Synthesis of Oligomeric Silicone Surfactant and its Interfacial Properties
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
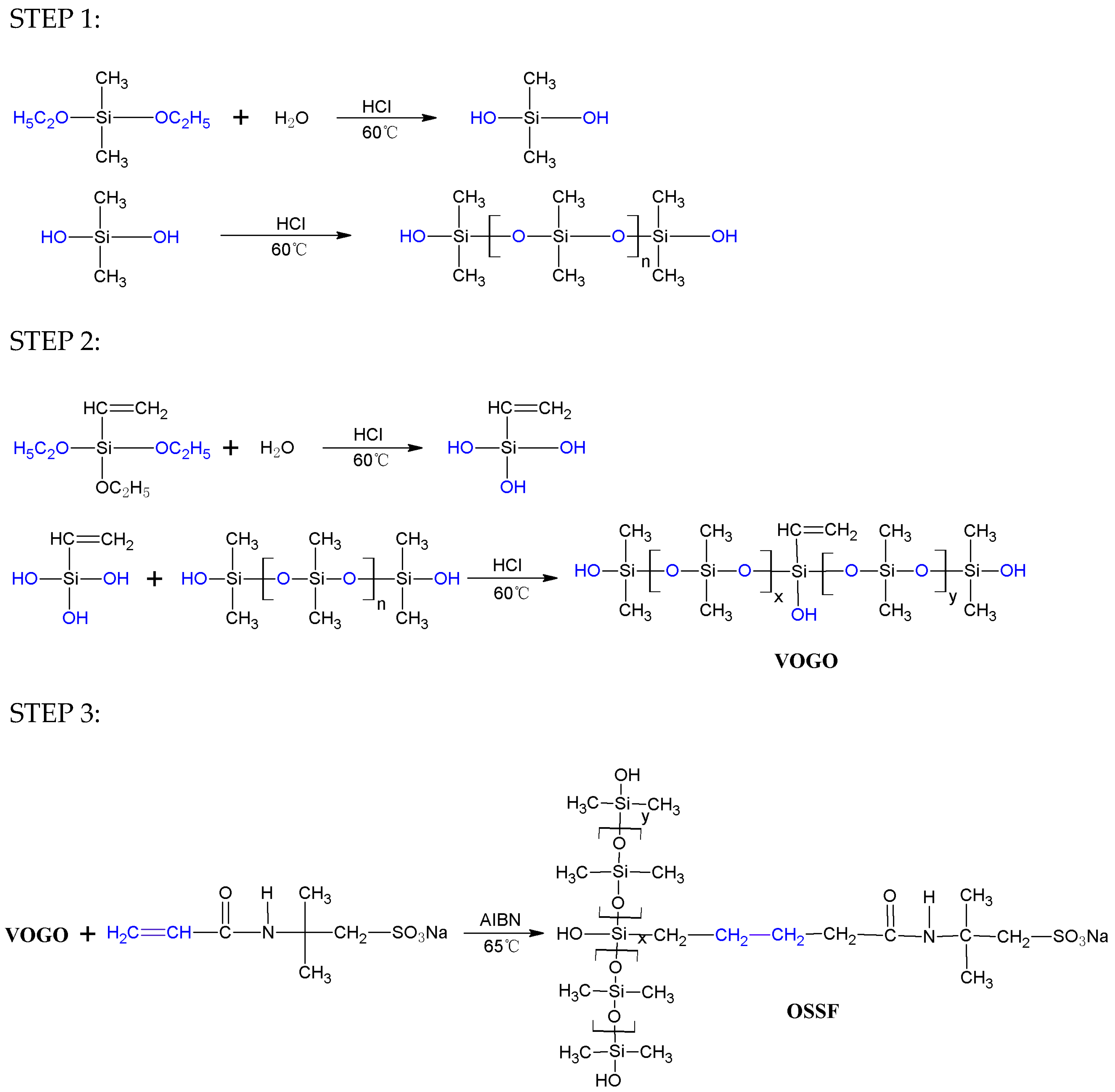
2.2. Synthesis of Vinylorganosilicon Oligomeric (VOGO)
2.3. Synthesis of Oligomeric Silicone Surfactant (OSSF)
2.4. Characterization Methods
3. Results and Discussion
