Electrospinning-Derived PLA/Shellac/PLA Sandwich—Structural Membrane Sensor for Detection of Alcoholic Vapors with a Low Molecular Weight
Abstract
Featured Application
Abstract
1. Introduction
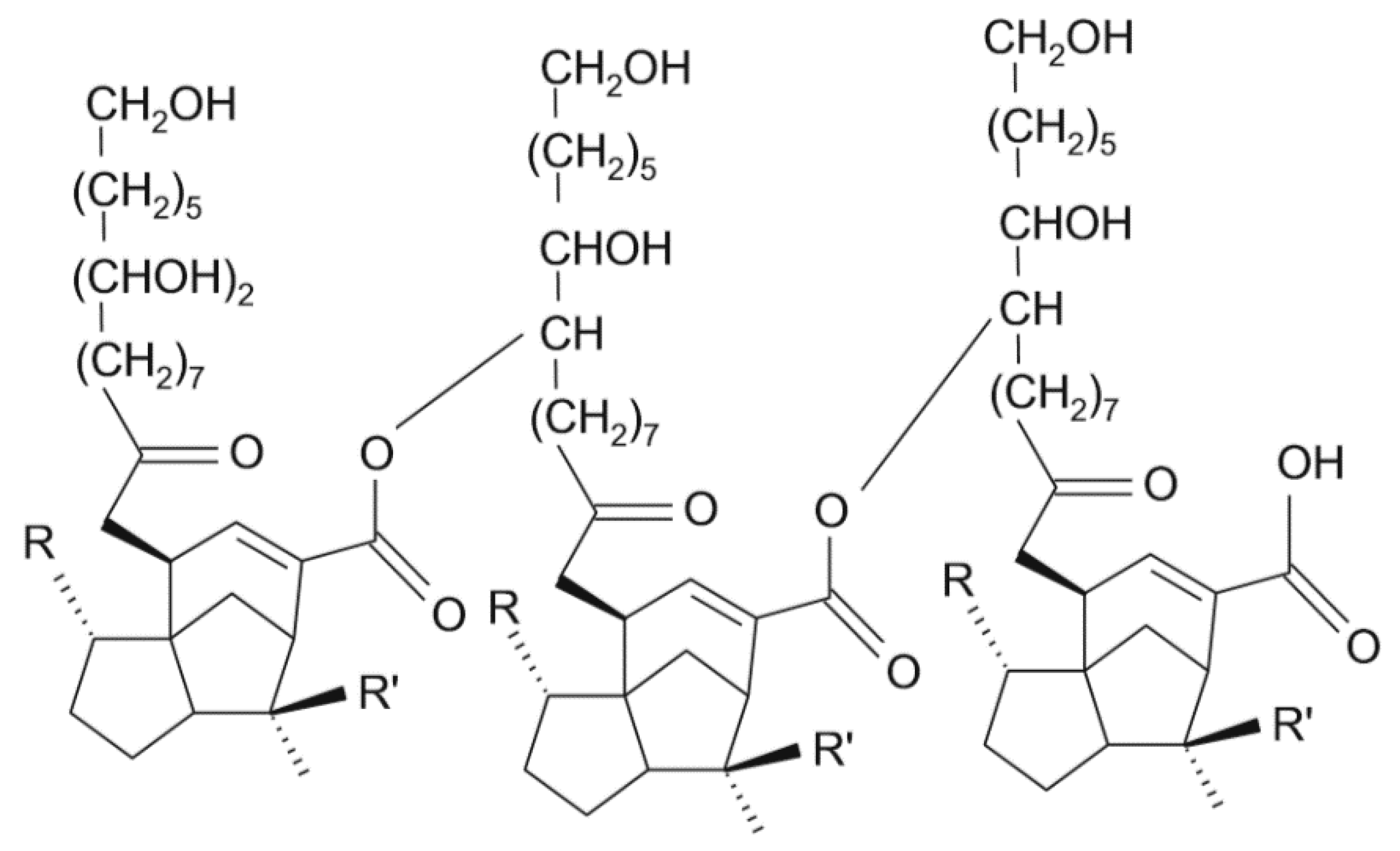
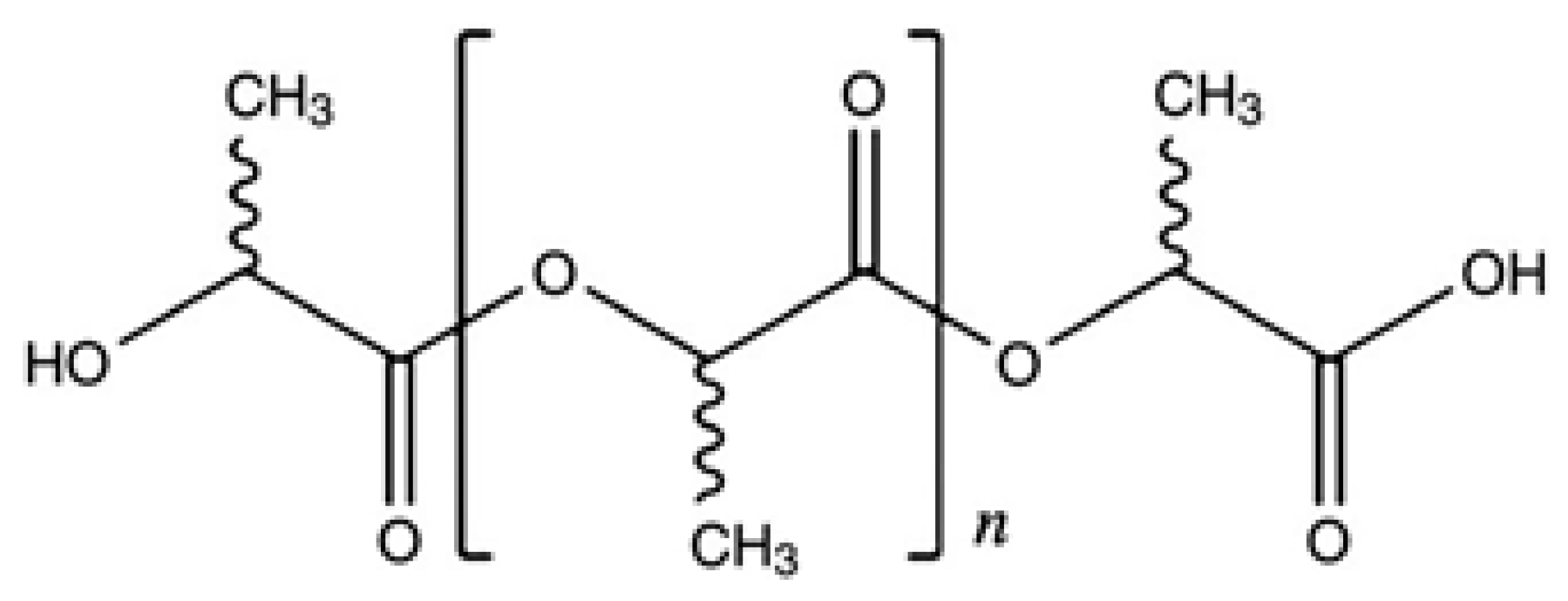
2. Materials and Methods
3. Results and Discussion
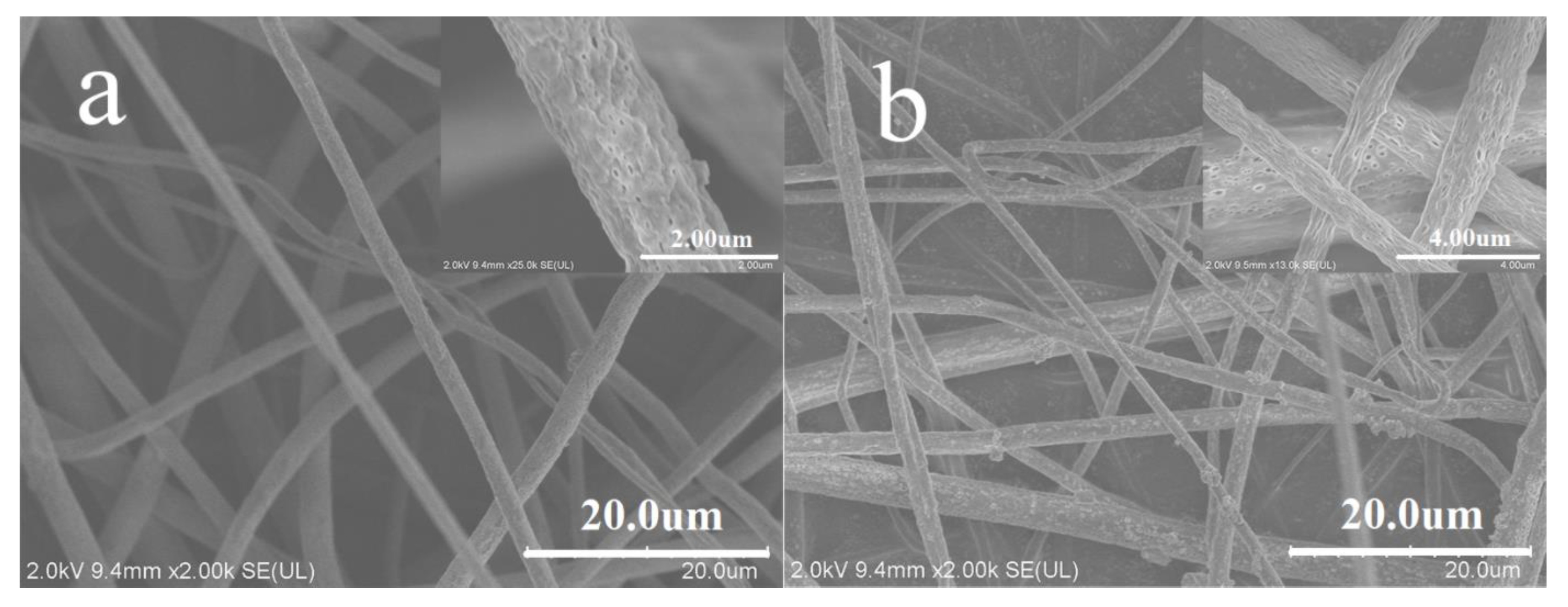
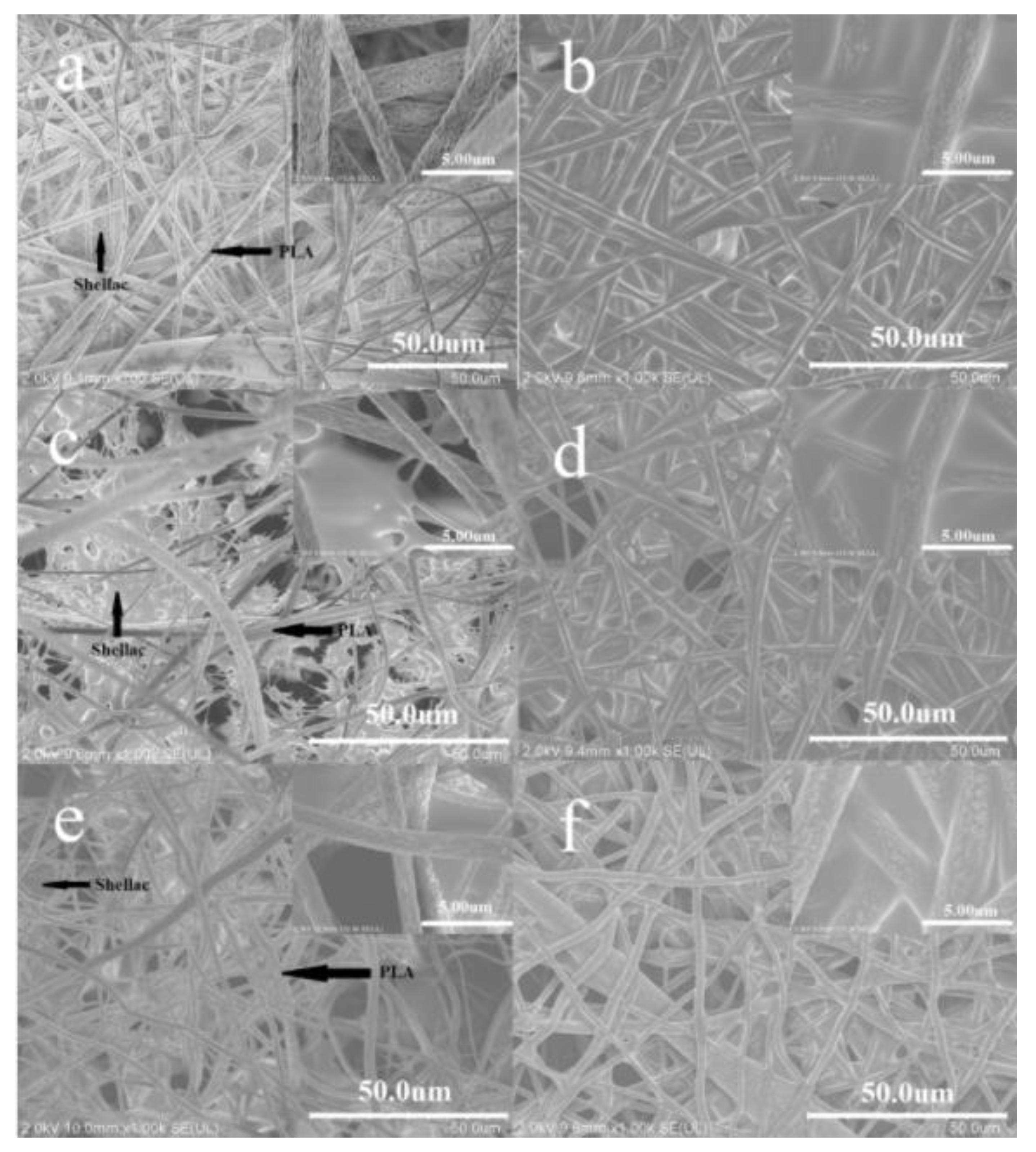
3.1. Morphology
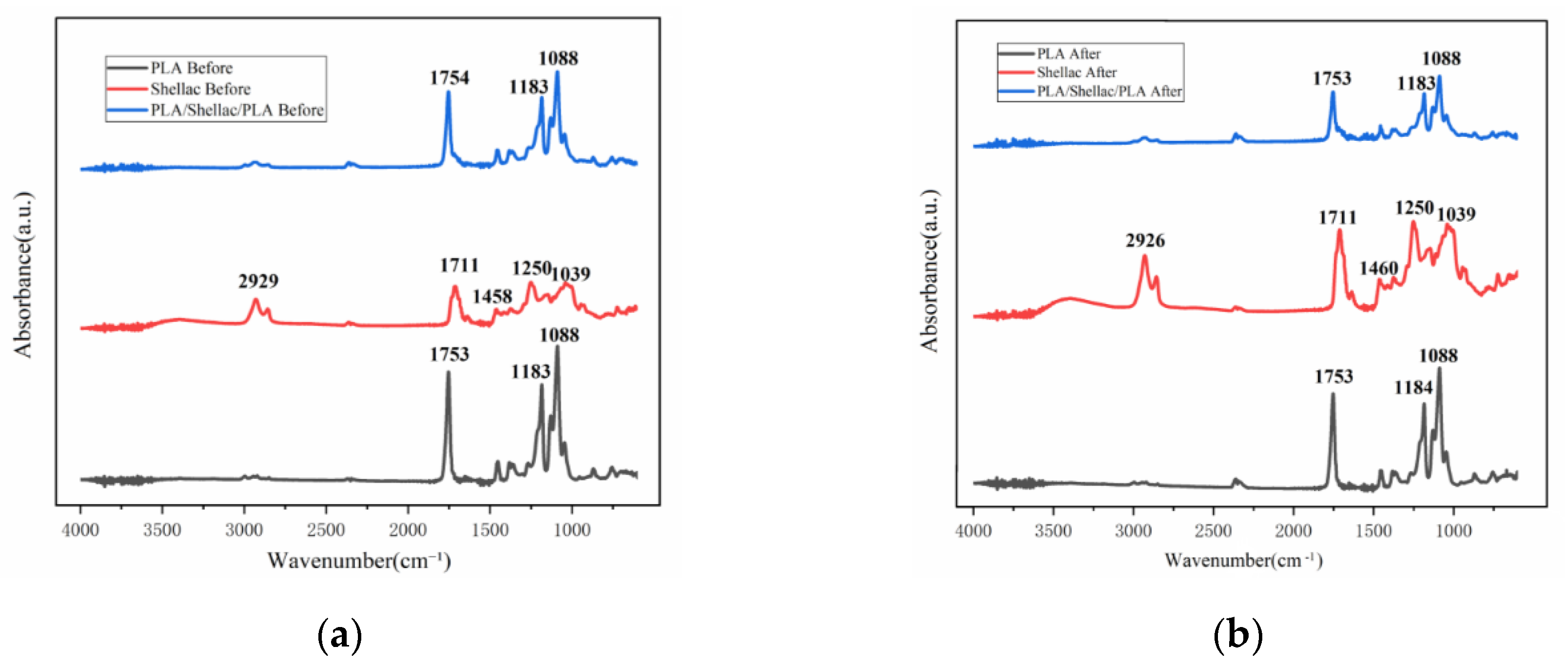
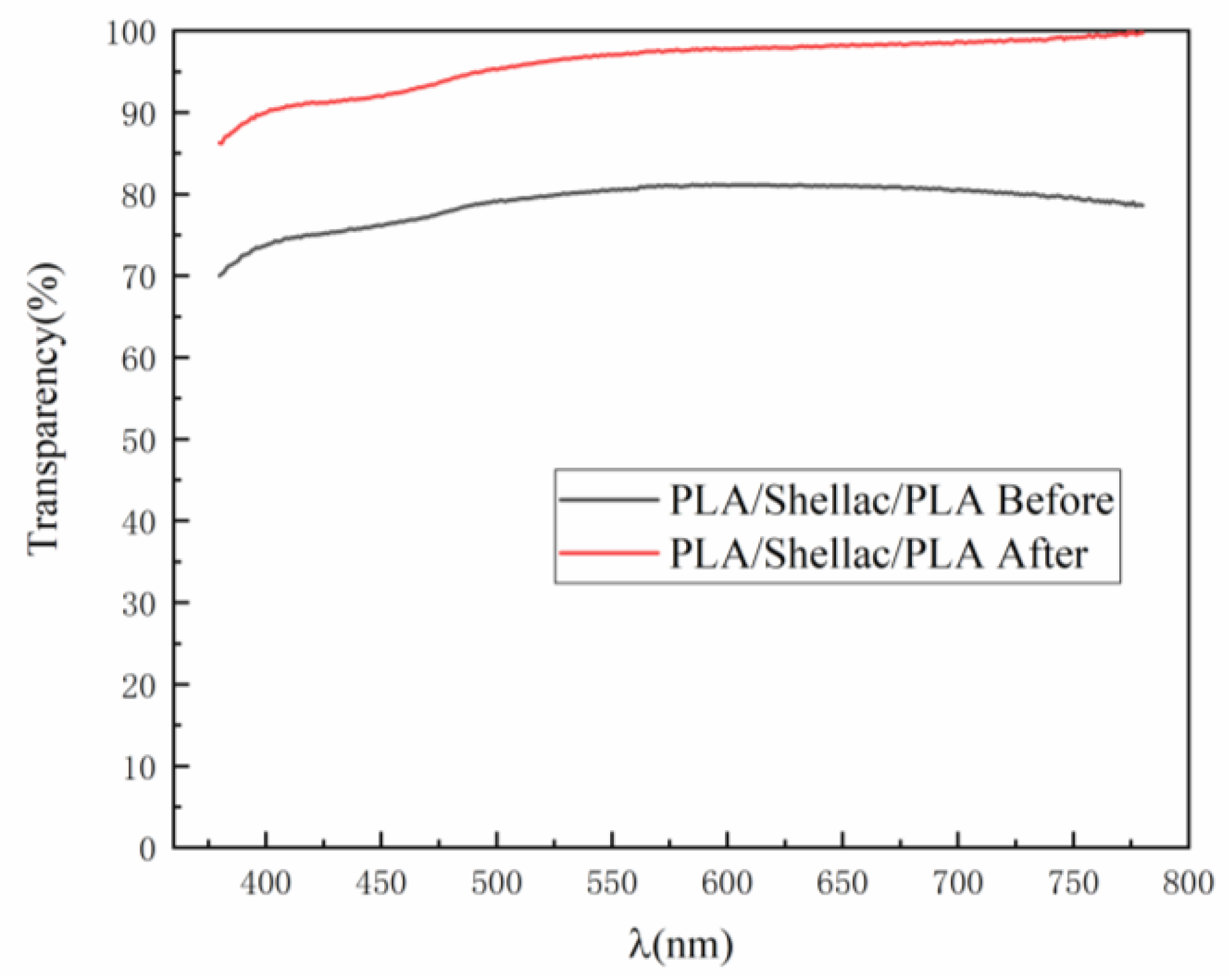
3.2. Influence of the Vapor Treatment on the Structure and Transparency
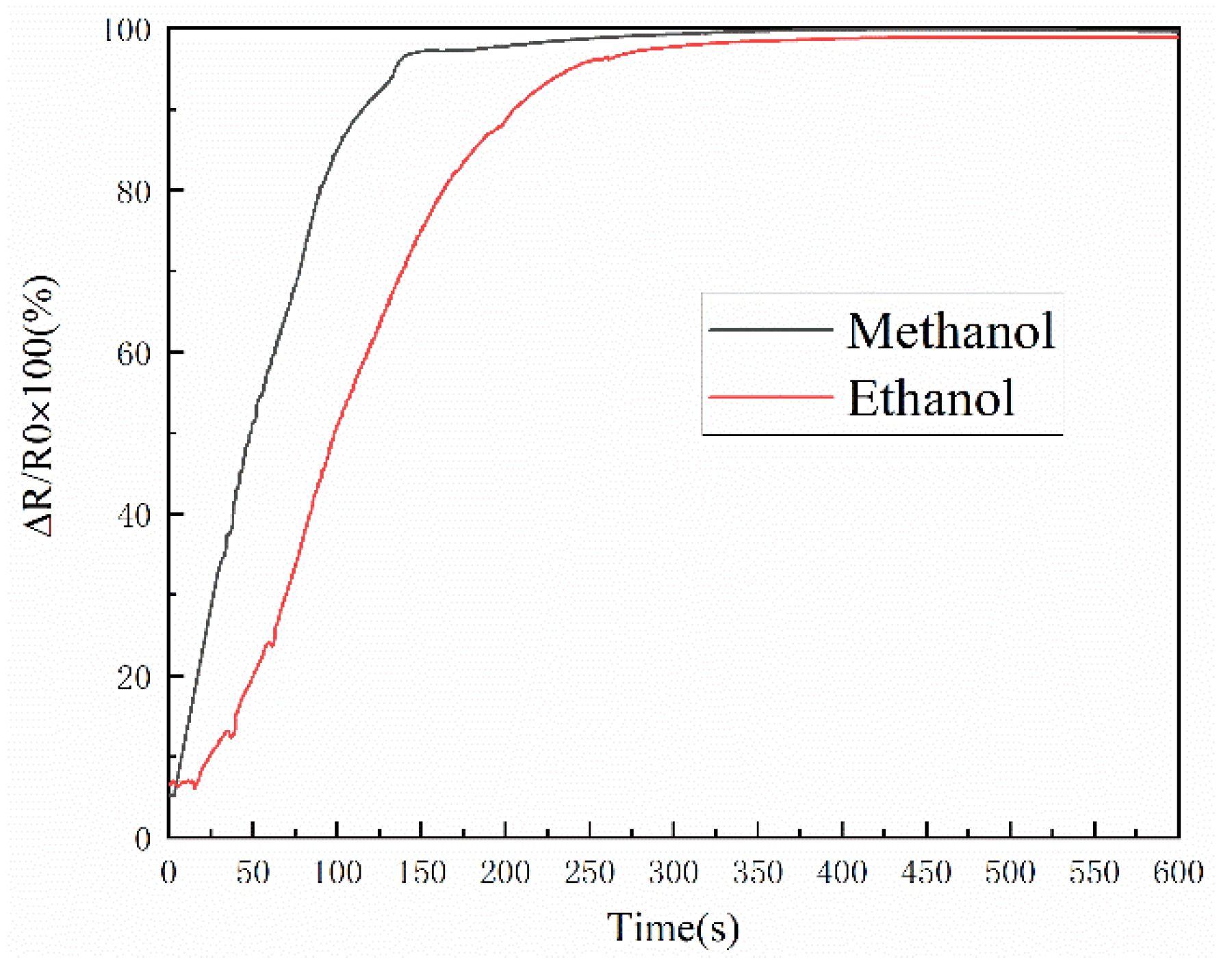
3.3. Alcoholic Vapor-Sensing Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Linnerud, I.; Kaspersen, P.; Jaeger, T. Gas monitoring in the process industry using diode laser spectroscopy. Appl. Phys. B-Lasers Opt. 1998, 67, 297–305. [Google Scholar] [CrossRef]
