Optimization of Triterpene Saponins Mixture with Antiproliferative Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Cell Culture
2.2.2. In Vitro Cytotoxicity Testing by MTT Assay
2.2.3. Mixture modeling using Design Expert 11 software
- Predictions of the response for any mixture or combination of the ingredients can be made empirically or statistically.
- Some measure of the influence on the response of each component and in combination with other components can be obtained.
- The optimization feature can be used to calculate the optimum composition for a mixture to maximize a certain effect.
2.2.4. Statistical Analysis
3. Results and Discussion
3.1. Influence of Studied Saponins on Cell Viability
3.1.1. Chemical Formula of Saponins
3.1.2. Cytotoxicity of Saponins in NCTC Cells
3.1.3. Antiproliferative Activity of Saponins in Hep-2 Cells
3.2. Mixture Modeling Using Design Expert 11 Software
- Model: Scheffe
- Lack of fit points: 5
- Replicate points: 3
- Additional model points: 0
- Additional center points: 0
- Blocks: 1
- Total runs: 14
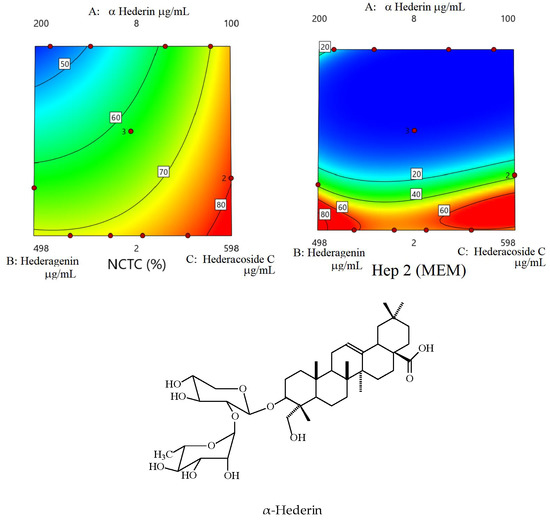
3.3. In Vitro Cytotoxicity and Antiproliferative Activity of the Saponin Mixtures
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cioaca, C.; Margineanu, C.; Cucu, V. The saponins of Hedera helix with antibacterial activity. Pharmazie 1978, 33, 609–610. [Google Scholar] [PubMed]
- Moulin-Traffort, J.; Favel, A.; Elias, R.; Regli, P. Study of the action of α-hederin on the ultrastructure of Candida albicans. Mycoses 1998, 41, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Julien, J.; Gasquet, M.; Maillard, C.; Balansard, G.; Timon-David, P. Extracts of the ivy plant, Hedera helix, and their antihelminthic activity on liver flukes. Planta Med. 1985, 3, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Majester-Savornin, B.; Elias, R.; Diaz-Lanza, A.M.; Balansard, G.; Gasquet., M.; Delmas, F. Saponins of the ivy plant, Hedera helix, and their leishmanicidic activity. Planta Med. 1991, 57, 260–262. [Google Scholar]
- Elias, R.; Lanza, A.M.D.; Vidal-Ollivier, E.; Balansard, G.; Faure, R.; Babadjamian, A. Triterpenoid saponins from the leaves of Hedera helix L. J. Nat. Prod. 1991, 54, 98–103. [Google Scholar] [CrossRef]
- Gulcin, I.; Mshvildaze, V.; Gepdiremen, A.; Elias, A. Antioxidant activity of saponins isolated from ivy: α-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F. Planta Med. 2004, 70, 561–563. [Google Scholar] [CrossRef]
- Evans, W.C. Saponins, cardioactive drugs and other steroids. In Trease and Evans Pharmacognosy, 16th ed.; Saunders Ltd.: Philadelphia, PA, USA; Elsevier: Edinburgh, UK, 2009; Volume 5, pp. 303–352. [Google Scholar]
- Koczurkiewicz, P.; Czyż, J.; Podolak, I.; Wójcik, K.; Galanty, A.; Janeczko, K.; Michalik, M. Review: Multidirectional effects of triterpene saponins on cancer cells—Mini-review of in vitro studies. Acta Biochim. Pol.(ARP) 2015, 62, 383–393. [Google Scholar] [CrossRef]
- Kokanova-Nedialkova, Z.; Nedialkov, P.T.; Momekov, G. Saponins from the roots of Chenopodium bonus-henricus L. Nat. Prod. Res. 2019, 33, 2024–2031. [Google Scholar] [CrossRef]
- Hussien, S.A.; Awad, Z.J. Isolation and characterization of triterpenoid saponin hederacoside C. present in the leaves of Hedera helix L. cultivated in Iraq. Iraqi J. Pharm. Sci. 2014, 23, 33–41. [Google Scholar]
- Liu, Y.Y.; Yang, Y.N.; Feng, Z.M.; Jiang, J.S.; Zhang, P.C. Eight new triterpenoid saponins with antioxidant activity from the roots of Glycyrrhiza uralensis Fisch. Fitoterapia 2019, 133, 186–192. [Google Scholar] [CrossRef]
- Podolak, I.; Galant, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.E. Pharmacological and therapeutic activities of Hedera helix-A review. IOSR J. Pharm. 2018, 8, 41–53. [Google Scholar]
