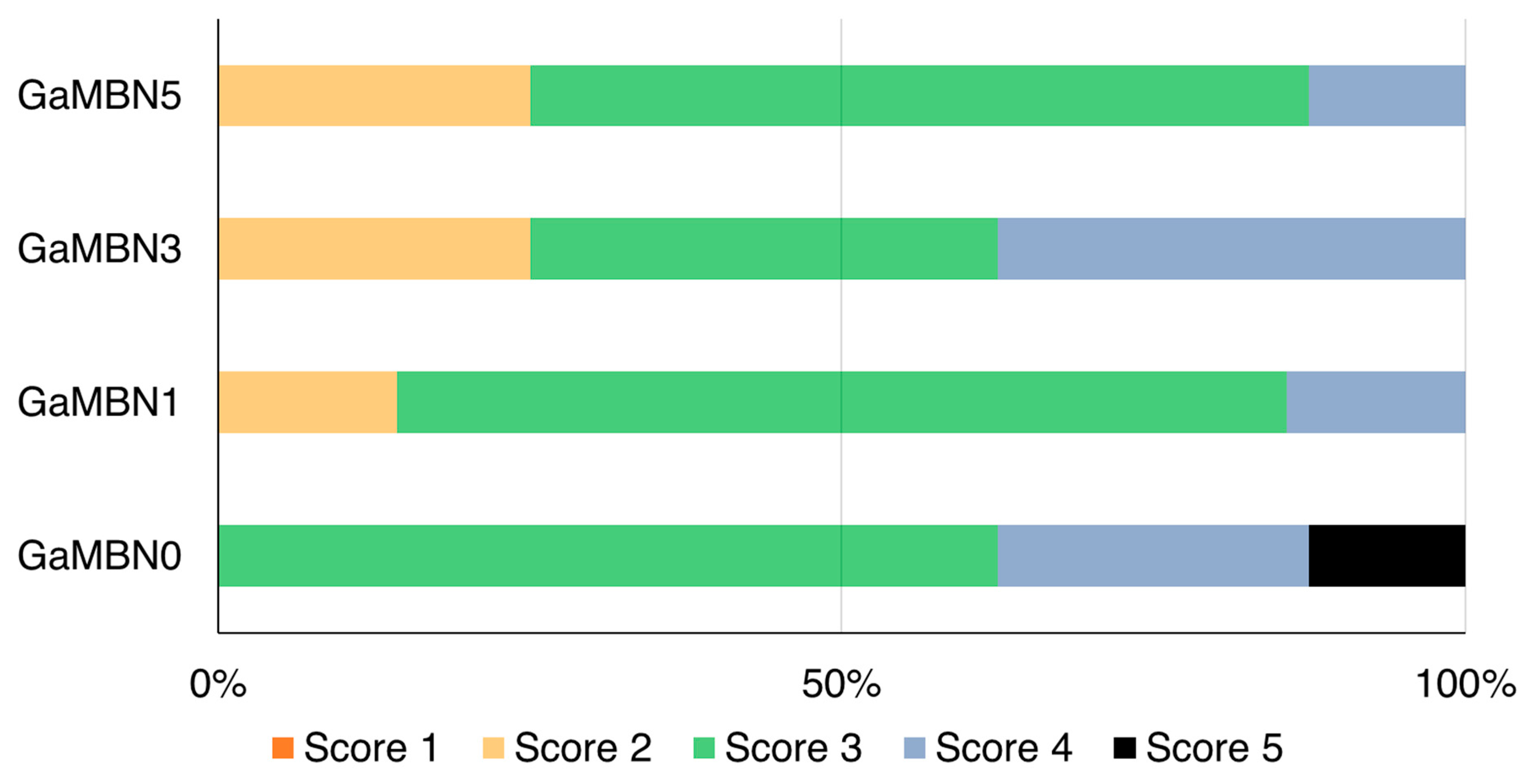
The ARI scores of the four GaMBN resins were not significantly different: GaMBN0 (3.5 ± 0.8), GaMBN1 (3.0 ± 0.5), GaMBN3 (3.1 ± 0.8), and GaMBN5 (2.9 ± 0.6) (Figure 9).
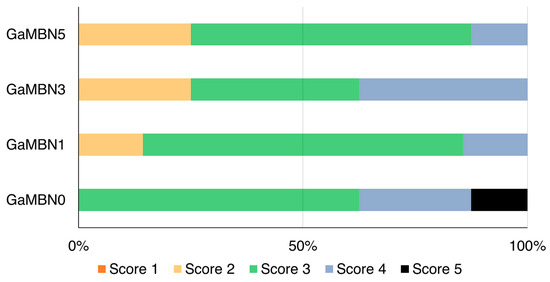
Figure 9.