- Vaseashta, A.; Vaclavikova, M.; Vaseashta, S.; Gallios, G.; Roy, P.; Pummakarnchana, O. Nanostructures in environmental pollution detection, monitoring, and remediation. Sci. Technol. Adv. Mater. 2007, 8, 47–59. [Google Scholar] [CrossRef]
- Di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.F.; Laskowski, D.; Deffenderfer, O.; Burch, T.; Zheng, S.; Mazzone, P.J.; Mekhail, T.; Jennings, C.; Stoller, J.K.; Pyle, J.; et al. Detection of lung cancer by sensor array analyses of exhaled breath. Am. J. Respir. Crit. Care Med. 2005, 171, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Bevenot, X.; Trouillet, A.; Veillas, C.; Gagnaire, H.; Clement, M. Hydrogen leak detection using an optical fibre sensor for aerospace applications. Sens. Actuator B-Chem. 2000, 67, 57–67. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. Highly stable and selective ethanol sensor based on α-Fe2O3 nanoparticles prepared by Pechini sol-gel method. Ceram. Int. 2016, 42, 6136–6144. [Google Scholar] [CrossRef]
- Yebo, N.A.; Lommens, P.; Hens, Z.; Baets, R. An integrated optic ethanol vapor sensor based on a silicon-on-insulator microring resonator coated with a porous ZnO film. Opt. Express 2010, 18, 11859–11866. [Google Scholar] [CrossRef]
- Hu, P.G.; Du, G.J.; Zhou, W.J.; Cui, J.J.; Lin, J.J.; Liu, H.; Liu, D.; Wang, J.Y.; Chen, S.W. Enhancement of Ethanol Vapor Sensing of TiO2 Nanobelts by Surface Engineering. ACS Appl. Mater. Interfaces 2010, 2, 3263–3269. [Google Scholar] [CrossRef]
- Patel, N.G.; Patel, P.D.; Vaishnav, V.S. Indium tin oxide (ITO) thin film gas sensor for detection of methanol at room temperature. Sens. Actuator B-Chem. 2003, 96, 180–189. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Pararneswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Bazrafshan, Z.; Stylios, G.K. One-Step Fabrication of Three-Dimensional Fibrous Collagen-Based Macrostructure with High Water Uptake Capability by Coaxial Electrospinning. Nanomaterials 2018, 8, 803. [Google