3.1. Surface Tension
3.2. Thermal Stability
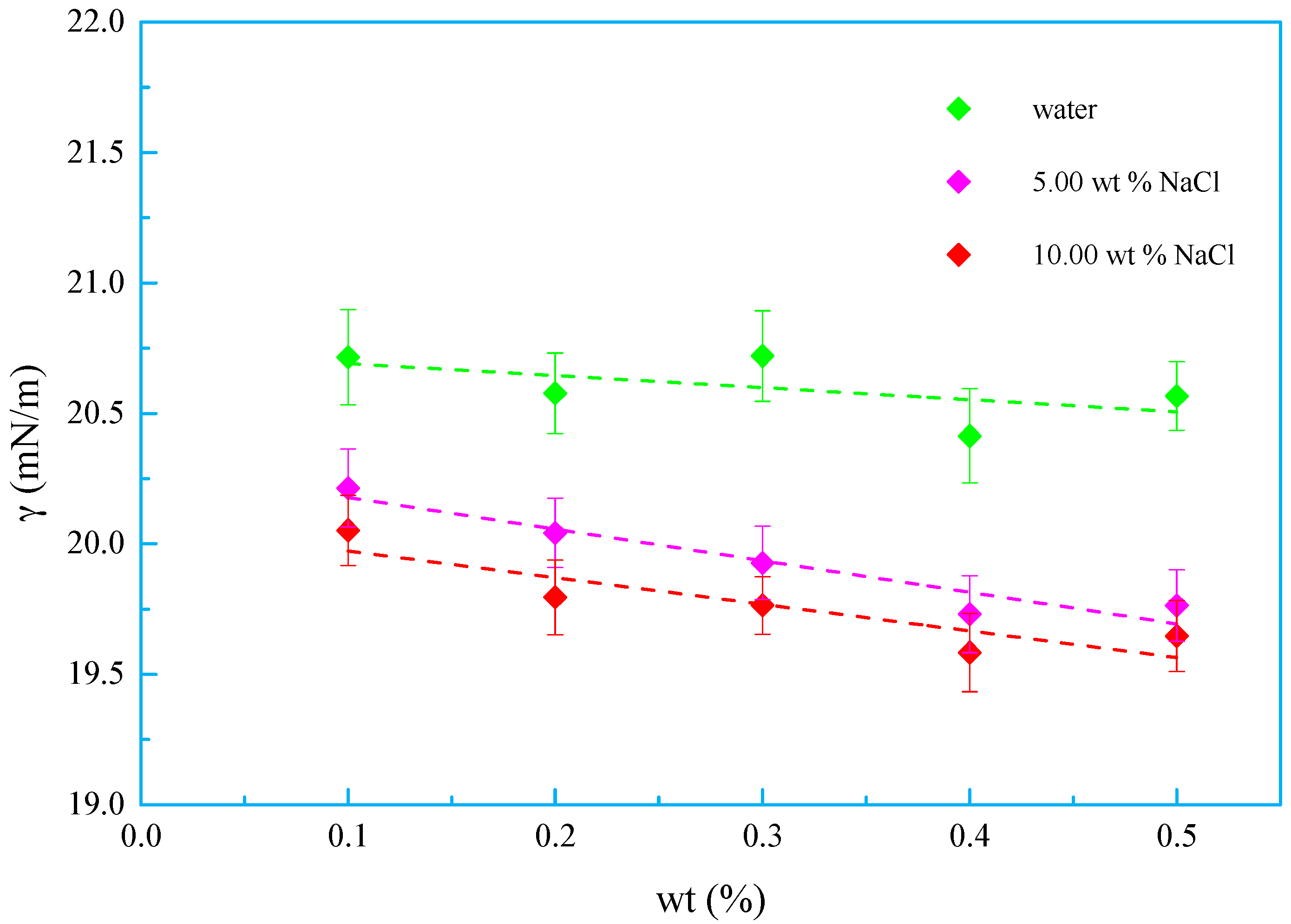
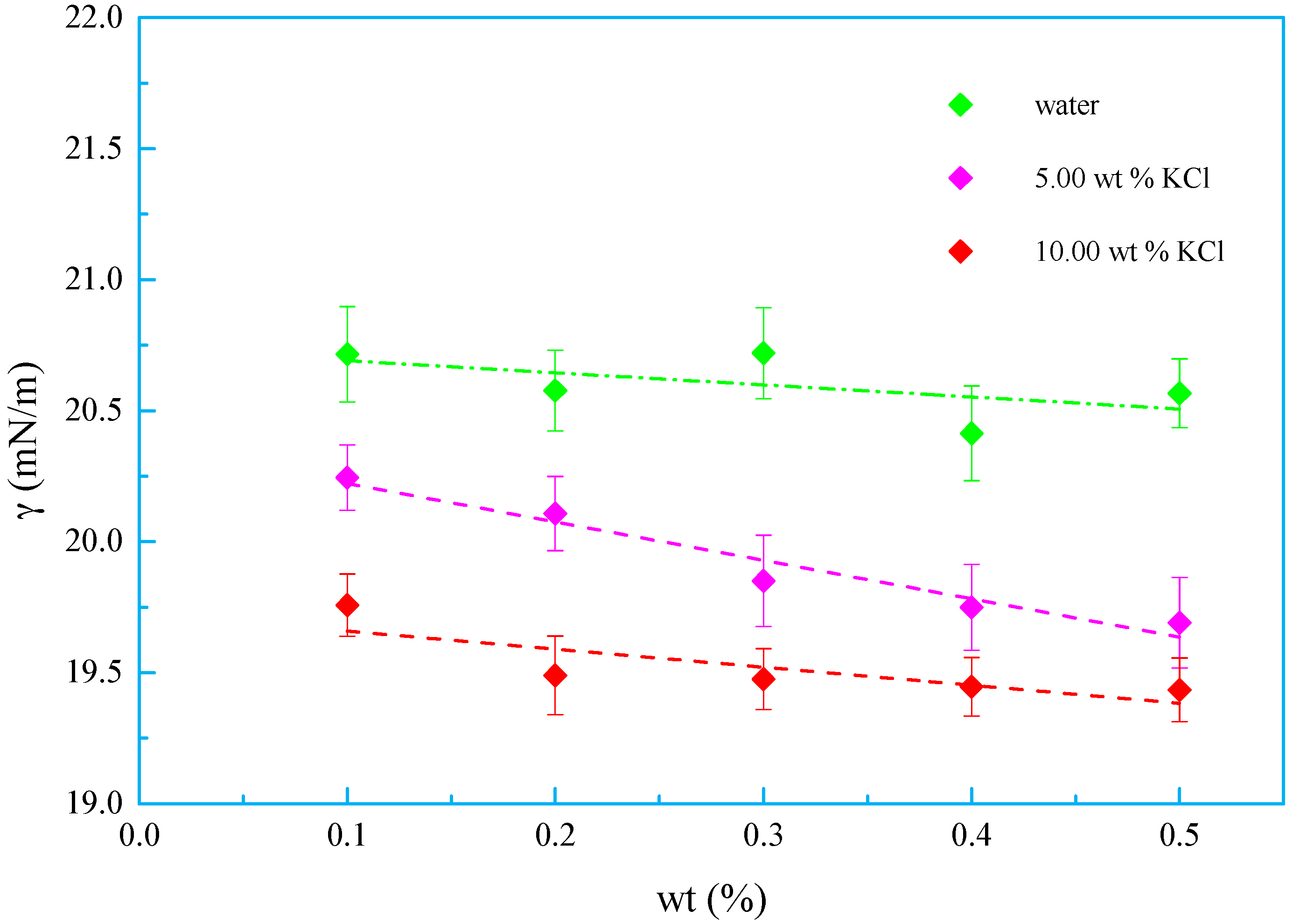
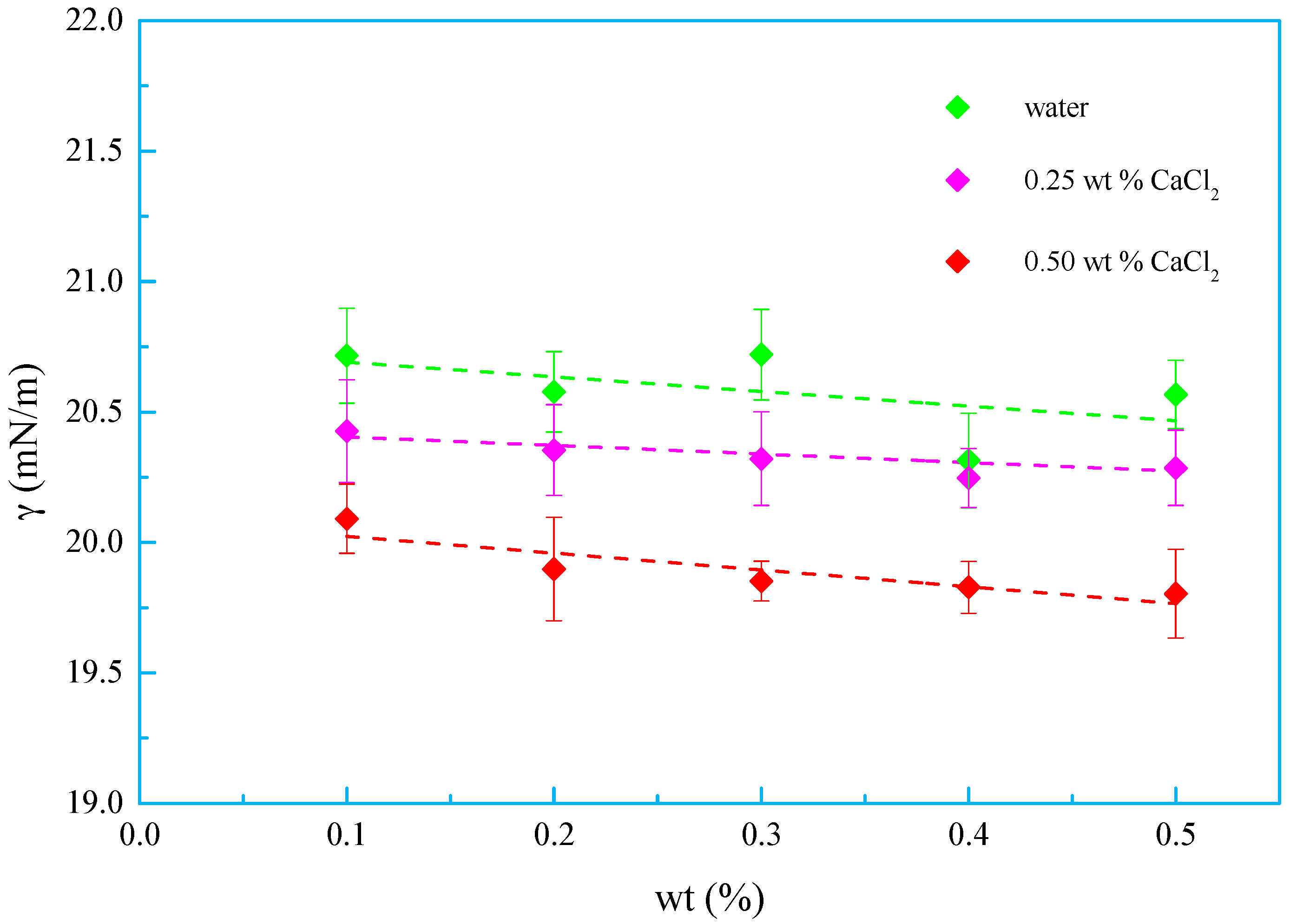
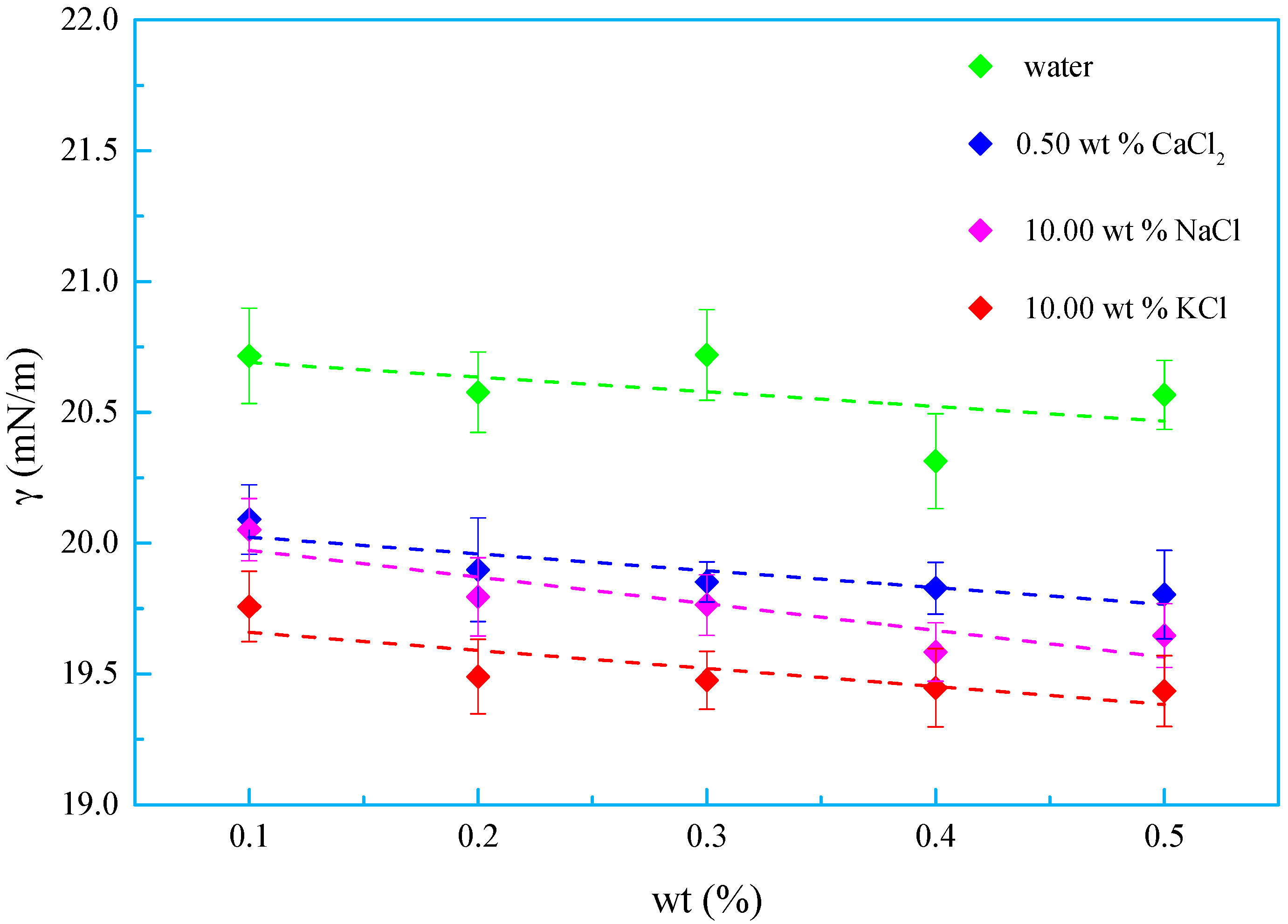
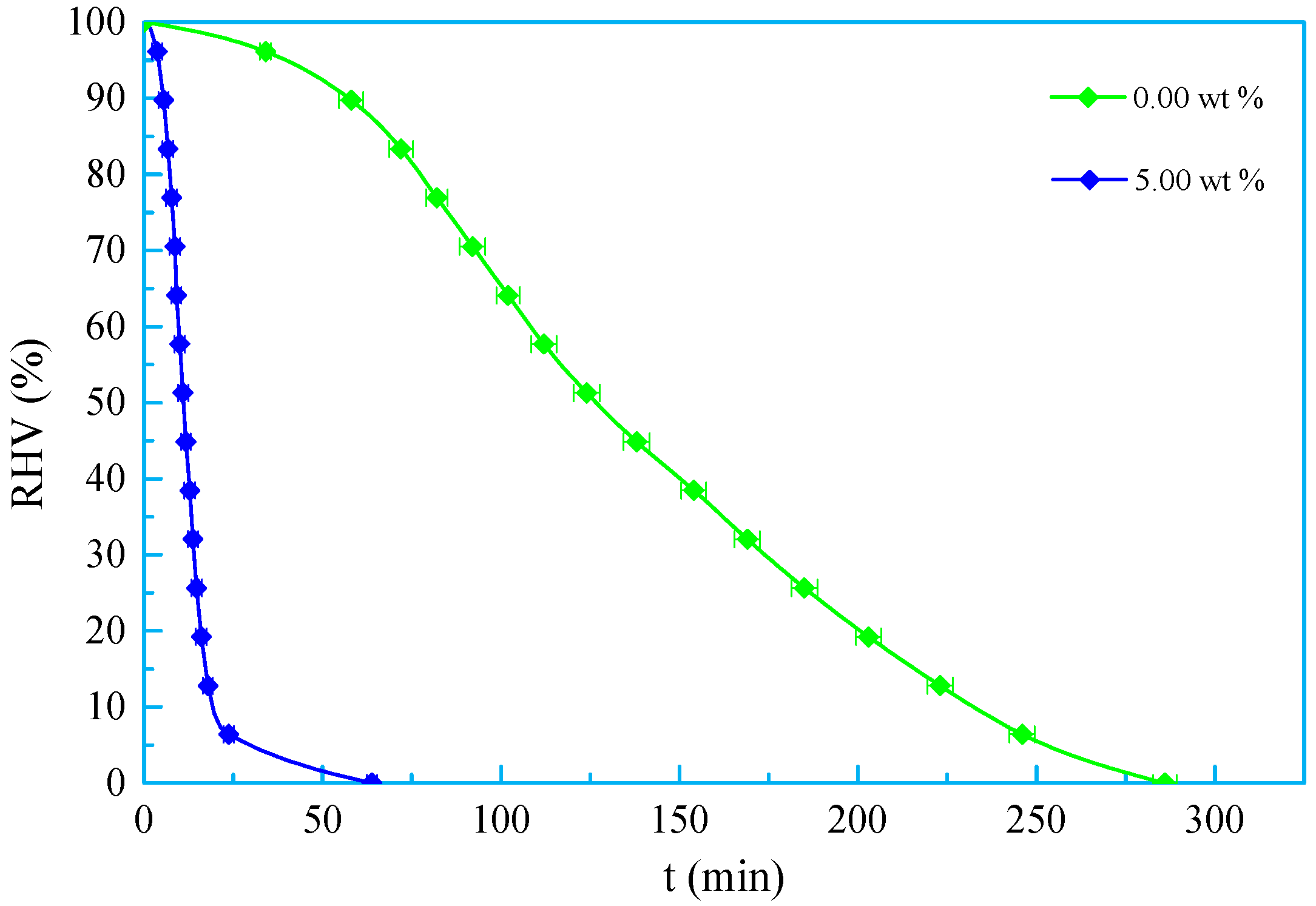
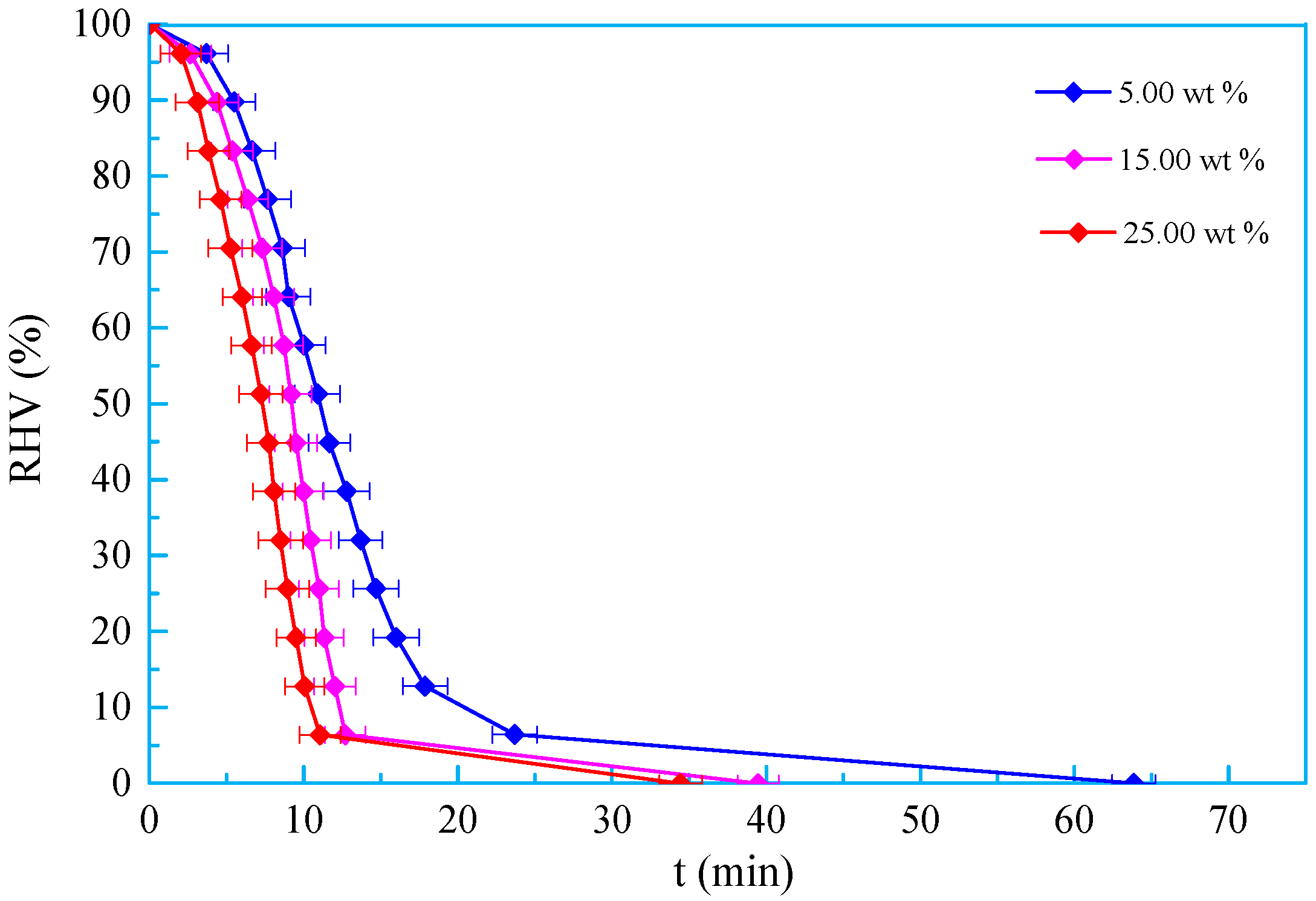
3.3. Salt Tolerance
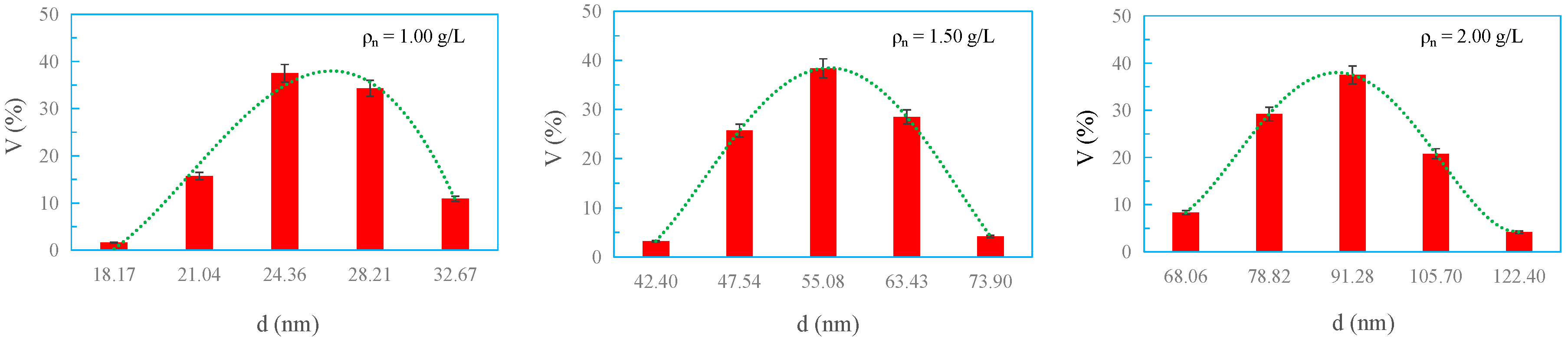