Adhesive remnant index (ARI) scores of four GaMBN resins used as orthodontic bonding adhesives. Based on the Kruskal–Wallis test at α = 0.05, the ARI scores were not significantly different (n = 8).
3.6. Cell Viability Test
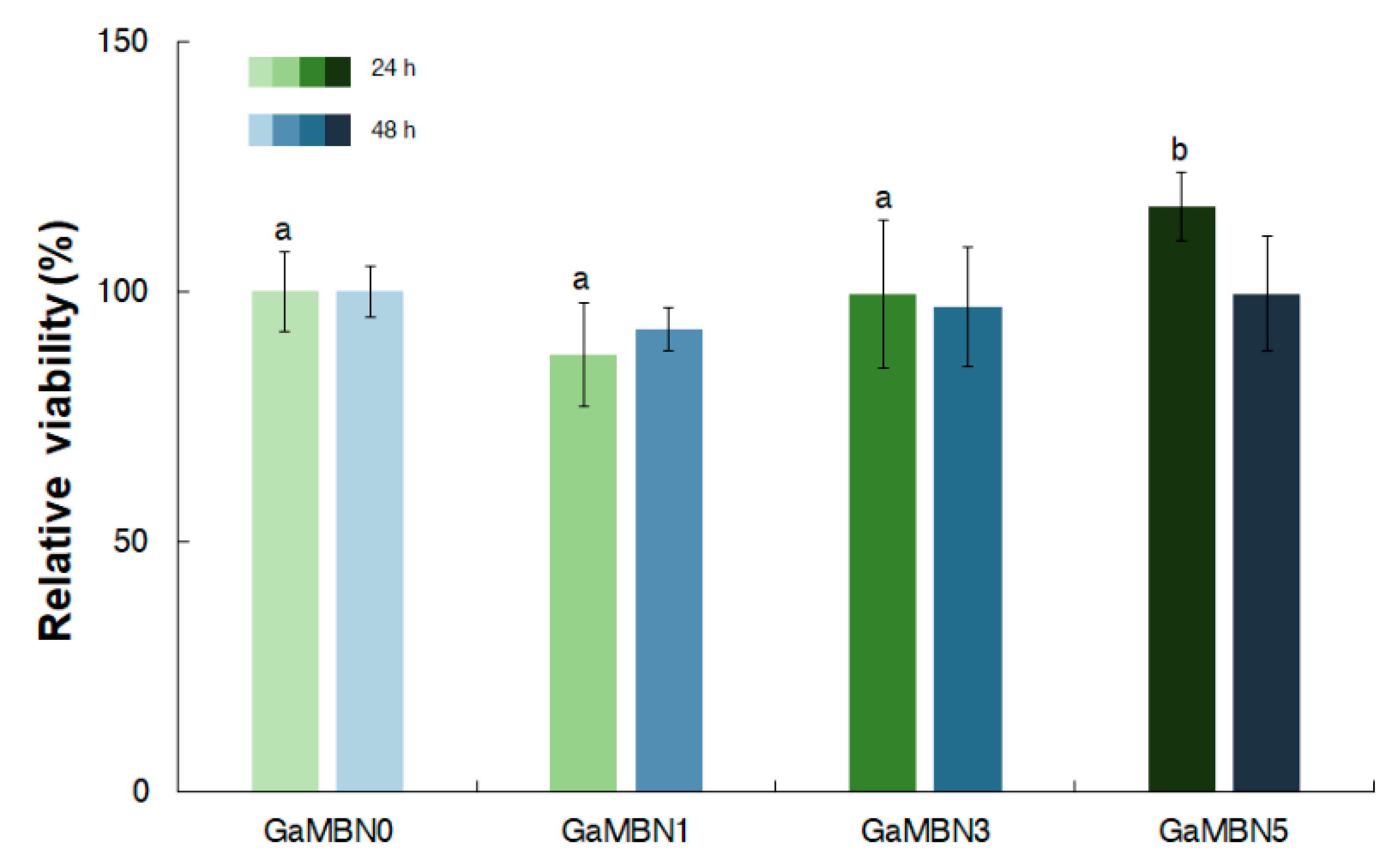
In terms of cell viability after 24 h, GaMBN1 (84.0 ± 10.5%), GaMBN3 (97.9 ± 14.6 %), and GaMBN5 (115.2 ± 6.7 %) did significantly change compared to GaMBN0 (100.0 %). After 48 h, the cell viability of the resins still did not exhibit significant differences: GaMBN1 (92.5 ± 4.3 %), GaMBN3 (97.1 ± 11.9 %), and GaMBN 5 (99.6 ± 11.5 %) (Figure 10).