Scholar] [CrossRef] [PubMed]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Popelka, S.; Machova, L.; Rypacek, F. Adsorption of poly (ethylene oxide)–block–polylactide copolymers on polylactide as studied by ATR-FTIR spectroscopy. J. Colloid Interface Sci. 2007, 308, 291–299. [Google Scholar] [CrossRef]
- Sharma, S.K.; Shukla, S.K.; Vaid, D.N. Shellac-Structure, Characteristics & Modification. Def. Sci. J. 2014, 33, 261–271. [Google Scholar]
- Sawalha, H.; Schroen, K.; Boom, R. Biodegradable polymeric microcapsules: Preparation and properties. Chem. Eng. J. 2011, 169, 1–10. [Google Scholar] [CrossRef]
- Ma, K.; Qiu, Y.P.; Fu, Y.Q.; Ni, Q.Q. Electrospun sandwich configuration nanofibers as transparent membranes for skin care drug delivery systems. J. Mater. Sci. 2018, 53, 10617–10626. [Google Scholar] [CrossRef]
- Ana, P.S.I.; Manuel, L.A.; Núria, C.; Rafael, L.B.; José, A.T. Drug delivery systems using sandwich congurations of electrospun poly(lactic acid) nanofiber membranes and ibuprofen. Mater. Sci. Eng. C-Mater. Biol. Appl. 2013, 33, 4002–4008. [Google Scholar]