- Lorent, J. Membrane Interactions of Lipopeptides and Saponins, Two Groups of Natural Amphiphiles, in Biomedical and Pharmaceutical. Ph.D. Thesis, Université catholique de Louvain, Leuven, Belgium, July 2013. [Google Scholar]
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical study and medical application of saponins as anti-cancer agents, Review. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Chwalek, M.; Lalun, N.; Bobichon, H.; Ple, K.; Voutquenne-Nazabadioko, L. Structure–activity relationships of some hederagenin diglycosides: Haemolysis, cytotoxicity and apoptosis induction. Biochim. Biophys. Acta 2006, 1760, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernandez, D.; Demuner, A.J.; Barbosa, L.C.A.; Csuk, R.; Heller, L. Hederagenin as a triterpene template for the development of new antitumor compounds. Eur. J. Med. Chem. 2015, 105, 57–62. [Google Scholar]
- Quetin-Leclercq, J.; Elias, R.; Balansard, G. Cytotoxic activity of some triterpenoid saponins. Planta Med. 1992, 58, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Lin, M.; Yu, J.Y.; Liu, B.; Bao, J.K. Targeting apoptotic and autophagic pathways for cancer therapeutics. Cancer Lett. 2011, 300, 105–114. [Google Scholar] [CrossRef]
- Reichard, S. Hedera helix L. In Invasive Plants of California’s Wildlands; Bossard, C.C., Randall, J.M., Hoshovsky, M.C., Eds.; University of California Press: Berkeley, CA, USA, 2000; pp. 212–216. [Google Scholar]
- Wang, J.; Zhao, X.-Z.; Qi, Q.; Tao, L.; Zhao, Q.; Mu, R.; Gu, H.-Y.; Wang, M.; Feng, X.; Guo, Q.-L. Macranthoside B, α-hederagenin, saponin extracted from Lonicera macranthoides and its anti-tumor activities in vitro and in vivo. Food Chem. Toxicol. 2009, 47, 1716–1721. [Google Scholar] [CrossRef]
- Chun, J.; Ha, I.J.; Kim, Y.S. Antiproliferative and apoptotic activities of triterpenoid saponins from the roots of Platycodon grandiflorum and their structure-activity relationships. Planta Med. 2013, 79, 639–645. [Google Scholar] [CrossRef]
- Swamy, S.M.; Huat, B.T. Intracellular glutathione depletion and reactive oxygen species generation are important in α-hederin-induced apoptosis of P388 cells. Mol. Cell. Biochem. 2003, 245, 27–139. [Google Scholar]
- Bomford, R. Saponin and other haemolysins (vitamin A, aliphatic amines, polyene antibiotics) as adjuvants for SRBC in the mouse. Evidence for a role for cholesterol-binding in saponin adjuvanticity. Int. Arch. Allergy Appl. Immunol. 1980, 63, 70–177. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.J.; Okoli, I.; Tegos, G.P.; Holson, E.B.; Wagner, F.F.; Hamblin, M.R.; Mylonakis, E. Characterization of plant-derived saponin natural products against Candida albicans. ACS Chem. Biol. 2010, 5, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Gepdiremen, A.; Mshvildadze, V.; Süleyman, H.; Elias, R. Acute anti-inflammatory activity of four saponins isolated from ivy: Alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F in carrageenan-induced rat paw edema. Phytomedicine 2005, 12, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Ridoux, O.; Di Giorgio, C.; Delmas, F.; Elias, R.; Mshvildadze, R.; Dekanosidze, G.; Kemertelidze, E.; Balansard, G.; Timon-David, P. In vitro antileishmanial activity of three saponins isolated from ivy, α-hederin, β-hederin and hederacolchiside A1, in association with pentamidine and amphotericin B. Phytother. Res. 2001, 15, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Comley, J. Design of Experiments: Useful Statistical Tool in Assay Development or Vendor Disconnect. DDW. 2010. Available online: http://www.ddw-online.com/drug-discovery/p146741-design-of-experiments (accessed on 26 November 2019).