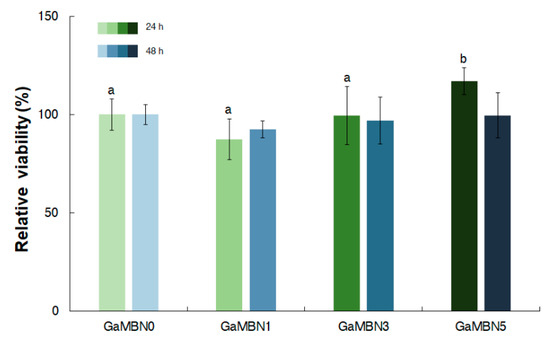
Figure 10.
Cell viability of human dental pulp stem cells (hDPSCs) on GaMBN-resin disks after 24 and 48 h. Based on one-way ANOVA (p > 0.01), the results were not significantly different at 48 h. Error bars represent the standard error (n = 7).
3.7. Antibacterial Properties
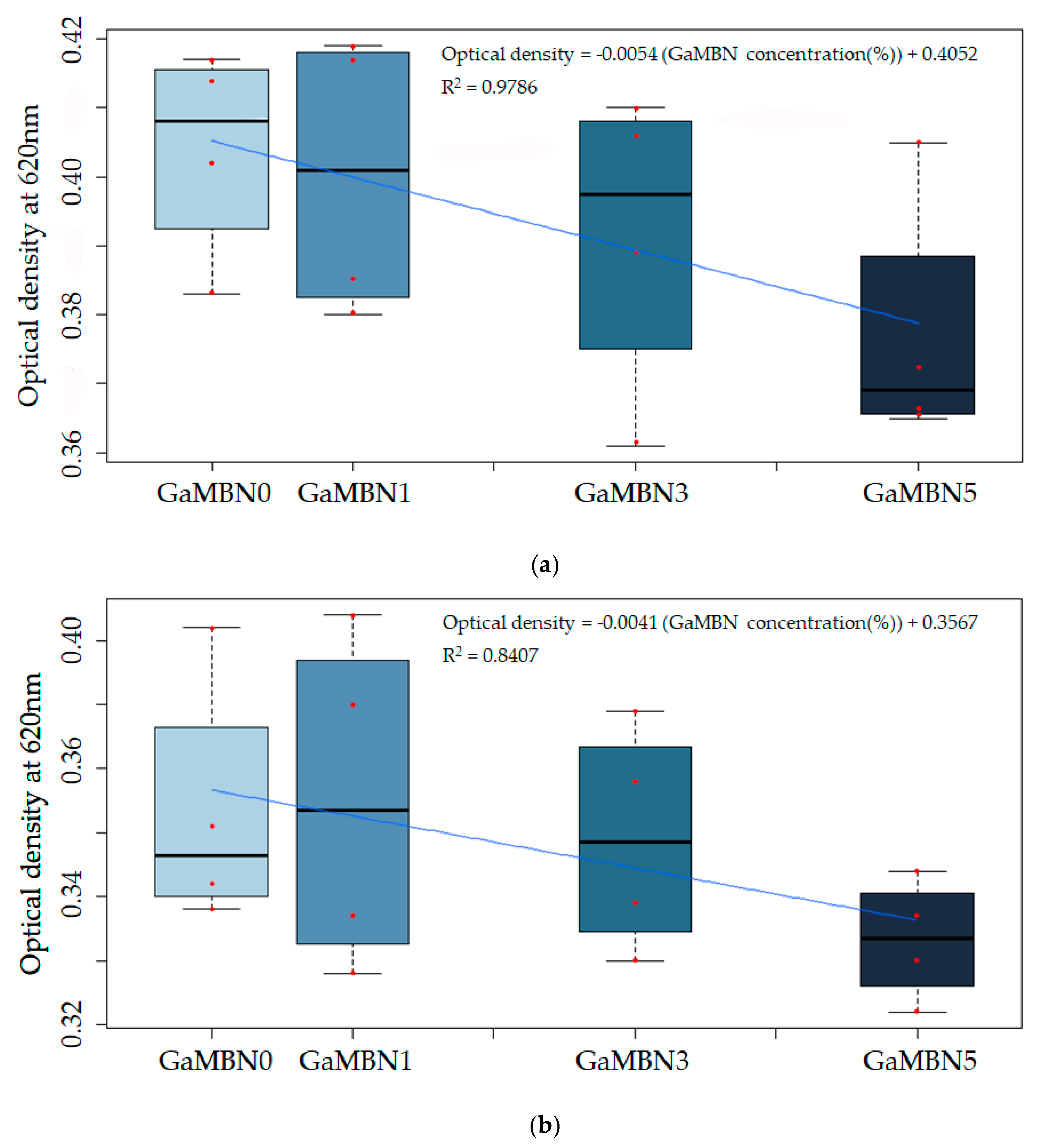
The change in viability was not statistically significant; however, the viability of S. mutans decreased as GaMBN concentration of the resin increased (Figure 11).