- Koenhen, D.M.; Smolders, C.A. The determination of solubility parameters of solvents and polymers by means of correlations with other physical quantities. J. Appl. Polym. Sci. 1975, 19, 1163–1179. [Google Scholar] [CrossRef]
- Small, P.A. Some factors affecting the solubility of polymers. J. Appl. Chem. 1953, 3, 71–80. [Google Scholar] [CrossRef]
- Hoy, K. New values of the solubility parameters from vapor pressure data. J. Paint Technol. 1970, 42, 76–118. [Google Scholar]
- Burrell, H. Solubility parameters for film formers. Off. Dig. 1955, 369, 726–758. [Google Scholar]
- Liang, J.W.; Gajula, P.; Wang, S.C.; Wu, J.L.; Lu, S.G. Enhancement of the Oil Absorption Capacity of Poly(Lactic Acid) Nano Porous Fibrous Membranes Derived via a Facile Electrospinning Method. Appl. Sci. 2019, 9, 1014. [Google Scholar] [CrossRef]
- Agarwal, M.; Koelling, K.W.; Chalmers, J.J. Characterization of the Degradation of Polylactic Acid Polymer in a solid substrate environment. Biotechnol. Prog. 1998, 14, 517–526. [Google Scholar] [CrossRef]
- Yang, S.-L.; Wu, Z.-H.; Yang, W.; Yang, M.-B. Thermal and mechanical properties of chemical crosslinked polylactide (PLA). Polym. Test. 2008, 27, 957–963. [Google Scholar] [CrossRef]
- Thanki, P.N.; Dellacherie, E.; Six, J.-L. Surface characteristics of PLA and PLGA films. Appl. Surf. Sci. 2006, 253, 2758–2764. [Google Scholar]
- Ahn, J.Y.; Chung, W.J.; Pinnau, I.; Guiver, M.D. Poly sulfone/silica nanoparticle mixed-matrix membranes for gas separation. J. Membr. Sci. 2008, 314, 123–133. [Google Scholar] [CrossRef]
- Zumbühl, S.; Hochuli, A.; Soulier, B.; Scherrer, N.C. Fluorination technique to identify the type of resin in aged vanishes and lacquers using infrared spectroscopy. Microchem. J. 2017, 134, 317–326. [Google Scholar] [CrossRef]
