Time-Domain Reflectometry (TDR) Monitoring at a Lab Scale of Aerobic Biological Processes in a Soil Contaminated by Diesel Oil
Abstract
1. Introduction
2. Theoretical Background
2.1. Complex Dielectric Permittivity
2.2. Models
2.3. Electrical Conductivity
2.4. Effect of Temperature
3. Materials and Methods
3.1. Soil Characterization
3.2. Set-up of Mesocosms
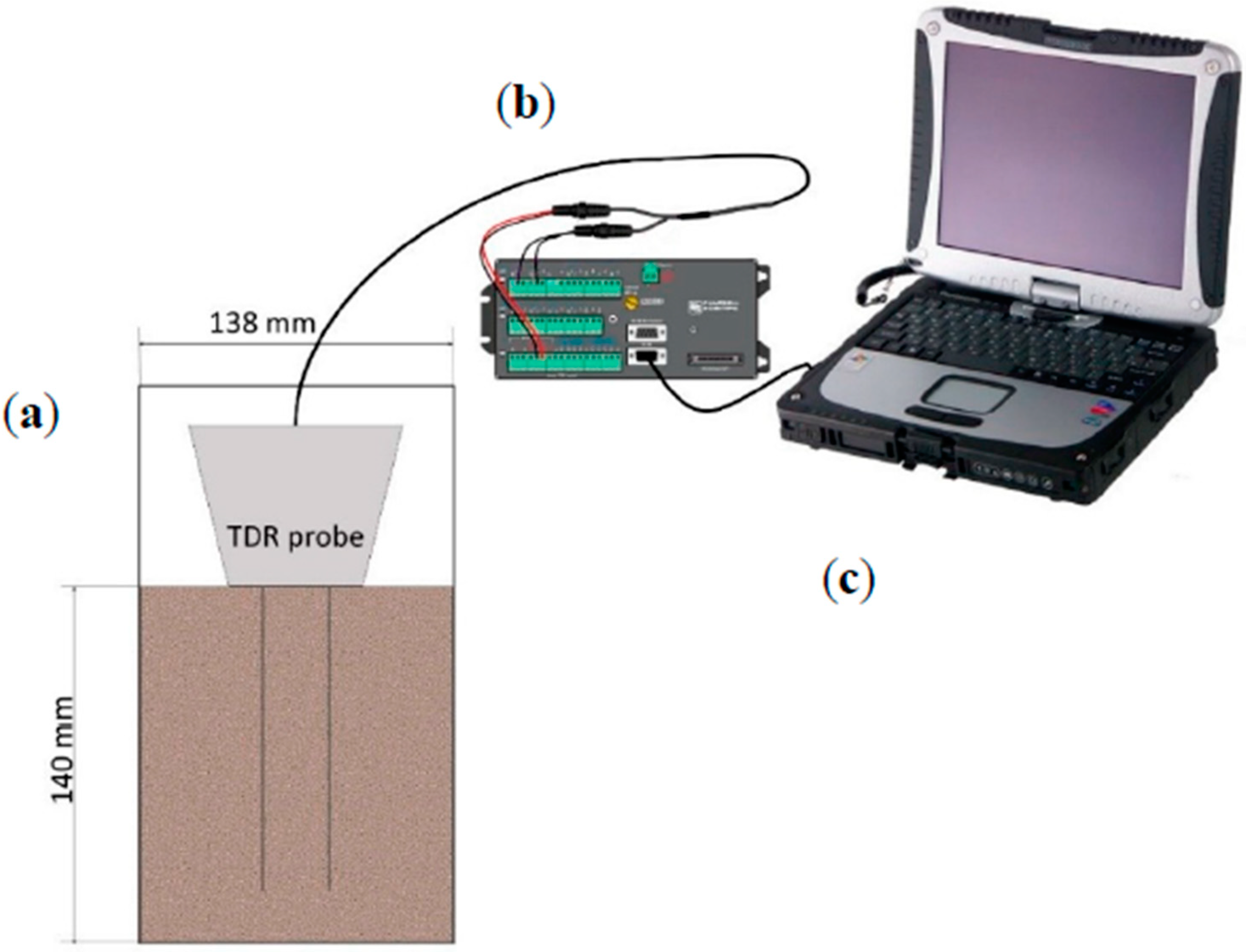
3.3. Time-Domain Reflectometry
3.4. Sampling
3.5. FDA Analysis
- Potassium phosphate buffer—8.7 g/L of K2HPO4 and 1.3 g/L of KH2PO4. The solution pH is 7.57, which falls within the acceptable range to get the FDA hydrolysis reaction, namely between pH 7–8;
- FDA stock solution in acetone at a concentration of 2 g/L.
