The Effect of Pharmaceutical Excipients for Applying to Spray-Dried Omega-3 Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Spray-Dried Powder
2.3. Determination of Particle Size of Spray-Dried Powder
2.4. Determination of Moisture Amount, Free Oil, Encapsulated Oil, and Total Oil of Spray-Dried Powder
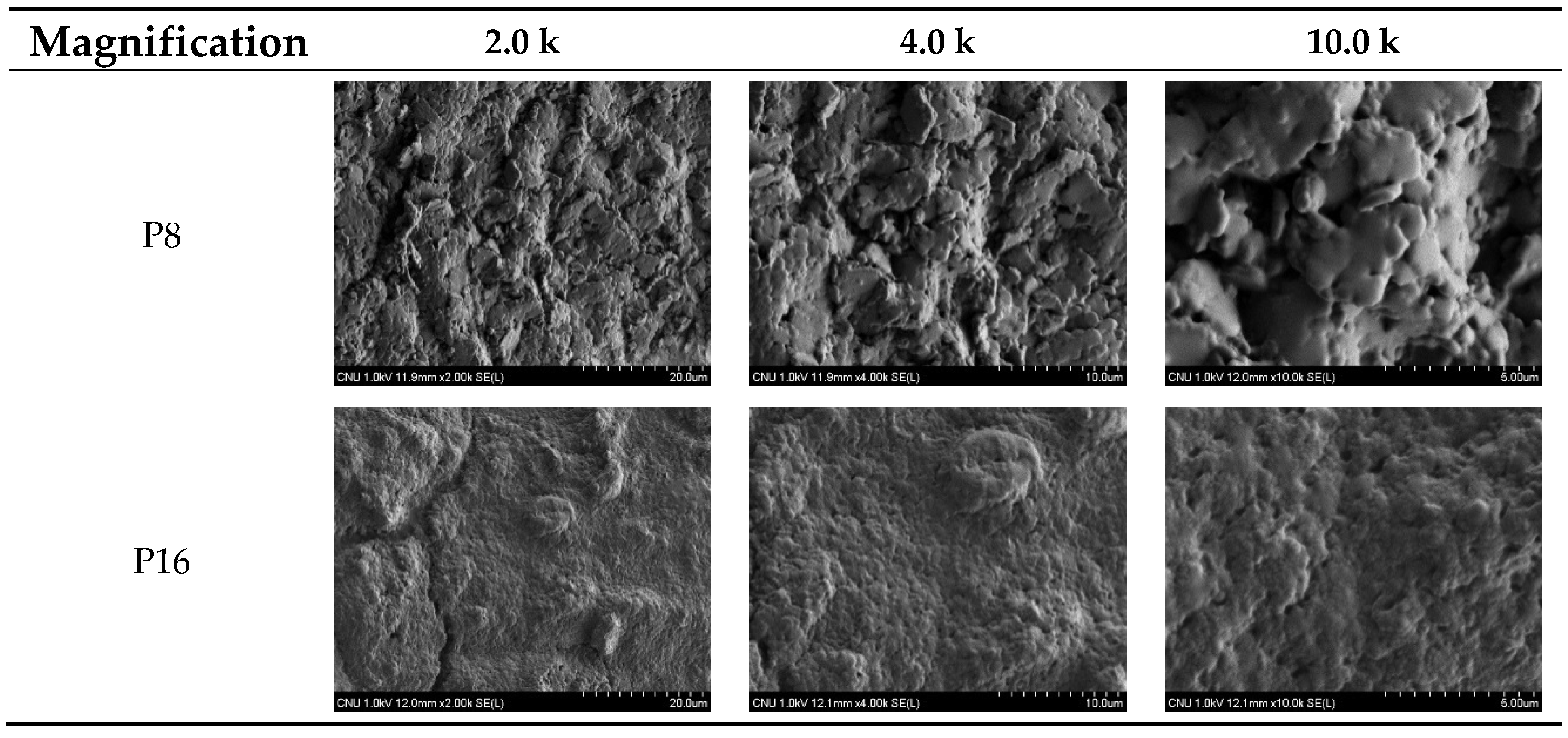
2.5. Scanning Electron Microscopy (SEM)
2.6. Chromatography Condition
2.7. Statistical Analysis
3. Results
3.1. Preparation of Spray-Dried Emulsion Powder
3.2. The Particle Size of Spray-Dried Powder
3.3. Determination of Moisture Content, Free Oil, Encapsulated Oil, and Total Oil
3.4. SEM Image
3.5. Characteristics of Spray-Dried Powder According to Ratio of Encapsulant To Surfactant
3.6. Determination of Particle Size, Moisture and Free Oil, Encapsulated Oil, and Total Oil
3.7. Characteristics of Omega-3 Fatty Acid Powder
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hung, P.; Kaku, S.; Yunoki, S.; Ohkura, K.; Gu, J.Y.; Ikeda, I.; Sugano, M.; Yazawa, K.; Yamada, K. Dietary effect of EPA-rich and DHA-rich fish oils on the immune function of Sprague-Dawley rats. Biosci. Biotechnol. Biochem. 1999, 63, 135–140. [Google Scholar] [CrossRef]
- Christensen, K.L.; Pedersen, G.P.; Kristensen, H.G. Physical stability of redispersible dry emulsions containing amorphous sucrose. Eur. J. Pharm. Biopharm. 2002, 53, 147–153. [Google Scholar] [CrossRef]
- Conquer, J.A.; Roelfsema, H.; Zecevic, J.; Graham, T.E.; Holub, B.J. Effect of exercise on FA profiles in n-3 FA-supplemented and-non supplemented premenopausal women. Lipids 2002, 37, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Dollo, G.; Le Corre, P.; Guėrin, A.; Chevanne, F.; Burgot, J.L.; Leverge, R. Spray-dried redispersible oil-in-water emulsion to improve oral bioavailability of poorly soluble drugs. Eur. J. Pharm. Sci. 2003, 19, 273–280. [Google Scholar] [CrossRef]
- Humberstone, A.J.; Charman, W.N. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Adv. Drug Deliv. Rev. 1997, 25, 103–128. [Google Scholar] [CrossRef]
- Jang, D.J.; Jeong, E.J.; Lee, H.M.; Kim, B.C.; Lim, S.J.; Kim, C.K. Improvement of bioavailability and photostability of amlodipine using redispersible dry emulsion. Eur. J. Pharm. Sci. 2006, 28, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Schrooyen, P.M.; van der Meer, R.; De Kruif, C.G. Microencapsulation: Its application in nutrition. Proc. Nutr. Soc. 2001, 60, 475–479. [Google Scholar] [CrossRef]
- Shah, A.V.; Serajuddin, A.T. Development of solid self-emulsifying drug delivery system (SEDDS) I: Use of poloxamer 188 as both solidifying and emulsifying agent for lipids. Pharm. Res. 2012, 29, 2817–2832. [Google Scholar] [CrossRef]