- Brajnicov, S.; Bercea, A.; Marascu, V.; Matei, A.; Mitu, B. Shellac Thin Films Obtained by Matrix-Assisted Pulsed Laser Evaporation (MAPLE). Coatings 2018, 8, 275. [Google Scholar] [CrossRef]
- Licchelli, M.; Malagodi, M.; Somaini, M.; Weththimuni, M.; Zanchi, C. Surface treatments of wood by chemically modified shellac. Surf. Eng. 2013, 29, 121–127. [Google Scholar] [CrossRef]
- Choi, J.; Park, E.J.; Park, D.W.; Shim, S.E. MWCNT-OH adsorbed electrospun nylon 6,6 nanofibers chemiresistor and their application in low molecular weight alcohol vapours sensing. Synth. Met. 2010, 160, 2664–2669. [Google Scholar] [CrossRef]





| Solvents | Molar Mass (g/mol) | Molar Volume (cm3/mol) | |
|---|---|---|---|
| Methanol | 32.04 | 40.7 | 22.3 |
| Ethanol | 46.07 | 58.5 | 19.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-C.; Liang, J.-W.; Yao, Y.-B.; Tao, T.; Liang, B.; Lu, S.-G. Electrospinning-Derived PLA/Shellac/PLA Sandwich—Structural Membrane Sensor for Detection of Alcoholic Vapors with a Low Molecular Weight. Appl. Sci. 2019, 9, 5419. https://doi.org/10.3390/app9245419
Wang S-C, Liang J-W, Yao Y-B, Tao T, Liang B, Lu S-G. Electrospinning-Derived PLA/Shellac/PLA Sandwich—Structural Membrane Sensor for Detection of Alcoholic Vapors with a Low Molecular Weight. Applied Sciences. 2019; 9(24):5419. https://doi.org/10.3390/app9245419
Chicago/Turabian StyleWang, Shi-Cai, Jun-Wei Liang, Ying-Bang Yao, Tao Tao, Bo Liang, and Sheng-Guo Lu. 2019. "Electrospinning-Derived PLA/Shellac/PLA Sandwich—Structural Membrane Sensor for Detection of Alcoholic Vapors with a Low Molecular Weight" Applied Sciences 9, no. 24: 5419. https://doi.org/10.3390/app9245419
APA StyleWang, S.-C., Liang, J.-W., Yao, Y.-B., Tao, T., Liang, B., & Lu, S.-G. (2019). Electrospinning-Derived PLA/Shellac/PLA Sandwich—Structural Membrane Sensor for Detection of Alcoholic Vapors with a Low Molecular Weight. Applied Sciences, 9(24), 5419. https://doi.org/10.3390/app9245419





