Sensitive Detection of E. coli in Artificial Seawater by Aptamer-Coated Magnetic Beads and Direct PCR
Abstract
:Featured Application
abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bacterial Strain and Growth Condition.
2.3. Drop Plate Method for Enumerating Bacteria
2.4. Aptamer
2.5. Magnetic Beads Functionalization
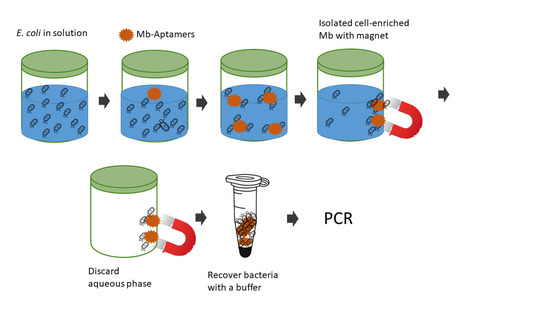
2.6. Bacterial Capturing by Apta-Magnetic Beads
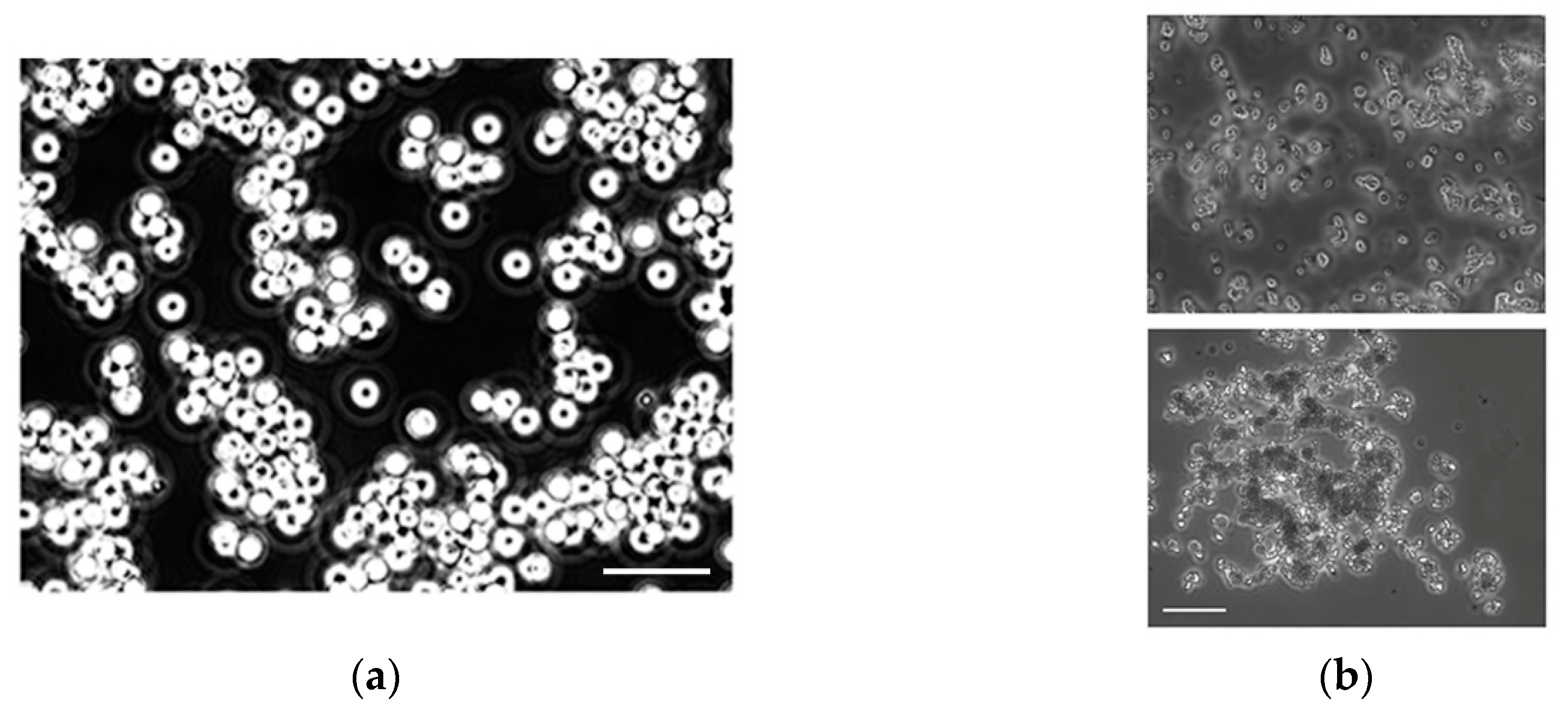
2.7. Optical Microscopy
2.8. PCR Tests
3. Results
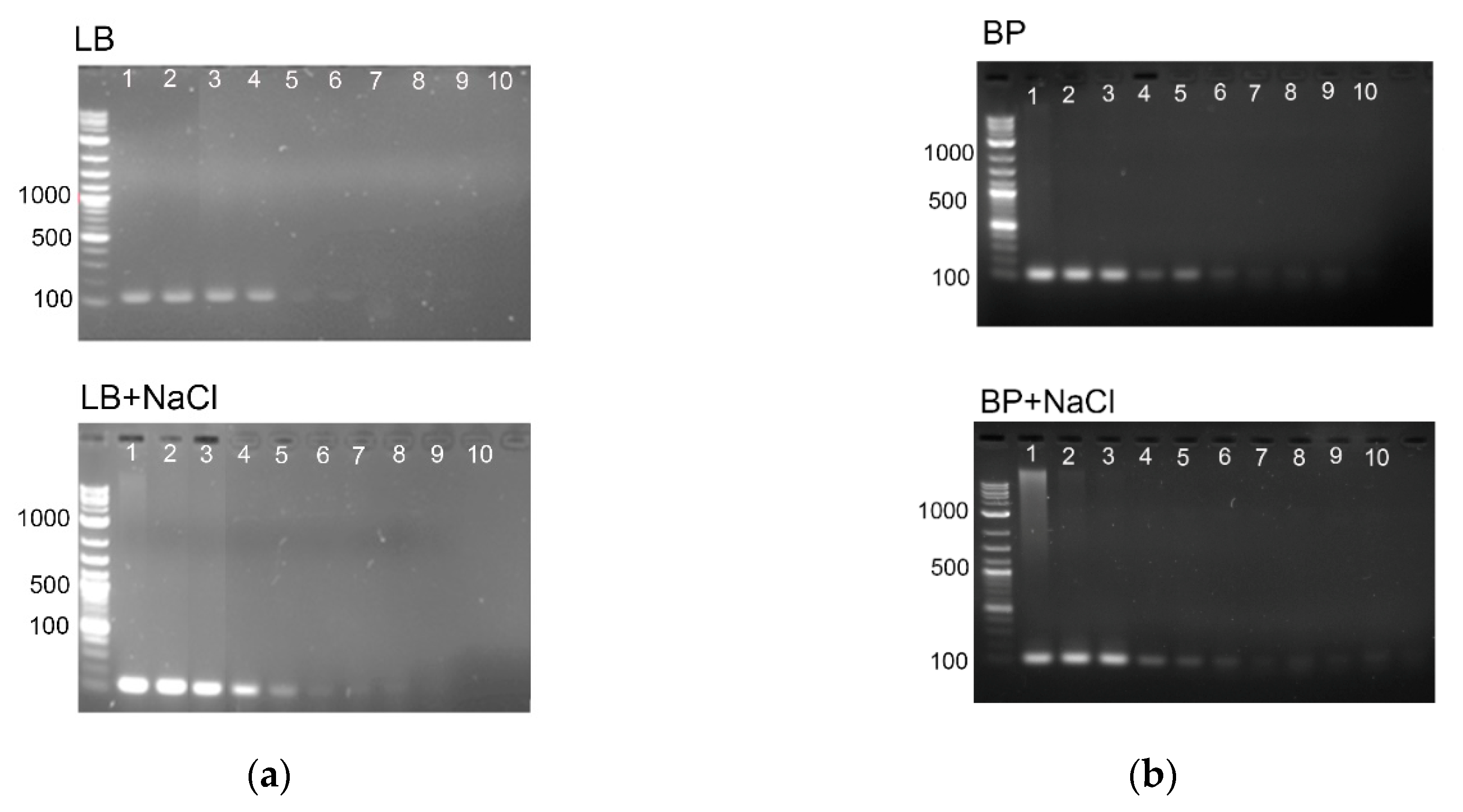
3.1. Survival of E. coli in Highly Salted Media
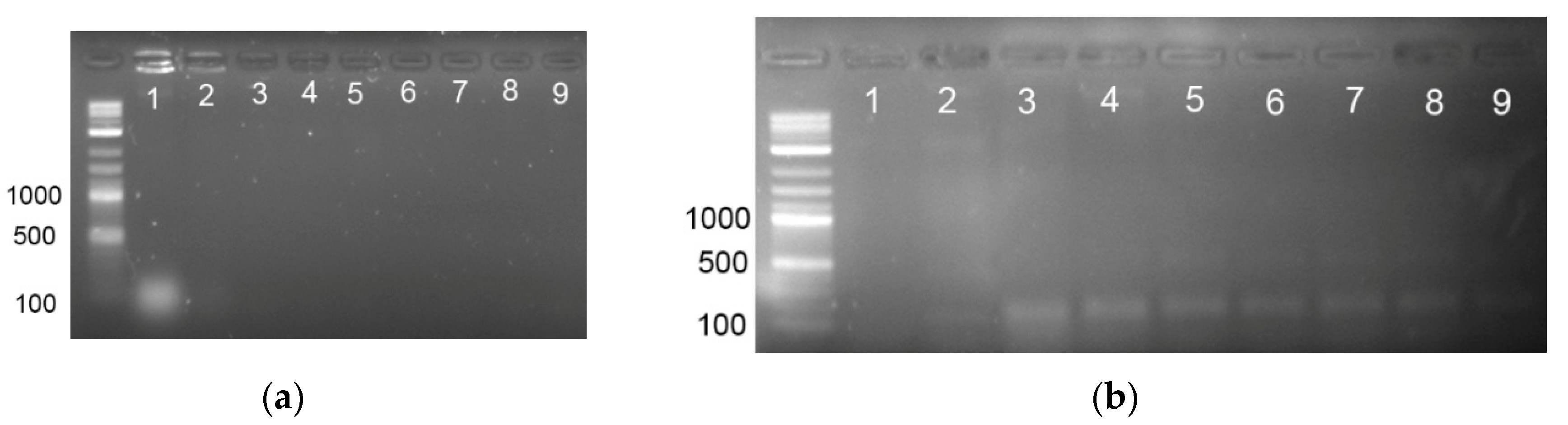
3.2. PCR Detection of E. coli Genomic DNA
