1. Introduction
Congenital anomalies of the kidney and urinary tract (CAKUT) are the most common congenital malformations, with a frequency of 3–6 per 1000 live births. They include: isolated kidney malformations (agenesis, hypo-dysplasia, multicystic renal disease, ureteropelvic junction obstruction) and urinary tract malformations (megaureters, megacystis, posterior urethral valve (PUV), urethral atresia/obstruction, urogenital sinus and cloacal malformations, obstructive ureterocele). CAKUT have also been reported in Prune-Belly syndrome (PBS) and associated genetic syndromes, mainly 13, 18 and 21 trisomy [
1].
The fetal bladder may be viewed and evaluated by ultrasound from the 12th gestational week, as a pelvic, oval, anechogen structure less than 6 mm of sagittal diameter.
An increased sagittal diameter of the fetal bladder has been considered as megacystis, regardless of etiologic factors and macroscopic features. Therefore, prenatal megacystis may be considered mainly an ultrasound diagnosis [
1,
2].
We aimed to evaluate the structural and histological differences between two megacystis diagnosed by routine prenatal ultrasound screening in the Obstetrical and Gynecological Clinic, University of Catania, Italy, and examined in the Pathologic Anatomy Section of G.F. Ingrassia Department, University of Catania.
2. Materials and Methods
Two fetuses with urinary tract malformations were examined in the Pathologic Anatomy Section of the G.F. Ingrassia Department, University of Catania, Italy. The study was conducted in accordance with with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Catania 1 (48102 of 7 November, according to national legislation about osservational studies, 20 March 2008, AIFA).
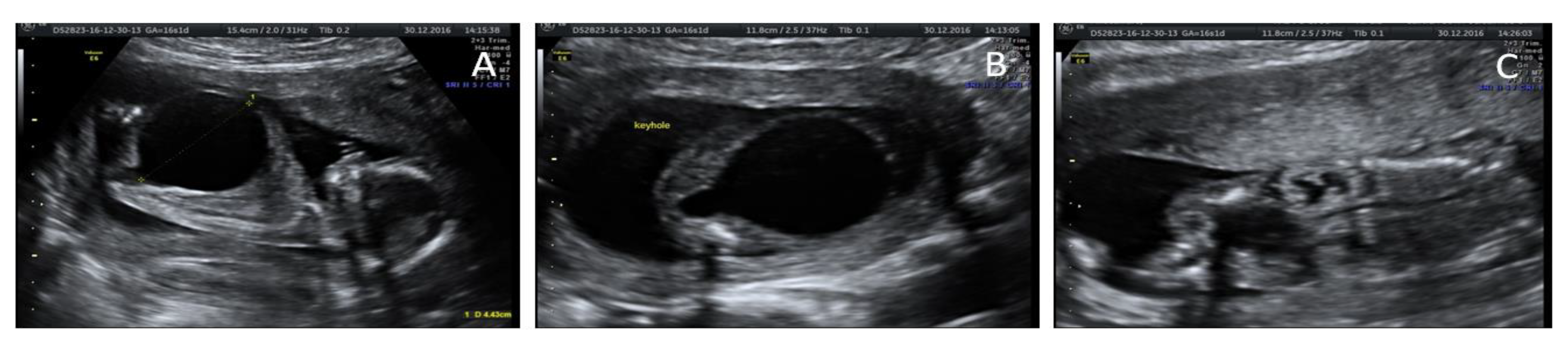
Case 1: 19th week termination of pregnancy (TOP); male, 330 gr; total length 24 cm; crown−rump (CR) length 15.3 cm; medial length of foot 3 cm; cranial circumference 15.5 cm; thoracic circumference 13 cm; abdominal circumference 11 cm. Ruby red skin; perforated orifices. Umbilical cord stump 10 × 0.7 cm; edema of penis. The anatomic relationship of thoracic organs was normal: heart in situs solitus with atrium−ventricular and ventricular−vascular concordance and lung development coherent with gestational age. Examination of the abdominal cavity showed: megacystis (25 mm sagittal diameter) with thickened wall (6 mm), megaureter with stenosis of ureteral orifices in the bladder, and bilateral hydronephrotic kidneys (both 18 mm maximum diameter).