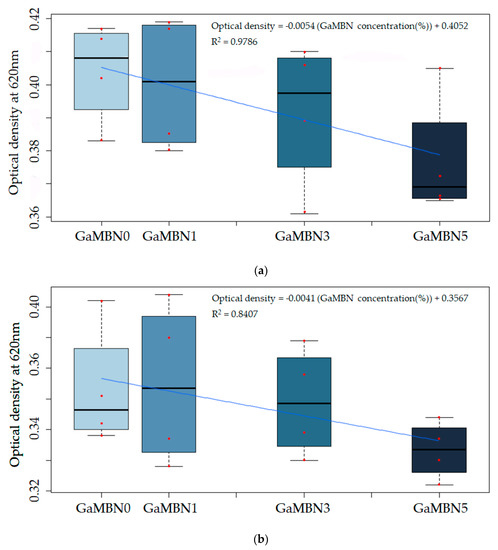
Figure 11.
The antibacterial properties of the cured GaMBN-containing orthodontic bonding pastes after (a) 24 and (b) 48 h were not statistically significantly different from that of GaMBN0 (p < 0.05) based on Duncan’s multiple comparison test. Error bars represent the standard error (n = x). Correlation between optical density and the GaMBN concentration of the resin disk.
3.8. Anti-Demineralization Properties
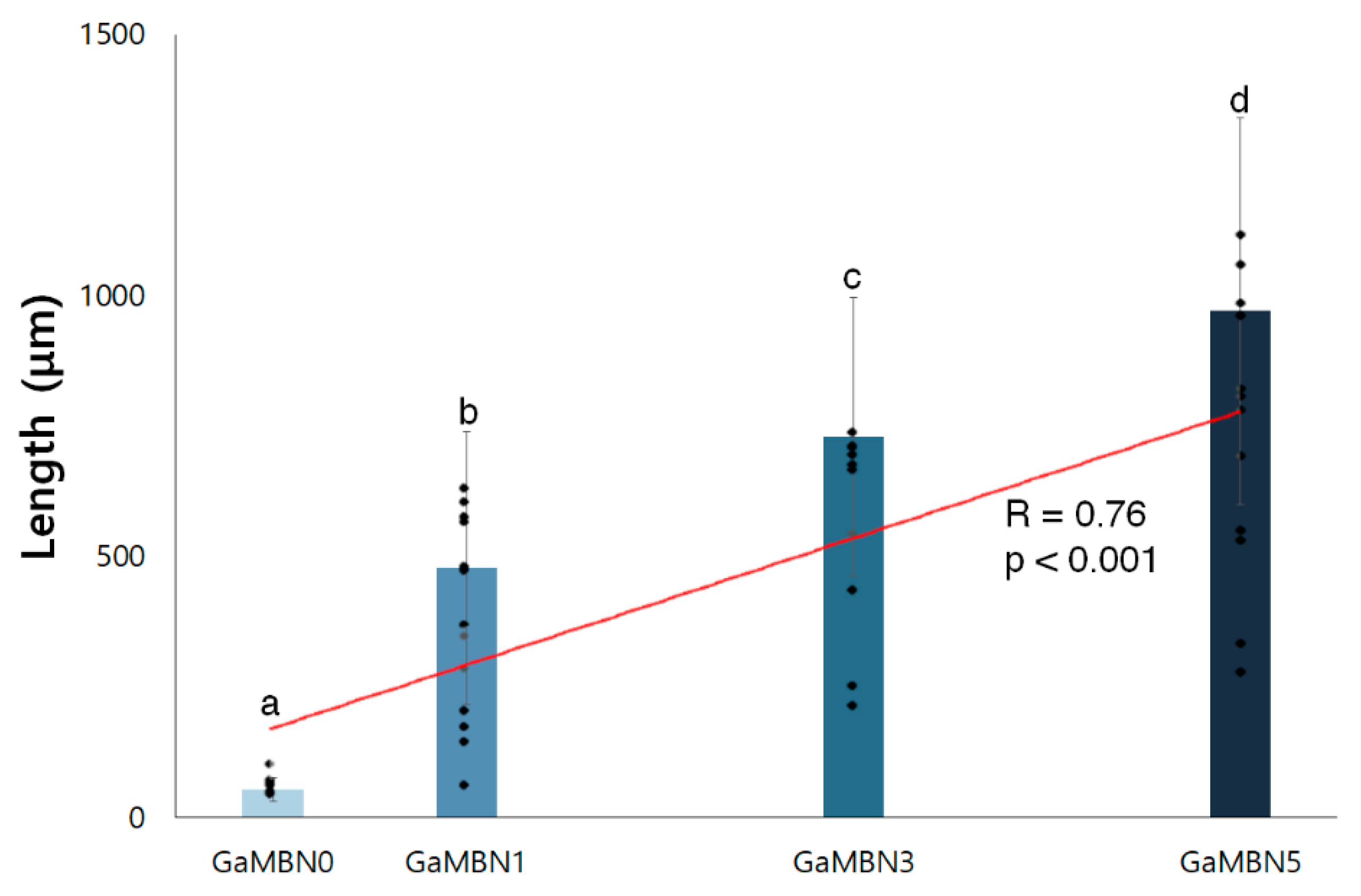
As the concentration of GaMBN increased, the remineralization length increased (R = 0.76, p < 0.001) relative to GaMBN0 (53.7 ± 22.2 μm): GaMBN1 (477.5 ± 260.5 μm), GaMBN3 (728.4 ± 266.8 μm), and GaMBN5 (970.3 ± 370.9 μm) (Figure 12 and Figure 13).