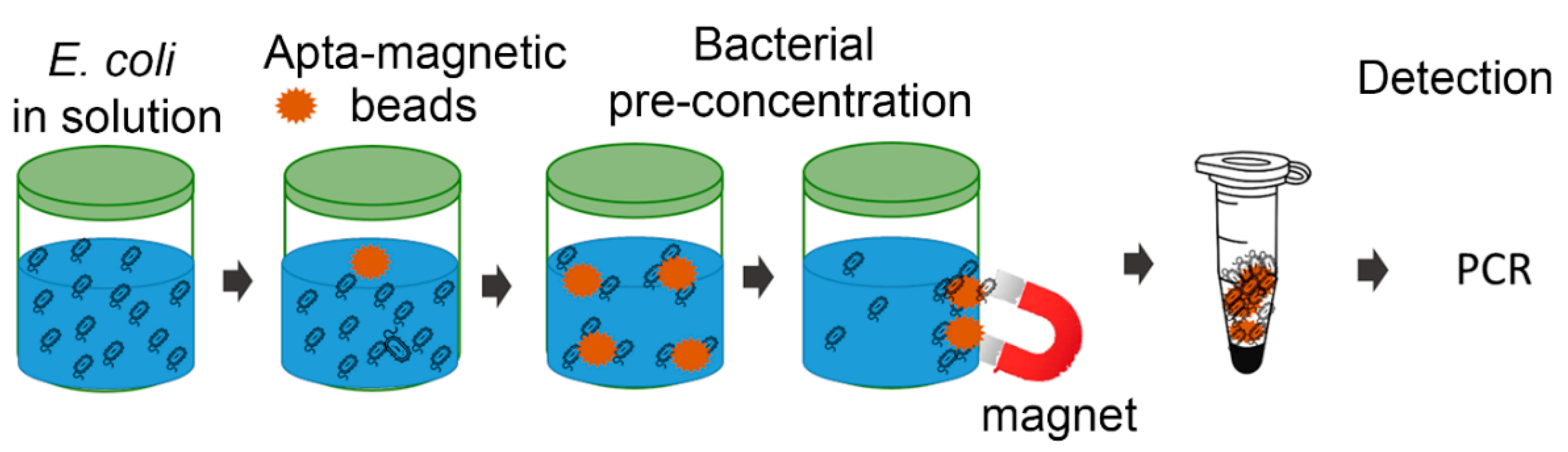
3.3. Bacterial Pre-Concentration with Aptamer Decorated Magnetic Beads
3.4. Direct PCR Detection of E. coli after Pre-Concentration
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- One Health. Available online: https://www.who.int/features/qa/one-health/en/ (accessed on 09 December 2019).