3.6. Microbial Counts
3.7. Diesel Oil Extraction and Gas Chromatograph Analysis
4. Results
4.1. Microbial Activity
4.2. SEM Images
4.3. Gas Chromatographic Analysis
4.4. TDR Measurements of Geoelectrical Properties
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Capcarova, M.; Slamecka, J.; Jurcik, R.; Sladecek, T.; Gren, A.; Argente, M.J.C.; Massanyi, P. The occurrence and dynamics of polychlorinated hydrocarbons in roe deer (Capreolus capreolus) in South-western Slovakia. J. Environ. Sci. Health Part A 2019, 54, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Fernando, E.Y.; Keshavarz, T.; Kyazze, G. The use of bioelectrochemical systems in environmental remediation of xenobiotics: A review. J. Chem. Technol. Biotechnol. 2019, 9, 2070–2080. [Google Scholar] [CrossRef]
- Bosco, F.; Casale, A.; Mazzarino, I.; Godio, A.; Ruffino, B.; Mollea, C.; Chiampo, F. Microcosm evaluation of bioaugmentation and biostimulation efficacy on diesel-contaminated soil. J. Chem. Technol. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Davis, C.A.; Pyrak-Nolte, L.J.; Atekwana, E.A.; Werkema, D.D., Jr.; Haugen, M.E. Acoustic and electrical property changes due to microbial growth and biofilm formation in porous media. J. Geophys. Res. 2010, 115, G00G06. [Google Scholar] [CrossRef]
- Álvarez, M.; Ruberto, L.; Balbo, L.; Cormack, M. Bioremediation of hydrocarbon-contaminated soils in cold regions: Development of a pre-optimized biostimulation biopile-scale field assay in Antarctica. Sci. Total Environ. 2017, 591, 194–203. [Google Scholar] [CrossRef]
- Arato, A.; Wehrer, M.; Birò, B.; Godio, A. Integration of geophysical, geochemical and microbiological data for a comprehensive small-scale characterization of an aged LNAPL-contaminated site. Environ. Sci. Pollut. Res. 2014, 21, 8948–8963. [Google Scholar] [CrossRef]
- Endres, A.L.; Redman, D.J. Modeling the electrical properties of porous rocks and soils containing immiscible contaminants. J. Environ. Eng. Geophys. 2009, 105–112. [Google Scholar] [CrossRef]
- Carcione, J.M.; Seriani, G. An electromagnetic modelling tool for the detection of hydrocarbons in the subsoil. Geophys. Prospect. 2000, 48, 231–256. [Google Scholar] [CrossRef]
- Feng, S.; Sen, P.N. Geometrical model of conductive and dielectric properties of partially saturated rocks. J. Appl. Phys. 1985, 58, 3236–3243. [Google Scholar] [CrossRef]
- Sen, P.N.; Scala, C.; Cohen, M.H. A self-similar model for sedimentary rocks with applications to the dielectric constant of fused glass beads. Geophysics 1981, 46, 781–795. [Google Scholar] [CrossRef]
- Comegna, A.; Coppola, A.; Dragonetti, G.; Sommella, A. Dielectric response of a variable saturated soil contaminated by Non-Aqueous Phase Liquids (NAPLs). Procedia Environ. Sci. 2013, 19, 701–710. [Google Scholar] [CrossRef]
- Cassiani, G.; Binley, A.; Kemna, A.; Wehrer, M.; Flores Orozco, A.; Deiana, R.; Boaga, J.; Rossi, M.; Dietrich, P.; Werban, U.; et al. Noninvasive characterization of the Trecate (Italy) crude-oil contaminated site: Links between contamination and geophysical signals. Environ. Sci. Pollut. Res. 2014, 21, 8914–8931. [Google Scholar] [CrossRef] [PubMed]
- Godio, A.; Arato, A.; Stocco, S. Geophysical characterization of a non-aqueous-phase liquid-contaminated site. Environ. Geosci. 2010, 17, 141–162. [Google Scholar] [CrossRef]