3.4. Size and Distribution of Micelles
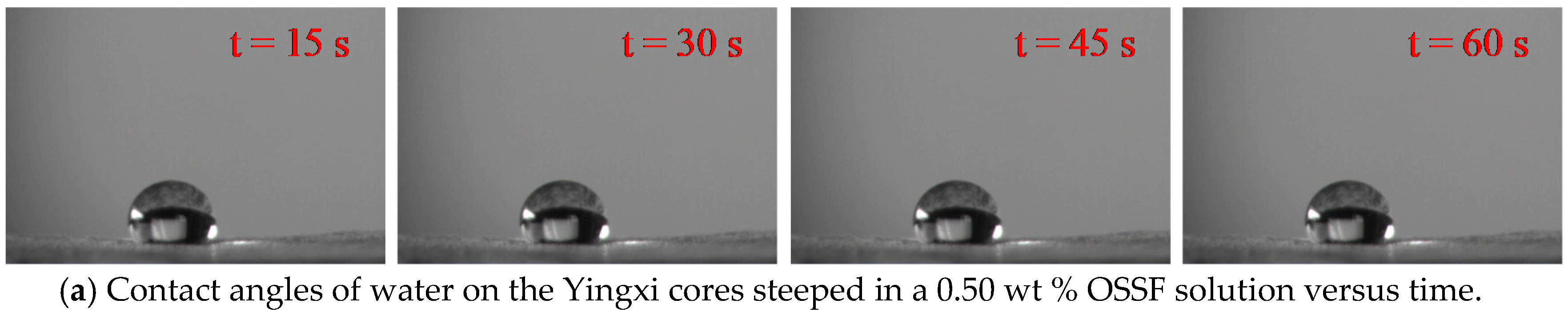
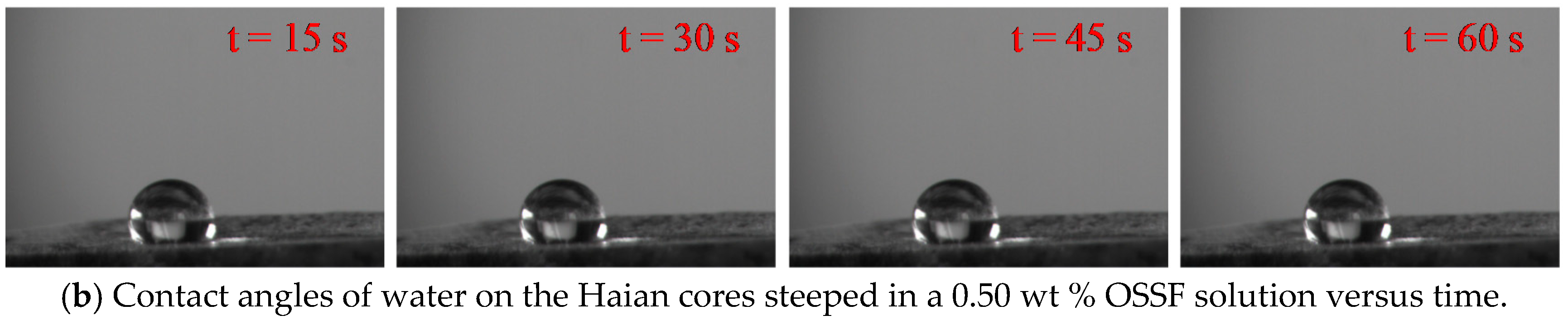
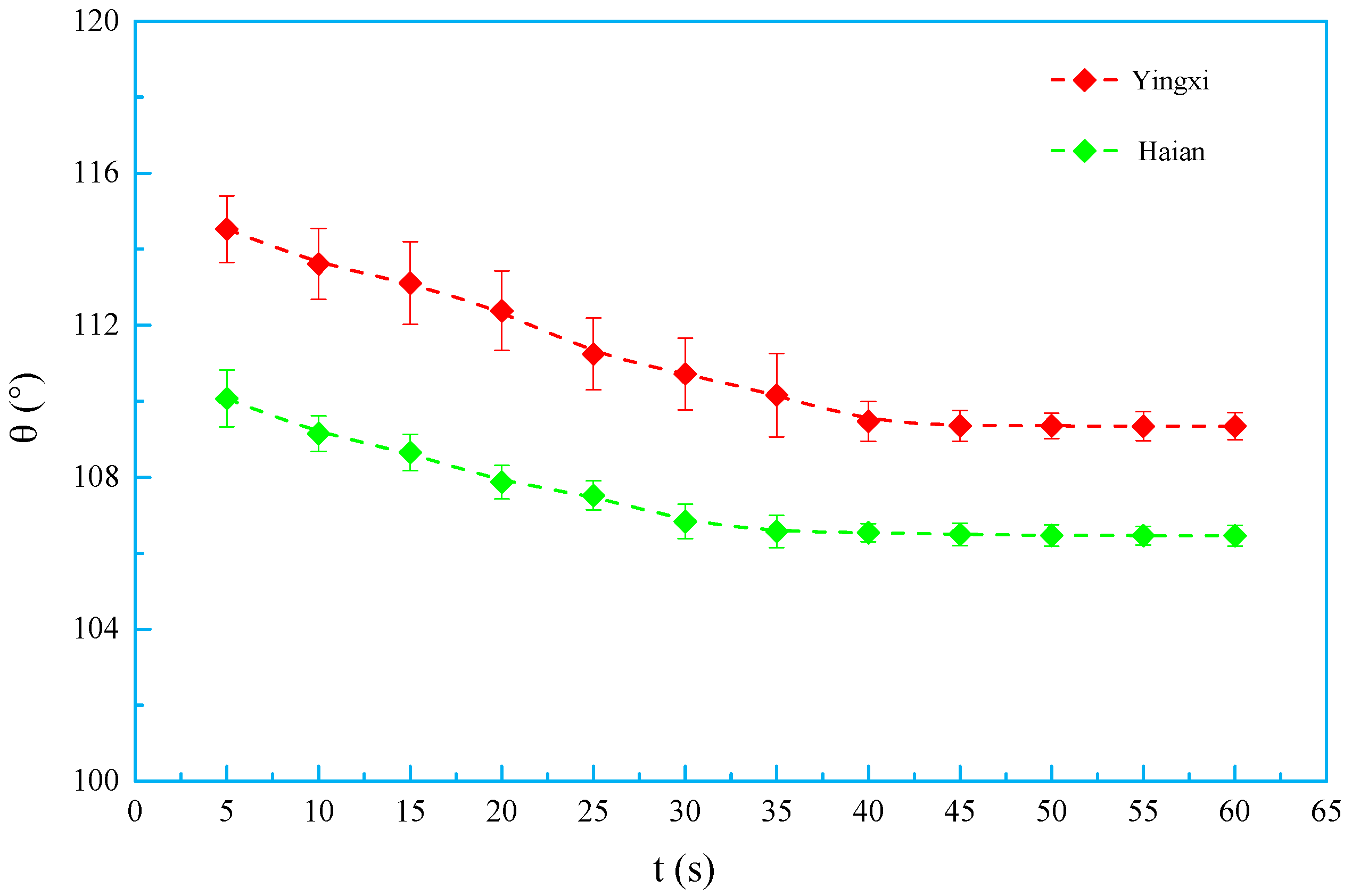
3.5. Contact Angle and Surface Energy
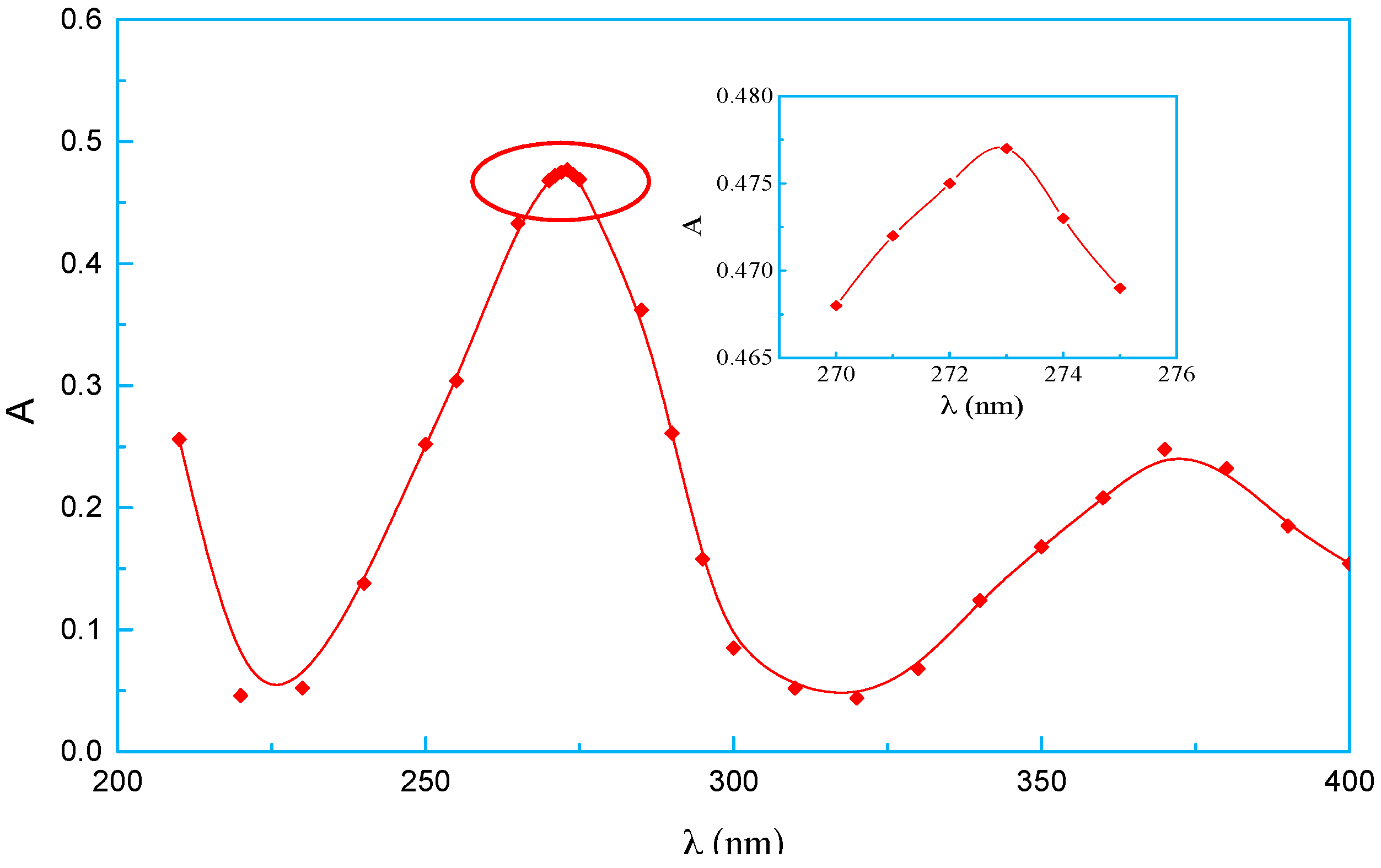
3.6. Surface Element Content
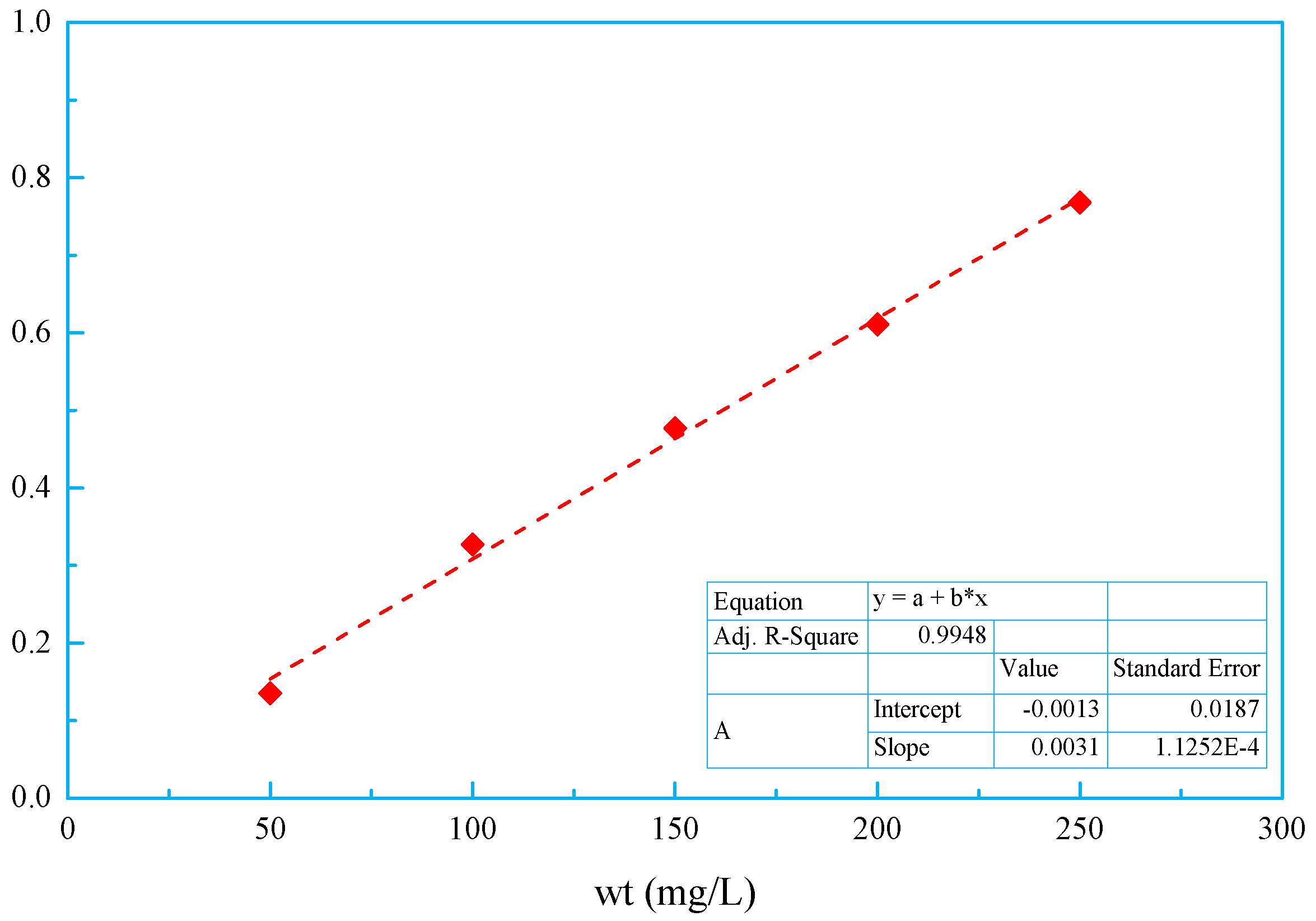
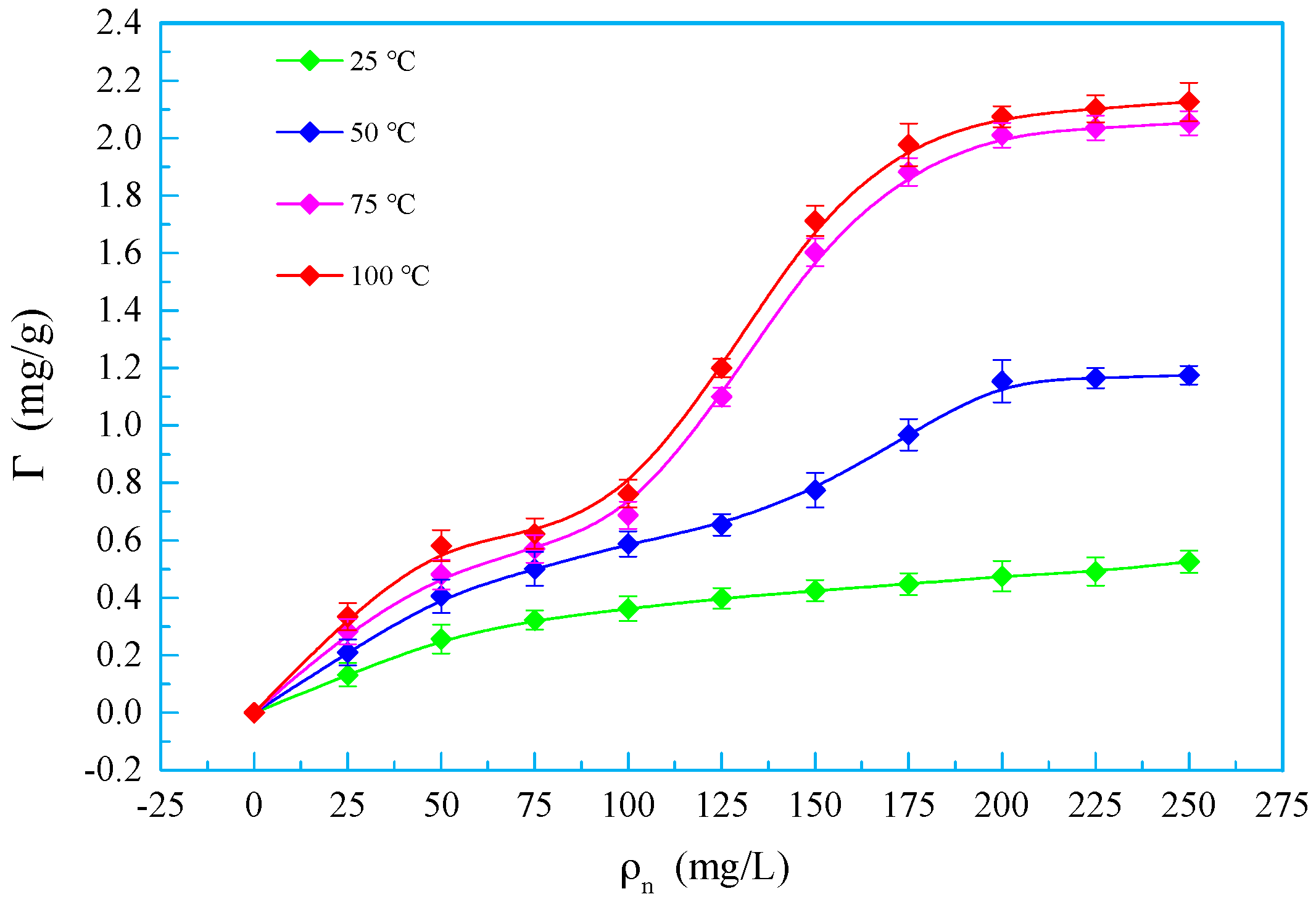