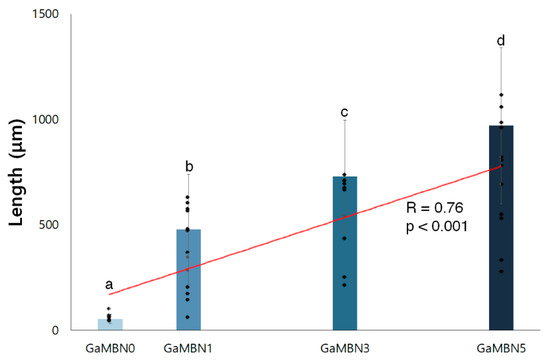
Figure 12.
Remineralization length of GaMBN-containing resins, measured using ImageJ. The same alphabet letter indicates no statistically significant difference between the groups (p < 0.05) by Duncan’s multiple comparison test. Error bars represent the standard deviation (n = 10).
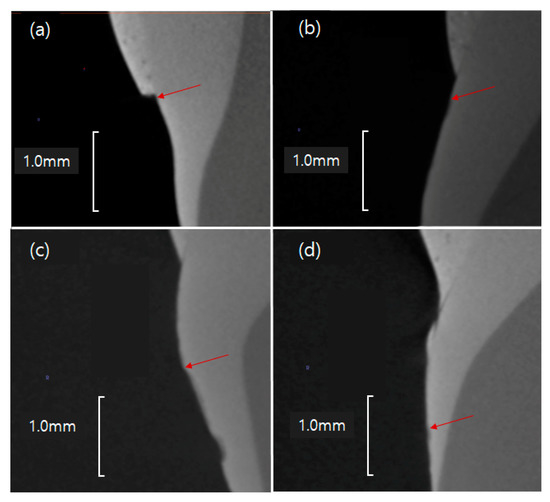
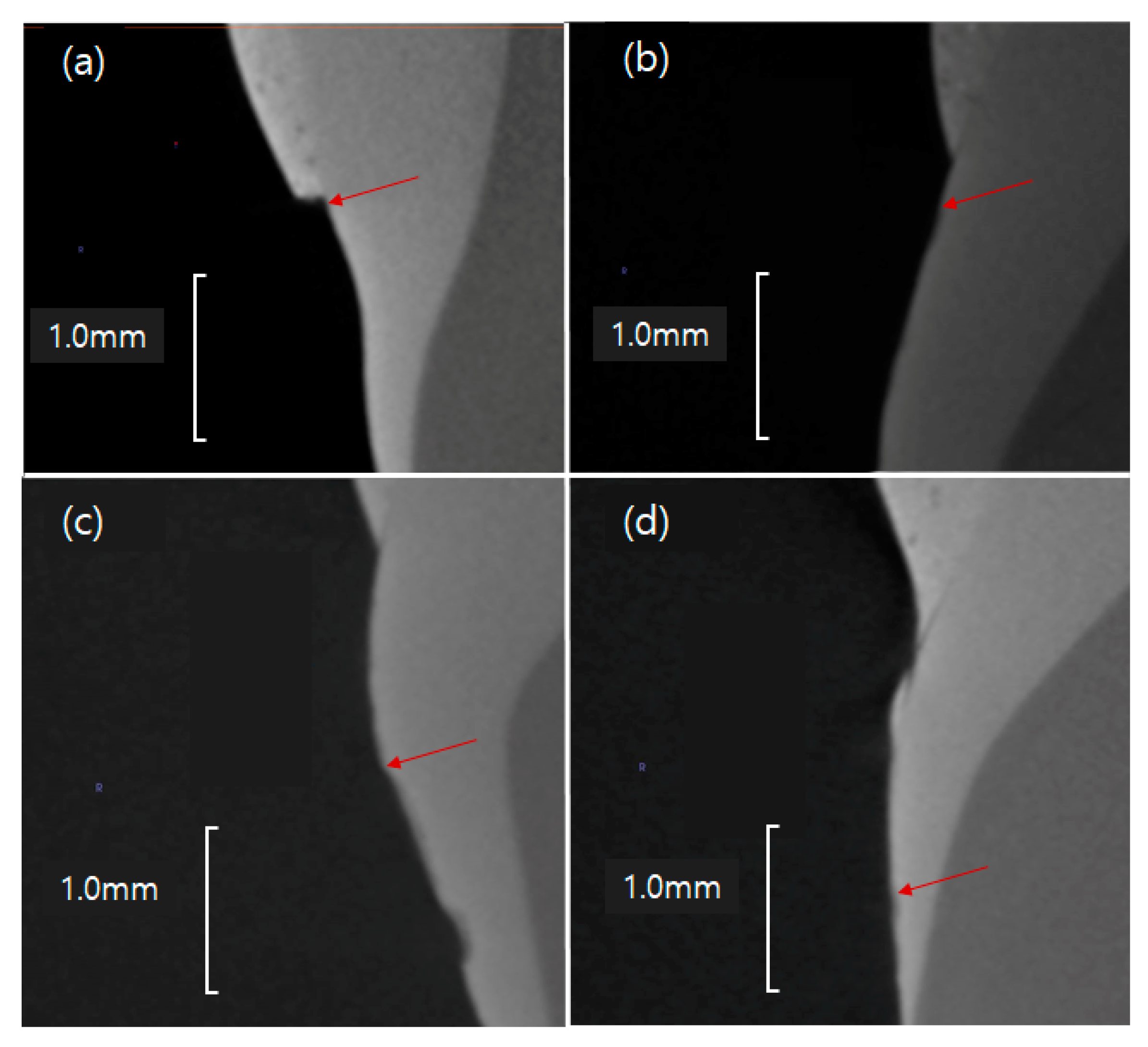
Figure 13.
Remineralization points (red arrows) of the GaMBN-containing resins determined by micro-CT: (a) GaMBN0, (b) GaMB1, (c) GaMBN3, and (d) GaMBN5.
4. Discussion
Orthodontic treatment seeks to enhance occlusal function and improve aesthetic appearance by improving the arrangement of teeth. However, the fixed orthodontic appliances used to shift teeth lead to plaque deposits that cause bacteria to grow on the enamel surface, thereby creating WSLs or caries that contradict the aesthetic purpose of orthodontic treatment [7]. While there are conservative and prosthodontic treatments that can treat WSLs, these methods can damage natural teeth; thus, it is important to prevent WSLs before they occur [4]. Previous studies have developed preventive methods by adding biomaterials to the dental resins used to attach fixed orthodontic appliances [8,9,10,11]. This study added GaMBN to dental resin to investigate whether the antibacterial properties of Ga and remineralization effect of Ca2+ and PO43− can prevent WSLs.
Characterization of the GaMBN-containing resins confirmed that the Ga3+, Ca2+, and PO43− ions formed the same heterogeneous structure as the previous study [18]. Moreover, the N2 adsorption–desorption isotherm results determined that the resins have uniform pore structures (type IV isotherms) as shown in the previous study [17].