- Revil, A.; Mendonc, C.A.; Atekwana, E.A.; Kulessa, B.; Hubbard, S.S.; Bohlen, K.J. Understanding biogeobatteries: Where geophysics meets microbiology. J. Geophys. Res. 2010, 115, G00G02. [Google Scholar] [CrossRef]
- Atekwana, E.A.; Slater, L.D. Biogeophysics: A new frontier in Earth science research. Rev. Geophys. 2009, 47, RG4004. [Google Scholar] [CrossRef]
- Mori, Y.; Suetsugu, A.; Matsumoto, Y.; Fujihara, A.; Suyama, K. Enhancing bioremediation of oil-contaminated soils by controlling nutrient dispersion using dual characteristics of soil pore structure. Ecol. Eng. 2013, 51, 237–243. [Google Scholar] [CrossRef]
- Masy, T.; Caterina, D.; Tromme, O.; Lavigne, B.; Thonart, P.; Hiligsmann, S.; Nguyen, F. Electrical resistivity tomography to monitor enhanced biodegradation of hydrocarbons with Rhodococcus erythropolis T902.1 at a pilot scale. J. Contam. Hydrol. 2016, 184, 1–13. [Google Scholar] [CrossRef]
- Abdel Aal, G.Z.; Atekwana, E.A.; Atekwana, E.A. Effect of bioclogging in porous media on complex conductivity signatures. J. Geophys. Res. 2010, 115, G00G07. [Google Scholar] [CrossRef]
- Bosco, F.; Casale, A.; Chiampo, F.; Godio, A. Removal of Diesel Oil in Soil Microcosms and Implication for Geophysical Monitoring. Water 2019, 11, 1661. [Google Scholar] [CrossRef]
- Ledieu, J.; De Bidder, P.; De Clerck, P.; Dautrebande, S. A method of measuring soil moisture by time-domain reflectometry. J. Hydrol. 1986, 88, 319–328. [Google Scholar] [CrossRef]
- Keysight Technologies. Basic of Measuring the Dielectric Properties of Materials; Application note; Keysight Technologies: Santa Rosa, CA, USA, 2019; literature number: 5989-2589EN. [Google Scholar]
- Kaatze, U.; Uhlendorf, V. The dielectric properties of water at microwave frequencies. Z. Phys. Chem. 1981, 126, 151–165. [Google Scholar] [CrossRef]
- Hilhorst, M.A. A pore water conductivity sensor. Soil Sci. Soc. Am. J. 2000, 64, 1922–1925. [Google Scholar] [CrossRef]
- Knight, R.; Endres, A. A new concept in modeling the dielectric response of sandstones: Defining a wetted rock and bulk water system. Geophysics 1990, 55, 586–594. [Google Scholar] [CrossRef]
- Rinaldi, V.A.; Francisca, F.M. Impedance analysis of soil dielectric dispersion (1 MHz–1 GHz). ASCE J. Geotech. Geoenviron. Eng. 1999, 125, 111–121. [Google Scholar] [CrossRef]
- Seyfried, M.S.; Grant, L.E. Temperature Effects on Soil Dielectric Properties Measured at 50 MHz. Vadose Zone J. 2007, 6, 759–765. [Google Scholar] [CrossRef]
- Wharton, R.P.; Hazen, G.A.; Rau, R.N.; Best, D.L. Electromagnetic propagation logging: Advances in technique and interpretation. In Proceedings of the 55th Annual Fall Technical Conference and Exibition of the SPE of AIME, Dallas, TX, USA, 21–24 September 1980. [Google Scholar]
- Archie, G.E. The electrical resistivity log as an aid in determining some reservoir characteristics. Pet. Trans. AIME 1942, 146, 54–62. [Google Scholar] [CrossRef]
- Martinsen, O.G. Bioimpedance and Bioelectricity Basics, 2nd ed.; House, L., Hill, J., Eds.; Elsevier: Oxford, UK, 2008; pp. 67–68. [Google Scholar]
- Vergnano, A. Analysis of Biodegradation of Diesel in Contaminated Soil Using Laboratory Scale Tests. Master’s Thesis, Politecnico di Torino, Torino, Italy, 2018. [Google Scholar]
- Ministero Delle Politiche Agricole e Forestali. Metodi Ufficiali di Analisi Chimica del Suolo; Gazzetta Ufficiale Serie Generale n. 248 (21.10.1999)—Supplemento Ordinario n. 185; Ministero Delle Politiche Agricole e Forestali: Roma RM, Italy, 1999.