3.7. Adsorption Isotherms
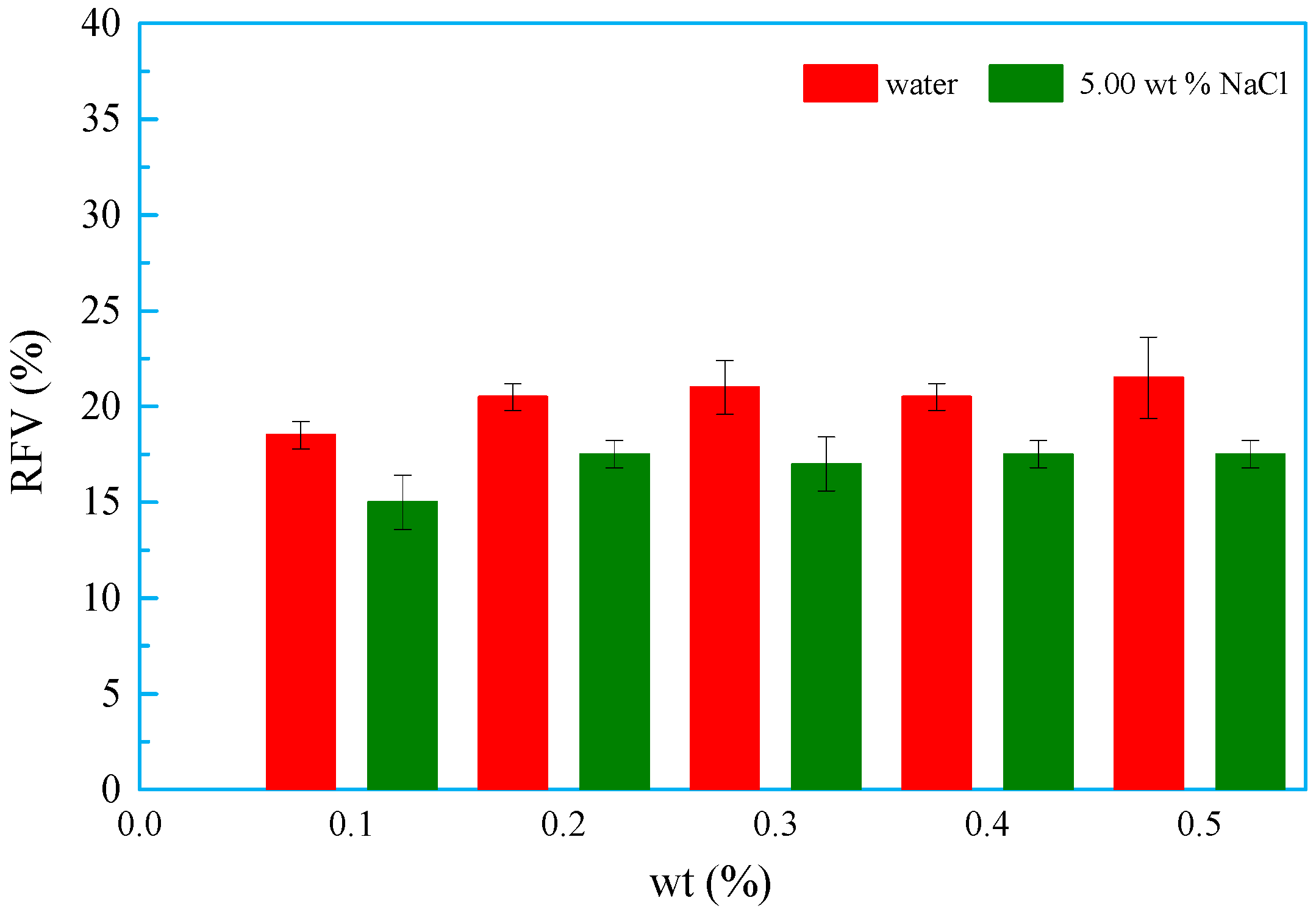
3.8. Foaming and Defoaming Property
3.8.1. Foaming Property
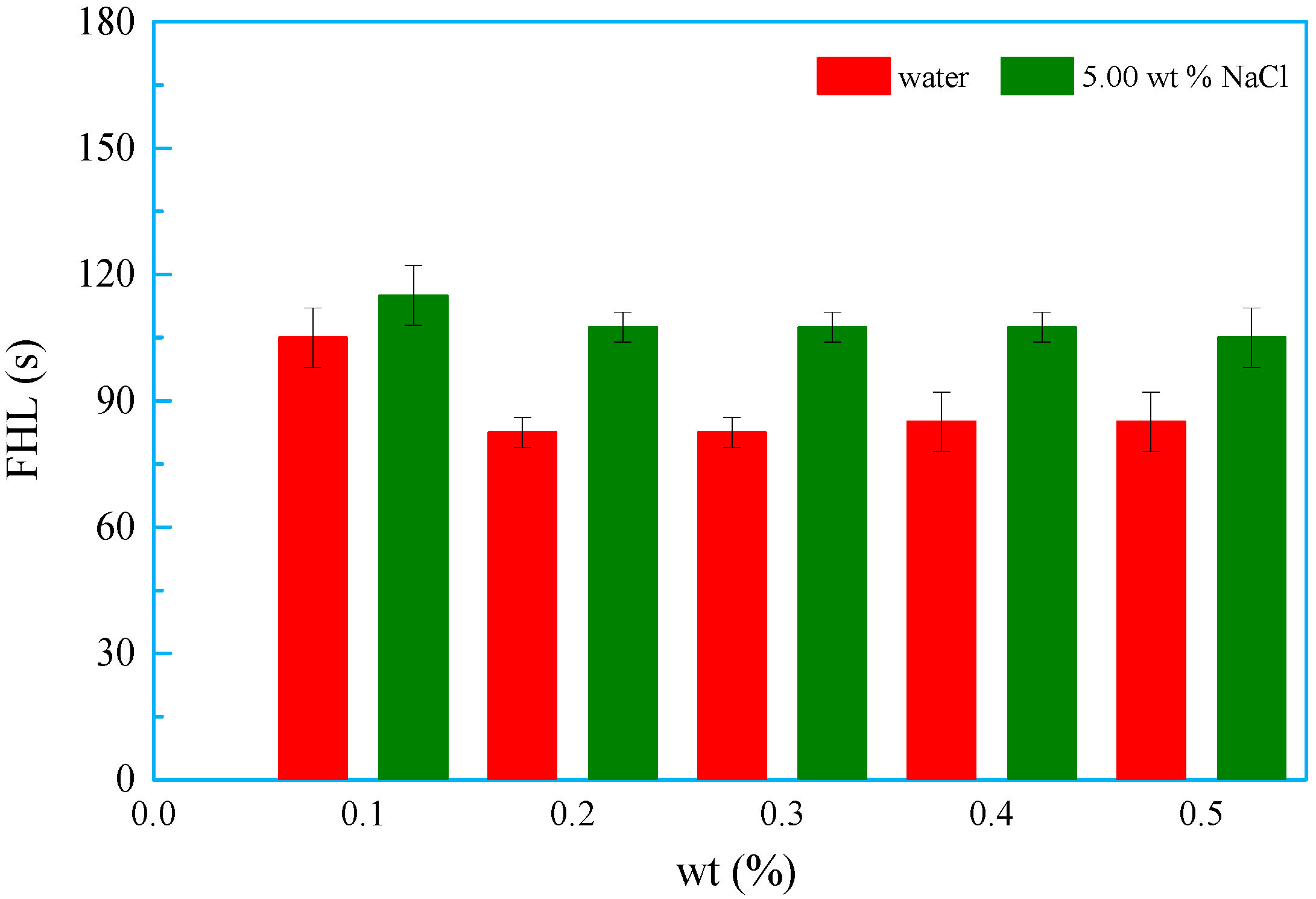
3.8.2. Defoaming Property
3.8.3. Foam-Suppressing Property
4. Conclusions
- (1)
- A new kind of oligomeric silicone surfactant (OSSF) containing sulfonic acid groups was synthesized. The critical micelle mass concentration was 0.980 g/L and the critical surface tension was 20.631 mN/m. The surface tension of OSSF increased with increasing hot rolling temperature and decreased with the addition of NaCl, KCl, or CaCl2.
- (2)
- OSSF adsorption transferred the wettability of cores from water-wet to preferential gas-wet. A change in the OSSF adsorption layers’ surface chemical composition occurred and exhibited lower interface energy than that of the cores. The adsorption isotherm of quartz sands changed from Langmuir type (L-type) to “double plateau” type (LS-type) in the OSSF solution due to the chemical adsorption at high temperature.
- (3)
- The foaming volume and property of OSSF began to stabilize as the weight percentage increased. The presence of NaCl decreased the foaming volume and improved the OSSF foam stability. At the same time, OSSF decreased the initial foaming volume and stability in the induction period and accelerated sodium dodecyl benzene sulfonate (SDBS) formation.