Ecographic diagnosis: urinary obstruction syndrome due to posterior urethral valve (PUV). The US (ultrasonographic) examination showed a megacystis with a maximum diameter of 44 mm and mild hydroureteronephrosis with bright hyperechogenic kidneys. The bladder had the so-called “key hole sign”, suggesting a possible PUV. Fetal biometry and amniotic fluid were appropriate for gestational age (GA). No other major abnormalities were detected (
Figure 1).
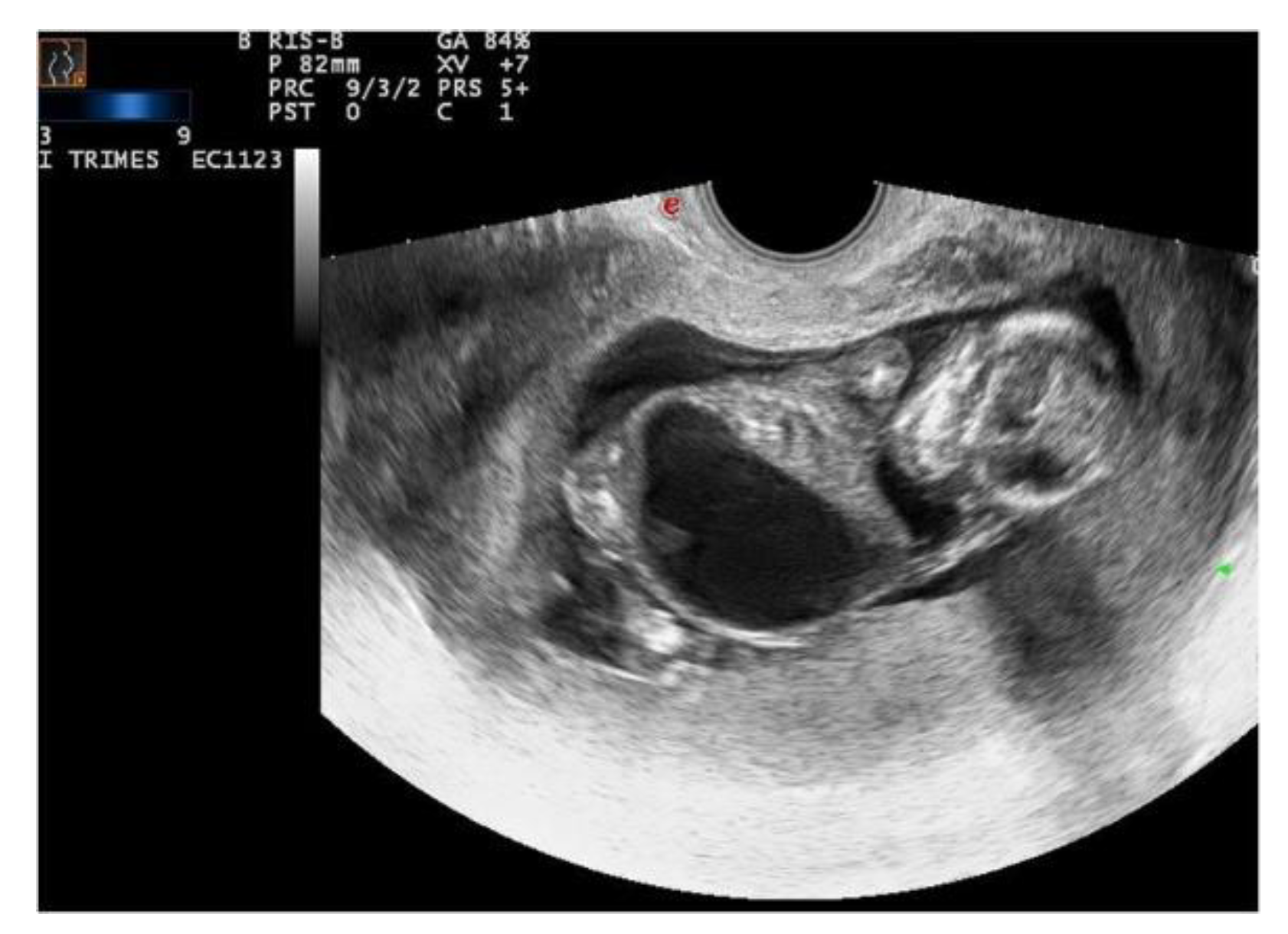
Case 2: 15th week TOP; XXY karyotype; 40 gr; total length 11 cm; crown−rump (CR) length 8.5 cm; medial length of foot 1.5 cm; cranial circumference 7 cm; thoracic circumference 6 cm; abdominal circumference 7 cm. Ruby red skin; imperforated orifices. Turricephal and anencephal skull, prognathism, prominent ocular bulbs and low implant of the ears. The abdominal wall was flaccid due to the incomplete development of the diaphragm and ribs. The abdominal organs were herniated in the thoracic cavity and in the neck across the skin. Megacystis (65 mm sagittal diameter) with a thinned wall (2 mm) occupied the abdominal and thoracic cavities; both kidneys and surrenal glands were underdeveloped (both with a maximum diameter of 10 mm).