- Turetta, A. Utilizzo di metodi biologici a scala di laboratorio per la bonifica di terreni inquinati da idrocarburi. Master’s Thesis, Politecnico di Torino, Torino, Italy, 2017. [Google Scholar]
- Palanisamy, N.; Ramya, J.; Kumar, S.; Vasanthi, N.S.; Chandran, P.; Khan, S. Diesel biodegradation capacities of indigenous bacterial species isolated from diesel contaminated soil. J. Environ. Health Sci. Eng. 2014, 12, 142. [Google Scholar] [CrossRef]
- Raffa, C.M. Ottimizzazione delle condizioni chimico-fisiche di un processo di bonifica a supporto del monitoraggio geofisico. Master’s Thesis, Politecnico di Torino, Torino, Italy, 2019. [Google Scholar]
- Becker, R.; Scheuermann, A.; Schlaeger, S.; Huebner, C.; Wagner, N. Spatial Time Domain Reflectometry (Spatial TDR)—Principles, limitations and accuracy. In Unsaturated Soils: Advances in Geo-Engineering; Toll, D.G., Augarde, C.E., Gallipoli, D., Wheeler, S.J., Eds.; © Taylor & Francis Group: London, UK, 2008; ISBN 978-0-415-47692-8. [Google Scholar]
- Schnurer, J.; Rosswall, T. Fluorescein Diacetate Hydrolysis as a Measure of Total Microbial Activity in Soil and litter. Appl. Environ. Microbiol. 1982, 43, 1256–1261. [Google Scholar]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Method 3546 “Microwave Extraction”; Environmental Protection Agency: Georgetown, Guyana, 2007.
- Environmental Protection Agency. Method 8015 “Nonhalogenated Organics Using GC/FID”; Environmental Protection Agency: Georgetown, Guyana, 2003.
- Atekwana, E.A.; Atekwana, E.; Legall, F.D.; Krishnamurthy, R.V. Field evidence for geophysical detection of subsurface zones of enhanced microbial activity. Geophys. Res. Lett. 2004, 31, l23603. [Google Scholar] [CrossRef]
- Hiebert, F.K.; Bennett, P.C. Microbial Control of Silicate Weathering in Organic-Rich Ground Water. Science 1992, 258, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Olchawa, A.; Kumor, M. Time Domain Reflectometry (TDR)—Measuring Dielectric Constant of Polluted Soil to Estimate Diesel Oil Content. Arch. Hydro Eng. Environ. Mech. 2008, 55, 55–62. [Google Scholar]








| Parameter | Value |
|---|---|
| pH (-) | 7.32 ± 0.04 |
| EC (μS/cm) at 25 °C | 165 ± 5 |
| Bicarbonate (mg/kg) | 66.9 ± 10.8 |
| Ammonia (mg/kg) | 2.18 ± 0.11 |
| Nitrate (mg/kg) | 68.0 ± 0.4 |
| Chloride (mg/kg) | 26.2 ± 0.3 |
| Sulfate (mg/kg) | 211 ± 3 |
| Parameter | Value |
|---|---|
| Diameter of columns (cm) | 13.8 |
| Soil mass (kg) | 3.4 |
| Soil particle size (mm) | 0.15–2 |
| Porosity (−) | 0.4 |
| Volumetric water content (V/V) | 0.20 |
| Volumetric diesel content (V/V) | 0.19 |
| C/N ratio (W/W) | 450 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergnano, A.; Godio, A.; Raffa, C.M.; Chiampo, F.; Bosco, F.; Ruffino, B. Time-Domain Reflectometry (TDR) Monitoring at a Lab Scale of Aerobic Biological Processes in a Soil Contaminated by Diesel Oil. Appl. Sci. 2019, 9, 5487. https://doi.org/10.3390/app9245487
Vergnano A, Godio A, Raffa CM, Chiampo F, Bosco F, Ruffino B. Time-Domain Reflectometry (TDR) Monitoring at a Lab Scale of Aerobic Biological Processes in a Soil Contaminated by Diesel Oil. Applied Sciences. 2019; 9(24):5487. https://doi.org/10.3390/app9245487
Chicago/Turabian StyleVergnano, Andrea, Alberto Godio, Carla Maria Raffa, Fulvia Chiampo, Francesca Bosco, and Barbara Ruffino. 2019. "Time-Domain Reflectometry (TDR) Monitoring at a Lab Scale of Aerobic Biological Processes in a Soil Contaminated by Diesel Oil" Applied Sciences 9, no. 24: 5487. https://doi.org/10.3390/app9245487
APA StyleVergnano, A., Godio, A., Raffa, C. M., Chiampo, F., Bosco, F., & Ruffino, B. (2019). Time-Domain Reflectometry (TDR) Monitoring at a Lab Scale of Aerobic Biological Processes in a Soil Contaminated by Diesel Oil. Applied Sciences, 9(24), 5487. https://doi.org/10.3390/app9245487