Author Contributions
Funding
Conflicts of Interest
References
- Bennion, D.B.; Thomas, F.B.; Ma, T. Formation damage processes reducing productivity of low permeability gas reservoirs. In Proceedings of the SPE Rocky Mountain Regional/Low-Permeability Reservoirs Symposium and Exhibition, Denver, CO, USA, 12–15 March 2000. SPE 60325. [Google Scholar]
- Brian, D.; William, D.W. Maximizing economic return by minimizing or preventing aqueous phase trapping during completion and stimulation operations. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 26–29 September 2004. SPE 90170. [Google Scholar]
- Zhou, X.P.; Sun, L.; Chen, C.G. Research on water block effect of low permeability gas reservoir. Spec. Oil Gas Reserv. 2005, 12, 52–54. [Google Scholar]
- Haimson, B.; Fairhurst, C. Hydraulic Fracturing in Porous-Permeability Materials. J. Pet. Technol. 1969, 21, 811–817. [Google Scholar] [CrossRef]
- Al-Anazi, H.A.; Walker, J.G.; Pope, G.A.; Sharma, M.M.; Hackney, D.F. A Successful Methanol Treatment in a Gas-Condenste Reservoir: Field Application. SPE Prod. Facil. 2005, 20, 60–69. [Google Scholar] [CrossRef]
- Li, G.; Meng, Y.F.; Tang, H.M. Clean Up Water Blocking in Gas Reservoirs by Microwave Heating: Laboratory Studies. In Proceedings of the International Oil & Gas Conference and Exhibition in China, Beijing, China, 5–7 December 2006. SPE 101072. [Google Scholar]
- Li, K.; Firoozabadi, A. Experimental Study of Wettability Alteration to Preferential Gas-Wetness in Porous Media and its Effect. SPE Reserv. Eval. Eng. 2000, 3, 139–149. [Google Scholar]
- Ren, L.W. Research and application progress of surfactant for drilling fluid. Sino-Glob. Energy 2016, 21, 34–39. [Google Scholar]
- Li, G.Z.; Xu, J. Application of surfactant in oil field and its principle. Adv. Fine Petrochem. 2004, 5, 1–6. [Google Scholar]
- Zhong, X.R.; Huang, L.; Wang, L.H. Research progress of water block effect in low permeability gas reservoirs. Spec. Oil Gas Reserv. 2008, 15, 12–23. [Google Scholar]
- Yao, T.Y.; Li, J.S.; Yao, F.Y. Influence of gas-wetting on seepage characteristics of condensate gas reservoir. Oilfield Chem. 2008, 25, 101–104. [Google Scholar]
- Barnum, R.S.; Brinkman, F.P.; Richardson, T.W. Gas condensate reservoir behavior: Productivity and recovery reduction due to condensation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 22–25 October 1995. SPE 30767. [Google Scholar]
- Li, K.W.; Firoozabadi, A. Phenomenological modeling of critical-condensate saturation and relative permeabilities in gas-condensate systems. SPE J. 2000, 5, 138–147. [Google Scholar] [CrossRef]
- Brook, M.A.; Zelisko, P.M.; Walsh, M.J.; Crowley, J.N. Silicone-protein surfactants; stability of water-in-silicone oil emulsions. Silicon Chem. 2002, 1, 99–106. [Google Scholar] [CrossRef]
- Xiao, J.X.; Zhao, Z.G. Application Principle of Surfactant; Chem. Ind. Press: Beijing, China, 2003; pp. 327–330. [Google Scholar]
- Wang, J.; Wang, P.Y. Special Surfactant; China Light Ind. Press: Beijing, China, 1997; pp. 440–447. [Google Scholar]
- Yang, T.T.; Peng, H.; Cheng, S.Y.; Park, I.J. Surface immobilization of perfluorinated acrylate copolymers by self-crosslinking. J. Fluor. Chem. 2005, 126, 1570–1577. [Google Scholar]
- Colas, A.R.; Renauld, F.A. Organosilicon Sulfosuccinate(s) Preparation by Reaction of Organosilicon Compounds with Base and Sodium Bisulfite, Useful as Surfactants. US Patent No. 4777277, 3 March 1988. [Google Scholar]
- Huang, L.X.; Li, S.Q.; Li, T. Synthesis and properties of sulfonate and polyether trisiloxane. Text. Aux. 2015, 32, 25–28. [Google Scholar]
- Zhang, L.; Huang, L.X.; Li, S.Q. Preparation and Properties of Sulfonate Fluorosilicone Surfactant. Fine Chem. 2017, 34, 45–51. [Google Scholar]
- Wang, H.; Gu, G.H.; Qiu, G.Z. Evaluation of surface free energy of polymers by contact angle goniometry. J. Cent. South Univ. Sci. Technol. 2006, 37, 942–947. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Zhang, W.C.; You, L.J. The adsorption characteristics of fluorocarbon surfactants on rock mineral surface. J. Yangtze Univ. Nat. Sci. Ed. 2016, 13, 6–12. [Google Scholar]
- Zhao, T.T.; Gong, H.J.; Xu, G.Y. Salt tolerance mechanism of anionic surfactant in aqueous solution. Oilfield Chem. 2010, 27, 112–118. [Google Scholar]
- Strauss, U.P.; Siegel, A. Counterion binding by polyelectrolytes VI the binding of magnesium ion by polyphosphates in aqueous electrolyte solutions. J. Phys. Chem. 1963, 67, 683–2687. [Google Scholar] [CrossRef]
- Wei, J.F.; Wu, D.Q. Surface ionization and complex reaction patterns of mineral water interface. Adv. Earth Sci. 2012, 35, 34–37. [Google Scholar]
- Wang, Q. Foaming and defoaming. Drill. Prod. Technol. 1994, 14, 9–13. [Google Scholar]
- Tang, J.K. Review on Influence Factors and Measurement Techniques of Foam Stability. Chem. Def. Ships 2008, 4, 1–8. [Google Scholar]
- Li, F.Q.; Tang, H.; Zhuang, K. Preparation and evaluation of polyether modified polysiloxane defoamer. Appl. Chem. Ind. 2017, 46, 1695–1699. [Google Scholar]






| Cores | Weight Percentage/wt % | Contact Angles/° | Dispersion Force/(mJ/m2) | Polar Force (mJ/m2) | Surface Energy/(mJ/m2) | |
|---|---|---|---|---|---|---|
| Water | Ethylene Glycol | |||||
| Haian | 0.0 | infiltration | infiltration | - | - | - |
| Yingxi | 0.0 | 13.47 | spreading | - | - | - |
| Haian | 0.1 | 46.86 | 11.32 | 16.53 | 35.09 | 51.63 |
| Haian | 0.2 | 109.35 | 25.28 | 2.96 | 20.55 | 23.51 |
| Haian | 0.3 | 108.76 | 24.82 | 2.30 | 19.80 | 22.09 |
| Haian | 0.4 | 110.12 | 27.14 | 3.47 | 20.80 | 24.27 |
| Haian | 0.5 | 110.01 | 25.37 | 3.91 | 21.58 | 25.49 |
| Yingxi | 0.1 | 52.21 | 12.67 | 21.48 | 26.93 | 48.42 |
| Yingxi | 0.2 | 114.34 | 39.58 | 5.87 | 20.98 | 26.85 |
| Yingxi | 0.3 | 113.61 | 38.72 | 4.97 | 20.37 | 25.34 |
| Yingxi | 0.4 | 113.94 | 39.20 | 5.33 | 20.59 | 25.92 |
| Yingxi | 0.5 | 114.53 | 39.19 | 6.44 | 21.52 | 27.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, D.; Luo, P.; Zhang, J.; Yao, X.; Wang, R.; Wang, L.; Wang, S. Synthesis of Oligomeric Silicone Surfactant and its Interfacial Properties. Appl. Sci. 2019, 9, 497. https://doi.org/10.3390/app9030497
Yin D, Luo P, Zhang J, Yao X, Wang R, Wang L, Wang S. Synthesis of Oligomeric Silicone Surfactant and its Interfacial Properties. Applied Sciences. 2019; 9(3):497. https://doi.org/10.3390/app9030497
Chicago/Turabian StyleYin, Da, Pingya Luo, Jie Zhang, Xuyang Yao, Ren Wang, Lihui Wang, and Shuangwei Wang. 2019. "Synthesis of Oligomeric Silicone Surfactant and its Interfacial Properties" Applied Sciences 9, no. 3: 497. https://doi.org/10.3390/app9030497
APA StyleYin, D., Luo, P., Zhang, J., Yao, X., Wang, R., Wang, L., & Wang, S. (2019). Synthesis of Oligomeric Silicone Surfactant and its Interfacial Properties. Applied Sciences, 9(3), 497. https://doi.org/10.3390/app9030497