Ecographic diagnosis: Prune-Belly syndrome, PBS. The ultrasonographic (US) examination was very difficult due to maternal BMI > 35 and the anhydramnios—absence of amniotic fluid. The fetus showed a megacystis with a maximum diameter of 38 mm. The whole fetal body had a very uncommon posture and it was impossible to assess other fetal structures because of the anhydramnios (
Figure 2).
The organs of both fetuses were carefully dissected, separated from each other, fixed in 10% buffered formalin and processed until paraffin embedding. 5 µm sections were stained with hematoxylin-eosin (EE). Additional immunohistochemical stainings with smooth muscular actin (SMA, Clone 1A4, Dako, Glostrup, Denmark, Dil: 1:100), S100 protein (polyclonal, Dako, Glostrup, Denmark, Dil. 1:100) and WT1c (clone WT 6F-H2, Dako, Glostrup, Denmark, prediluted) were performed on bladder and bowel slides. Bladder and bowel samples from a fetus of XXI gestational age were considered as a normal control.
4. Discussion
The kidney and urinary tract develops from two different embryonal sheets: kidneys, ureters and bladder trigone from the mesodermal sheet, and bladder and urethra from the endodermal sheet. The bladder develops from the fourth to seventh week of gestational age from the urogenital sinus. At the ninth week, after the involution of cloaca, the urogenital sinus opens into the amniotic cavity. CAKUT represent up to 20%–30% of all major congenital pre and postnatal defects [
3], including both sporadic and familial cases and associated anomalies, such as syndromic malformations.
Among all CAKUT, our paper focuses on mainly bladder and urethral malformations. Isolated bladder anomalies, such as bladder agenesis, complete/incomplete duplication or bladder extrophy, are rare. Instead, morphologic bladder anomalies, known as megacystis, are more frequent and related to urethral or neuromuscular anomalies.
Fetal bladder is defined as megacystis if its longitudinal diameter is >10% of crown−rump length in different gestational ages. In the first trimester, the longitudinal diameter may be more than 6 mm.
Prenatal detection of a larger bladder could suggest an outlet obstruction, mainly due to urethral obstruction, but also other congenital complex malformations of the kidney and urinary tract. Moreover, in the case of megacystis, microcolon syndrome as well as neuro-muscular malformations should be considered.
PUV has been considered the most common cause of urethral obstruction in newborn males, while obstruction of the anterior urethral valve is less common and its complications are less severe than PUV.
Three types of PUV have been described: type 1, the most common (95%), with two mucosal folds from the bottom of veromontanum to the membranous urethra; type 2, with mucosal folds extending along posterolateral urethral wall from the ureteric orifice to the veromontanum; type 3, with a circular diaphragm with a central opening in the membranous urethra. In any type of urethral obstruction, the high intravesical pressure leads to defects in the muscular differentiation, with fibrotic tissue interposed among muscular fibers and a thickened bladder wall [
1,
2].
PBS occurs mainly in baby or infant males (97%) and is characterized by atrophy of the anterior abdominal wall due to muscular absence, anomalies in the urinary tract, such as megaureters and bilateral hydronephrosis, and testicular agenesis or cryptorchidism. More often, the bladder shows thickened walls with dilated ureteral orifices in the vesical trigone and vesicoureteral reflux. However, bladder histology is variable, showing both increased and decreased muscular fibers, with or without interposed connective tissue. In a previous study, Volmar et al. [
4] described PBS with increased muscular thickness of the bladder in a case of intravesical obstruction, while a more recent work [
5] reported PBS with decreased muscular thickness of the bladder in a case of urethral obstruction. In the latter case, the higher intravesical pressure, together with atrophy of the abdominal wall, could cause decreased muscular thickness, increased fibroblastic activity and overproduction of collagen type I, inhibiting muscular contractility and electrical impulse diffusion through the muscular layers.