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Costa Andrade, V.; Zampieri, B.D.B.; Ballesteros, E.R.; Pinto, A.B.; De Oliveira, A.J.F.C. Densities and antimicrobial resistance of Escherichia coli isolated from marine waters and beach sands. Environ. Monit. Assess. 2015, 187, 342. [Google Scholar] [CrossRef] [PubMed]
- Pommepuy, M.; Butin, M.; Derrien, A.; Gourmelon, M.; Colwell, R.; Cormier, M. Retention of enteropathogenicity by viable but nonculturable Escherichia coli exposed to seawater and sunlight. Appl. Environ. Microbiol. 1996, 62, 4621–4626. [Google Scholar] [PubMed]
- Sokolova, E.; Åström, J.; Pettersson, T.J.; Bergstedt, O.; Hermansson, M. Estimation of pathogen concentrations in a drinking water source using hydrodynamic modelling and microbial source tracking. J. Water Health 2012, 10, 358–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Song, M.Y.; Jurng, J.; Kim, B.C. Isolation and characterization of DNA aptamers against Escherichia coli using a bacterial cell–systematic evolution of ligands by exponential enrichment approach. Anal. Biochem. 2013, 436, 22–28. [Google Scholar] [CrossRef]
- Vidic, J.; Noiray, M.; Bagchi, A.; Slama-Schwok, A. Identification of a Novel Complex between the Nucleoprotein and PA (1–27) of Influenza A Virus Polymerase. Biochemistry 2016, 55, 4259–4262. [Google Scholar] [CrossRef]
- Molina, F.; López-Acedo, E.; Tabla, R.; Roa, I.; Gómez, A.; Rebollo, J.E. Improved detection of Escherichia coli and coliform bacteria by multiplex PCR. BMC Biotechnol. 2015, 15, 48. [Google Scholar] [CrossRef] [Green Version]
- Rozen, Y.; Belkin, S. Survival of enteric bacteria in seawater. FEMS Microbiol. Rev. 2001, 25, 513–529. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, J.; Zhu, X.; Song, M.; Zhang, T.; Xin, F.; Dong, W.; Ma, J.; Jiang, M. Expression of global regulator IrrE for improved succinate production under high salt stress by Escherichia coli. Bioresour. Technol. 2018, 254, 151–156. [Google Scholar] [CrossRef]
- Hansell, D.A.; Carlson, C.A.; Repeta, D.J.; Schlitzer, R. Dissolved organic matter in the ocean: A controversy stimulates new insights. Oceanography 2009, 22, 202–211. [Google Scholar] [CrossRef]
- Heery, B.; Briciu-Burghina, C.; Zhang, D.; Duffy, G.; Brabazon, D.; O’Connor, N.; Regan, F. ColiSense, today’s sample today: A rapid on-site detection of β-D-Glucuronidase activity in surface water as a surrogate for E. coli. Talanta 2016, 148, 75–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawil, N.; Sacher, E.; Mandeville, R.; Meunier, M. Surface plasmon resonance detection of E. coli and methicillin-resistant S. aureus using bacteriophages. Biosens. Bioelectron. 2012, 37, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Vidic, J.; Manzano, M.; Chang, C.-M.; Jaffrezic-Renault, N. Advanced biosensors for detection of pathogens related to livestock and poultry. Vet. Res. 2017, 48, 11. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Shelton, D.R.; Karns, J.S.; Sundaram, A.; Li, S.; Amstutz, P.; Tang, C.-M. Detection of water-borne E. coli O157 using the integrating waveguide biosensor. Biosens. Bioelectron. 2005, 21, 678–683. [Google Scholar] [CrossRef] [Green Version]
- Manzano, M.; Viezzi, S.; Mazerat, S.; Marks, R.S.; Vidic, J. Rapid and label-free electrochemical DNA biosensor for detecting hepatitis A virus. Biosens. Bioelectron. 2018, 100, 89–95. [Google Scholar] [CrossRef]