Ion release tests demonstrated that ions were barely released by GaMBN0, and that ion release increased with the GaMBN concentration. In particular, the release of Ca2+ ions by GaMBN5 continued to increase after the first 7 days, reaching a solution concentration of 27.2 ppm after 14 days. This result implies that the GaMBN resin can supply Ca ions for remineralization. Although the amount of Ca and PO43− ions released GaMBN1 and GaMBN3 did not change after 7 days, GaMBN5 exhibited the potential to form the ion layer necessary for hydroxyapatite reformation by continuously releasing PO43− ions [19]. In terms of Ga, the amount of ions released by GaMBN5 was initially 0.5 ppm in solution and increased over the first 7 days to 6.7 ppm.
The microhardness of the resins increased with the GaMBN concentration, which is in agreement with a previous study [11]. This increase in microhardness occurs because GaMBN acts as a filler in the resin and its strength increases with its concentration. Moreover, there were no statistically significant differences between the bracket retention force and ARI of the GaMBN-containing resins, which has also been reported by previous studies [8,9,10,11]; therefore, GaMBN-containing resins are not expected to cause clinical problems when maintaining a fixed orthodontic appliance.
Human dental pulp stem cells were used to evaluate the cell viability of the resins, which resulted in stricter cell viability compared to previous studies because stem cells have higher sensitivity than other cells. The cell viability tests also demonstrated that there were no statistical differences between the GaMBN-containing resins and GaMBN0, a commercial product.