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- NIST/SEMATECH e-Handbook of Statistical. 2012. Available online: http://www.itl.nist.gov/div898/handbook (accessed on 26 November 2019).
- Myers, R.H.; Montgomery, D.C.; Anderson-Kook, C.M. D.C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; Wiley Series in Probability and Statistics; Wiley: New York, NY, USA, 2016; pp. 591–605. [Google Scholar]
- Cheever, M.; Master, A.; Versteegen, R. Differentiating Fetal Bovine Serum from Newborn Calf Serum. 2017. Available online: https://www.serumindustry.org/uploads/cms/nav-37-5b038a8f440f1 (accessed on 26 November 2019).
- Harborne, J.B. The Terpenoids. In Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis, 3rd ed.; Chapman & Hall: London, UK, 1998; pp. 107–138. [Google Scholar]
- Liu, B.X.; Zhou, J.Y.; Li, Y.; Zou, X.; Wu, J.; Gu, J.F.; Yuan, J.R.; Zhao, B.J.; Feng, L.; Jia, X.B.; et al. Hederagenin from the leaves of ivy (Hedera helix L.) induces apoptosis in human LoVo colon cells through the mitochondrial pathway. BMC Complement. Altern. Med. 2014, 14, 412–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Standard | IC50 (µg/mL) | |
|---|---|---|
| Hep-2 MEM | Hep-2 MEM + FBS | |
| α-Hederin | 2 | 25 |
| Hederagenin | 413 | >500 |
| Hederacoside C | >400 | >400 |
| Component | Name | Min. | Max. |
|---|---|---|---|
| A | α-Hederin | 2 | 8 |
| B | Hederagenin | 100 | 498 |
| C | Hederacoside C | 200 | 598 |
| Total= | 700.00 | ||
| Run | Mixture Composition, µg/mL | ||
|---|---|---|---|
| A: α-Hederin | B: Hederagenin | C: Hederacoside C | |
| 1 | 8.000 | 381.029 | 310.971 |
| 2 | 3.824 | 100.000 | 596.176 |
| 3 | 2.000 | 279.100 | 418.900 |
| 4 | 3.514 | 496.486 | 200.000 |
| 5 | 8.000 | 139.800 | 552.200 |
| 6 | 2.000 | 343.630 | 354.370 |
| 7 | 2.000 | 188.104 | 509.896 |
| 8 | 5.305 | 301.292 | 393.403 |
| 9 | 3.824 | 100.000 | 596.176 |
| 10 | 5.305 | 301.292 | 393.403 |
| 11 | 2.000 | 424.557 | 273.443 |
| 12 | 8.000 | 462.150 | 229.850 |
| 13 | 8.000 | 230.898 | 461.102 |
| 14 | 5.305 | 301.292 | 393.403 |
| Mixture Variants | Cell Viability (%) | ||
|---|---|---|---|
| NCTC | Hep-2 (MEM) | Hep-2 (MEM + FBS) | |
| 1 | 44 ± 2.04 | 4 ± 1.59 | 49 ± 2.82 |
| 2 | 77 ± 1.15 | 37 ± 1.24 | 77 ± 3.44 |
| 3 | 72 ± 3.59 | 60 ± 3.84 | 66 ± 3.59 |
| 4 | 63 ± 1.41 | 38 ± 3.68 | 69 ± 1.55 |
| 5 | 69 ± 1.82 | 2 ± 0.95 | 61 ± 3.00 |
| 6 | 73 ± 4.04 | 49 ± 3.31 | 64 ± 2.97 |
| 7 | 79 ± 2.58 | 57 ± 3.74 | 73 ± 3.98 |
| 8 | 60 ± 0.61 | 2 ± 1.24 | 63 ± 0.35 |
| 9 | 81 ± 0.31 | 36 ± 1.34 | 77 ± 3.08 |
| 10 | 65 ± 2.32 | 2 ± 0.41 | 67 ± 2.56 |
| 11 | 71 ± 2.49 | 63 ± 3.69 | 69 ± 3.35 |
| 12 | 50 ± 3.02 | 17 ± 0.31 | 44 ± 2.98 |