- Zhao, Y.-W.; Wang, H.-X.; Jia, G.-C.; Li, Z. Application of aptamer-based biosensor for rapid detection of pathogenic Escherichia coli. Sensors 2018, 18, 2518. [Google Scholar] [CrossRef] [Green Version]
- Vidic, J.; Vizzini, P.; Manzano, M.; Kavanaugh, D.; Ramarao, N.; Zivkovic, M.; Radonic, V.; Knezevic, N.; Giouroudi, I.; Gadjanski, I. Point-of-need DNA testing for detection of foodborne pathogenic bacteria. Sensors 2019, 19, 1100. [Google Scholar] [CrossRef] [Green Version]
- Vizzini, P.; Braidot, M.; Vidic, J.; Manzano, M. Electrochemical and Optical Biosensors for the Detection of Campylobacter and Listeria: An Update Look. Micromachines 2019, 10, 500. [Google Scholar] [CrossRef] [Green Version]
- Rocha, A.M.; Yuan, Q.; Close, D.M.; O’Dell, K.B.; Fortney, J.L.; Wu, J.; Hazen, T.C. Rapid detection of microbial cell abundance in aquatic systems. Biosens. Bioelectron. 2016, 85, 915–923. [Google Scholar] [CrossRef] [Green Version]
- Miodek, A.; Sauriat-Dorizon, H.; Chevalier, C.; Delmas, B.; Vidic, J.; Korri-Youssoufi, H. Direct electrochemical detection of PB1-F2 protein of influenza A virus in infected cells. Biosens. Bioelectron. 2014, 59, 6–13. [Google Scholar] [CrossRef]
- Nurliyana, M.; Sahdan, M.; Wibowo, K.; Muslihati, A.; Saim, H.; Ahmad, S.; Sari, Y.; Mansor, Z. The Detection Method of Escherichia Coli in Water Resources: A Review. J. Phys. Conf. Ser. 2018, 995, 012065. [Google Scholar] [CrossRef] [Green Version]
- Lepej, S.Z.; Poljak, M. Portable molecular diagnostic instruments in microbiology: Current status. Clin. Microbiol. Infect. 2019. [Google Scholar] [CrossRef]
- Andrews, D.; Chetty, Y.; Cooper, B.S.; Virk, M.; Glass, S.K.; Kelly, P.A.; Sudhanva, M.; Jeyaratnam, D. Multiplex PCR point of care testing versus routine, laboratory-based testing in the treatment of adults with respiratory tract infections: A quasi-randomised study assessing impact on length of stay and antimicrobial use. BMC Infect. Dis. 2017, 17, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreckenberger, P.C.; McAdam, A.J. Point-counterpoint: Large multiplex PCR panels should be first-line tests for detection of respiratory and intestinal pathogens. J. Clin. Microbiol. 2015, 53, 3110–3115. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsiri, Z.; Vantarakis, A.; Rizzotto, F.; Kavanaugh, D.; Ramarao, N.; Vidic, J. Sensitive Detection of E. coli in Artificial Seawater by Aptamer-Coated Magnetic Beads and Direct PCR. Appl. Sci. 2019, 9, 5392. https://doi.org/10.3390/app9245392
Kotsiri Z, Vantarakis A, Rizzotto F, Kavanaugh D, Ramarao N, Vidic J. Sensitive Detection of E. coli in Artificial Seawater by Aptamer-Coated Magnetic Beads and Direct PCR. Applied Sciences. 2019; 9(24):5392. https://doi.org/10.3390/app9245392
Chicago/Turabian StyleKotsiri, Zoi, Apostolos Vantarakis, Francesco Rizzotto, Devon Kavanaugh, Nalini Ramarao, and Jasmina Vidic. 2019. "Sensitive Detection of E. coli in Artificial Seawater by Aptamer-Coated Magnetic Beads and Direct PCR" Applied Sciences 9, no. 24: 5392. https://doi.org/10.3390/app9245392
APA StyleKotsiri, Z., Vantarakis, A., Rizzotto, F., Kavanaugh, D., Ramarao, N., & Vidic, J. (2019). Sensitive Detection of E. coli in Artificial Seawater by Aptamer-Coated Magnetic Beads and Direct PCR. Applied Sciences, 9(24), 5392. https://doi.org/10.3390/app9245392