The antibacterial test showed that bacterial activity on the GaMBN5 resin decreased relative to GaMBN0 after 24 and 48 h; however, the change was not statistically significant. This reduction in bacterial activity is attributed to the small amount of Ga (0.5 ppm in solution) released after 24 h. Another study has tested the antimicrobial activity of Ga released from Ga-doped MBN, and reported approximately 100 ppm Ga ion elution [13]. Thus, the amount of Ga ions released in this study was too low to result in significant antibacterial activity. Because ion release generally increases with time, it could be used to predict the long-term antibacterial effect of GaMBN-containing resins as orthodontic treatment is a long-term treatment. Therefore, long-term ion release tests of GaMBN-containing resins could be used to develop a guideline for clinical applications [20].
The micro-CT results demonstrated that the remineralization length increased with the GaMBN concentration. The amounts of Ca2+ and PO43– released by GaMBN5 were consistent with the amounts required for remineralization during the 14 days of the pH cycle [8,9,10,11,19].
The greatest advantage of MBN is its pore structure, which makes it a functional material that can be used to transport various genes and drugs. Non-ionic surfactants or cationic surfactants, such as CTAB, can be used to control the pore size of MBN. In this study, CTAB was used in order to produce MBN with high mesopore volume (PV) and surface area(SA) because PV and SA have been reported to provide sufficient space for transporting small chemicals, such as genes or drugs [21,22]. Furthermore, the size of the mesopores is influenced by the hydrolysis of alkyl silicate and can be controlled by adjusting the amount of TEOS [23]. Based on the results of this study, MBN is an efficient anti-demineralizing agent that should be considered for future research aiming to transport Ga or other antibacterial nanomaterials using mesopores of various sizes.
This study of GaMBN-containing resins demonstrated that these resins exhibit the physical and biological properties required for safe clinical use, as well as the ability to prevent demineralization. Future studies should focus on in vivo and clinical research.
5. Conclusions
When added to orthodontic bonding resin, GaMBN exhibits the physical and biological properties required for safe clinical use. The 5%-GaMBN-containing resin presented in this study releases the Ca2+ and PO43– ions needed for remineralization and has demonstrated in vitro remineralization. Based on the results of this study, resins containing GaMBN have the potential to prevent the formation of WSLs during orthodontic treatment.
Author Contributions
Conceptualization, H.-K.S., S.-M.L., and Y.-I.K.; Methodology, H.-K.S., K.-H.Y., S.-Y.Y. and H.S.N., J.C.; Software, Validation, H.-K.S., S.-M.L., and Y.-I.K.; Formal Analysis. H.-K.S., K.-H.Y., S.-Y.Y. and H.S.N., J.C.; Writing—Original Draft Preparation, H.-K.S., S.-M.L., W.-S.S. and Y.-I.K.; Writing—Review and Editing, H.-K.S., K.-H.Y., S.-Y.Y., W.-S.S., H.S.N., J.C., S.-M.L., and Y.-I.K.; Visualize; Supervision, H.-S.N., S.-M.L., and W.-S.S.; Project Administration, S.-M.L.; Funding Acquisition, Y.-I.K.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (2018R1D1A1B07042098).
Acknowledgments
In this section you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
Declare conflicts of interest or state “The authors declare no conflict of interest.” Authors must identify and declare any personal circumstances or interest that may be perceived as inappropriately influencing the representation or interpretation of reported research results. Any role of the funders in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results must be declared in this section. If there is no role, please state “The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results”.