Megacystis/megaureter syndrome is characterized by megacystis with thinned walls, vesicoureteral reflux, bilateral hydroureters and hydronephrosis and, often, dysplastic kidneys. It is frequently associated with microcolon and functional obstruction of the gastrointestinal and urinary tracts [
6].
However, gestational age and vesical longitudinal diameter may be considered the main prognostic factors: it has been reported [
2] that mild megacystis (8–12 mm) in early pregnancy (10–14 weeks) could spontaneously resolve, while severe megacystis (>17 mm) had a poor prognosis at any gestational age. Moreover, the ultrasound “keyhole sign”, which is more frequently but not exclusively linked to PUV, has been considered the only important discriminant criteria. Some surgical therapies have been suggested to treat fetal megacystis, including amnioinfusion, vescicoamniotic shunting and vescicocentesis, but their outcome has been considered uncertain because fetal megacystis is often associated with other adverse prognostic factors.
Our two cases showed different gestational age (19 and 15 weeks), severe megacystis (25 mm and 85 mm) and, above all, different development of the muscular layers (thickened and thinned muscular layers).
Due to the heterogeneity in the definition of megacystis, in bladder development evaluation, and in future outcomes, we proposed to evaluate the histological differences and potential relationship with enteric nervous system development.
The immunohistochemical expression of S100 protein, SMA and WT1c was assessed and their expression in the bladder and in the small and large bowel from normal fetus and fetuses with megacystis were compared.
Comparing normal bladder with the bladder in PUV, SMA staining highlighted a disarray of muscular fibers and increased embryonal mesenchyme, due to the higher intravesical pressure, while in PBS, it showed thinned muscular layers with only longitudinal fibers and poor interposed embryonal mesenchyme (
Figure 9).
To our knowledge, for the first time, the distribution of neural components among muscular fibers using S100 protein and WT1c has been assessed in the bladder [
7].
In the normal bladder, S100 protein showed small ganglionic plexuses running parallel to the muscular fibers. In PUV, S100 staining showed small and giant hyperplastic ganglionic structures running parallel to the muscular fibers, while in PBS, S100 was negative (
Figure 10).
As concerns WT1c expression, normal bladder and PUV showed poor positivity in ganglion cells, in neural cells and in their cytoplasmic extensions, while WT1c was negative in PBS (
Figure 11).
Considering the relationship between the enteric nervous system and urinary tract development [
6], SMA, S100 and WT1c expressions were also assessed in the enteric wall of the fetuses in order to to prove or deny their relationship and, above all, the pre or postnatal outcome as well as life expectancy.
In the normal fetus and PUV, SMA showed a double muscular layer and muscularis mucosae with interposed embryonal mesenchyme; in PBS, a single thickened muscular layer and disrupted muscularis mucosae are shown (
Figure 12).
S100 and WT1c expression are shown in myenteric and submucosal plexuses in the normal bowel and in PUV; both antibodies were not expressed in PBS, proving the same neuromuscular defect in the bladder and bowel (
Figure 13 and
Figure 14).
Recent literature reports interesting data about the relationship between the intrinsic enteric nervous system (ENS) and the central nervous system, communicating through the gut–brain axis. Both systems develop from the neural crest progenitor and are regulated by the interactions among enteric neurons, glia and enteric-endocrine cells [
8]. We hypothesized the same embryologic nature in enteric and vesical neural plexuses. Therefore, S100 and WT1c expression were evaluated in bladder and bowel muscular layers. Both markers were not expressed in the bladder and bowel of PBS associated with anencephaly, confirming a close relationship between encephalic and peripheral neural development.
In conclusion, megacystis could be considered only a macroscopic definition, concerning the size of the fetal bladder rather than the embryologic origin [
9]. A larger bladder may be a single malformation or multiple malformations associated with the bowel and/or encephalic malformations, which decide the outcome and prognosis in fetal megacystis.