| 13 | 65 ± 1.31 | 2 ± 0.72 | 70 ± 2.28 |
| 14 | 63 ± 3.03 | 2 ± 2.11 | 62 ± 1.20 |
| Response range | 44 to 81 | 2 to 64 | 44 to 77 |
| Ratio of max to min | 1.84 | 39.25 | 1.73 |
| Transformation | none | Natural log | none |
| Response | Model Type | Model | Lack of Fit | R2 | ||
|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | |||
| NCTC | Quadratic model | 22.77 | 0.0002 | 2.79 | 0.2142 | 0.9343 |
| Hep-2 (MEM) | Cubic model | 457.91 | <0.0001 | 5.18 | 0.1072 | 0.9990 |
| Hep-2(MEM+FBS) | Special Quartic model | 52.48 | 0.0002 | 0.191 | 0.8355 | 0.9882 |
| Model | Coefficients (Coded Equation) and p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | AB | AC | BC | ABC | AB(A-B) | AC(A-C) | ABC2 | A | |
| NCTC | 18,786.6 | 70.9735 | 83.3454 | −20,732.2 | −19,596.5 | 15.8615 | - | - | - | - | 18,786.6 |
| p-values | <0.0001 | <0.0001 | <0.0001 | 0.5880 | 0.6085 | 0.1430 | - | - | - | - | <0.0001 |
| ln Hep-2 (MEM) | 5.65968 E+06 | 4.51276 | 3.99676 | 8.53632 E+06 | 8.552 E+06 | 1.0551 | 5.76906 E+06 | 2.87669 E+06 | 2.89269 E+06 | - | 5.65968 E+06 |
| p-values | <0.0001 | <0.0001 | <0.0001 | 0.0052 | 0.0052 | 0.3829 | 0.0052 | 0.0053 | 0.0052 | - | <0.0001 |
| Hep-2 (MEM + FBS) | −9343.46 | 76.5045 | 88.5913 | 7533.95 | 6852.24 | 71.2811 | - | - | - | 16,716.9 | −9343.46 |
| p-values | <0.0001 | <0.0001 | <0.0001 | 0.9173 | 0.9248 | 0.0152 | - | - | - | 0.0391 | <0.0001 |
| Name | Goal | Lower Limit | Upper Limit |
|---|---|---|---|
| A: Hederin | is in range | 2 | 8 |
| B: Hederagenin | is in range | 100 | 498 |
| C: Hederacoside C | is in range | 200 | 598 |
| NCTC | maximize | 79.0 | 80.8879 |
| Hep-2 (MEM) | minimize | 1.614 | 50.0 |
| Hep-2 (MEM + FBS) | none | 44.3192 | 76.9953 |
| No. | α-Hederin | Hederagenin | Hederacoside C | NCTC | Hep-2 (MEM) | Hep-2 (MEM + SFB) | Desirability |
|---|---|---|---|---|---|---|---|
| 1 | 3.863 µg/mL | 100.000 µg/mL | 596.137 µg/mL | 80% | 35% | 76% | 0.182 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatia, R.; Zalaru, C.; Craciunescu, O.; Moldovan, L.; Oancea, A.; Calinescu, I. Optimization of Triterpene Saponins Mixture with Antiproliferative Activity. Appl. Sci. 2019, 9, 5160. https://doi.org/10.3390/app9235160
Tatia R, Zalaru C, Craciunescu O, Moldovan L, Oancea A, Calinescu I. Optimization of Triterpene Saponins Mixture with Antiproliferative Activity. Applied Sciences. 2019; 9(23):5160. https://doi.org/10.3390/app9235160
Chicago/Turabian StyleTatia, Rodica, Christina Zalaru, Oana Craciunescu, Lucia Moldovan, Anca Oancea, and Ioan Calinescu. 2019. "Optimization of Triterpene Saponins Mixture with Antiproliferative Activity" Applied Sciences 9, no. 23: 5160. https://doi.org/10.3390/app9235160