References
- Lovrov, S.; Hertrich, K.; Hirschfelder, U. Enamel demineralization during fixed orthodontic treatment − Incidence and correlation to various oral-hygiene parameters. J. Orofac. Orthop. 2007, 68, 353–363. [Google Scholar] [CrossRef]
- Bourbia, M.; Ma, D.; Cvitkovitch, D.G.; Santerre, J.P.; Finer, Y. Cariogenic bacteria degrade dental resin composites and adhesives. J. Dent. Res. 2013, 92, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Baka, Z.M.; Basciftci, F.A.; Arslan, U. Effects of 2 bracket and ligation types on plaque retention: a quantitative microbiologic analysis with real-time polymerase chain reaction. Am. J. Orthod. Dentofacial Orthop. 2013, 144, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.; Stewart, S.; Su, B.; Sandy, J.; Ireland, A. Prevention and treatment of demineralisation during fixed appliance therapy: a review of current methods and future applications. Br. Dent. J. 2013, 215, 505–511. [Google Scholar] [CrossRef]
- Lucchese, A.; Bondemark, L.; Marcolina, M.; Manuelli, M. Changes in oral microbiota due to orthodontic appliances: a systematic review. J. Oral Microbiol. 2018, 10, 1476645. [Google Scholar] [CrossRef] [PubMed]
- Bishara, SE, Ostby, AW, White Spot Lesions: Formation, Prevention, and Treatment. Semin Orthod. 2008, 14, 174–182. [CrossRef]
- Khalaf, K. Factors Affecting the Formation, Severity and Location of White Spot Lesions during Orthodontic Treatment with Fixed Appliances. J. Oral Maxillofac. Res. 2014, 5, e4. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kim, I.-R.; Park, B.-S.; Lee, D.J.; Ko, C.-C.; Son, W.-S.; Kim, Y.-I. Remineralization Property of an Orthodontic Primer Containing a Bioactive Glass with Silver and Zinc. Materials (Basel) 2017, 10, 1253. [Google Scholar] [CrossRef]
- Lee, S.-M.; Yoo, K.-H.; Yoon, S.-Y.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Ko, C.-C.; Son, S.-A.; Kim, Y.-I. Enamel Anti-Demineralization Effect of Orthodontic Adhesive Containing Bioactive Glass and Graphene Oxide: An In-Vitro Study. Materials (Basel) 2018, 11, 1728. [Google Scholar] [CrossRef]
- Nam, H.-J.; Kim, Y.-M.; Kwon, Y.H.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Lee, S.-M.; Kim, Y.-I. Enamel Surface Remineralization Effect by Fluorinated Graphite and Bioactive Glass-Containing Orthodontic Bonding Resin. Materials (Basel) 2019, 12, 1308. [Google Scholar] [CrossRef]
- Nam, H.-J.; Kim, Y.-M.; Kwon, Y.H.; Yoo, K.-H.; Yoon, S.-Y.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Lee, S.-M.; Kim, Y.-I. Fluorinated Bioactive Glass Nanoparticles: Enamel Demineralization Prevention and Antibacterial Effect of Orthodontic Bonding Resin. Materials (Basel) 2019, 12, 1813. [Google Scholar] [CrossRef] [PubMed]
- Kelson, A.B.; Carnevali, M.; Truong-Le, V. Gallium-based anti-infectives: targeting microbial iron-uptake mechanisms. Curr. Opin. Pharmacol. 2013, 13, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Valappil, S.P.; Owens, G.J.; Miles, E.J.; Farmer, N.L.; Cooper, L.; Miller, G.; Clowes, R.; Lynch, R.J.; Higham, S.M. Effect of gallium on growth of Streptococcus mutans NCTC 10449 and dental tissues. Caries Res. 2014, 48, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.C. Calcium phosphate-based remineralization systems: scientific evidence? Aust. Dent. J. 2008, 53, 268–273. [Google Scholar] [CrossRef]
- Andersson, O.H.; Kangasniemi, I. Calcium phosphate formation at the surface of bioactive glass in vitro. J. Biomed. Mater. Res. 1991, 25, 1019–1030. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef]
- Bae, J.; Son, W.-S.; Yoo, K.-H.; Yoon, S.-Y.; Bae, M.-K.; Lee, D.J.; Ko, C.-C.; Choi, Y.-K.; Kim, Y.-I. Effects of Poly(Amidoamine) Dendrimer-Coated Mesoporous Bioactive Glass Nanoparticles on Dentin Remineralization. Nanomaterials (Basel) 2019, 9, 591. [Google Scholar] [CrossRef]
- Shruti, S.; Salinas, A.J.; Malavasi, G.; Lusvardi, G.; Menabue, L.; Ferrara, C.; Mustarelli, P.; Vallet-Regì, M. Structural and in vitro study of cerium, gallium and zinc containing sol–gel bioactive glasses. J. Mater. Chem. 2012, 22, 13698–13706. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Kim, D.-H.; Song, C.W.; Yoon, S.-Y.; Kim, S.-Y.; Na, H.S.; Chung, J.; Kim, Y.-I.; Kwon, Y.H. Antibacterial and remineralization effects of orthodontic bonding agents containing bioactive glass. Korean J. Orthod. 2018, 48, 163–171. [Google Scholar] [CrossRef]
- Turkun, L.S.; Turkun, M.; Ertugru, l.F.; Ates, M.; Brugger, S. Long-term antibacterial effects and physical properties of a chlorhexidine-containing glass ionomer cement. J. Esthet. Restor. Dent. 2008, 20, 29–44. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, T.-H.; Kim, M.; Eltohamy, M.; Won, J.-E.; Lee, E.-J.; Kim, H.-W. Capacity of mesoporous bioactive glass nanoparticles to deliver therapeutic molecules. Nanoscale 2012, 4, 7475–7488. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Hua, Z.; Shi, J. One-pot synthesis of magnetic and mesoporous bioactive glass composites and their sustained drug release property. Acta Mater. 2008, 56, 3260–3265. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).