- Tan, A.; Rao, S.; Prestidge, C.A. Transforming lipid-based oral drug delivery systems into solid dosage forms: An overview of solid carriers, physicochemical properties, and biopharmaceutical performance. Pharm. Res. 2013, 30, 2993–3017. [Google Scholar] [CrossRef]
- Klinkesorn, U.; Sophanodora, P.; Chinachoti, P.; Decker, E.A.; McClements, D.J. Characterization of spray-dried tuna oil emulsified in two-layered interfacial membranes prepared using electrostatic layer-by-layer deposition. Food Res. Int. 2006, 39, 449–457. [Google Scholar] [CrossRef]
- Araujo, P.; Nguyen, T.T.; Frøyland, L.; Wang, J.; Kang, J.X. Evaluation of a rapid method for the quantitative analysis of fatty acids in various matrices. J. Chromatogr. A 2008, 1212, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Vestland, T.L.; Jacobsen, Ø.; Sande, S.A.; Myrset, A.H.; Klaveness, J. Characterization of omega-3 tablets. Food Chem. 2016, 197, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.L.; Pedersen, G.P.; Kristensen, H.G. Preparation of redispersible dry emulsions by spray drying. Int. J. Pharm. 2001, 212, 187–194. [Google Scholar] [CrossRef]
- Hansen, T.; Holm, P.; Rohde, M.; Schultz, K. In vivo evaluation of tablets and capsules containing spray-dried o/w-emulsions for oral delivery of poorly soluble drugs. Int. J. Pharm. 2005, 293, 203–211. [Google Scholar] [CrossRef]
- Rowe, R. Handbook of Pharmaceutical Excipients, 7th ed.; Pharmaceutical Press: London, UK, 2006. [Google Scholar]
- Buttini, F.; Soltani, A.; Colombo, P.; Marriott, C.; Jones, S.A. Multilayer PVA adsorption onto hydrophobic drug substrates to engineer drug-rich microparticles. Eur. J. Pharm. Sci. 2008, 33, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, Y.P.; Seo, E.M.; Choi, Y.K.; Kim, H.S. Antioxidant effect of natural plant extracts on the microencapsulated high oleic sunflower oil. J. Food Eng. 2008, 84, 327–334. [Google Scholar] [CrossRef]
- Sheu, T.Y.; Rosenberg, M. Microstructure of microcapsules consisting of whey proteins and carbohydrates. J. Food Sci. 1998, 63, 491–494. [Google Scholar] [CrossRef]
- Walton, D.E. The morphology of spray-dried particles: A qualitative view. Dry Technol. 2000, 18, 1943–1986. [Google Scholar] [CrossRef]
- Lee, J.H. Polyunsaturated Fatty Acids in Children. Pediatric Gastroenterol. Hepatol. Nutr. 2013, 16, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Vishnu, K.V.; Chatterjee, N.S.; Ajeeshkumar, K.K.; Lekshmi, R.G.K.; Tejpal, C.S.; Mathew, S.; Ravishankar, C.N. Microencapsulation of sardine oil: Application of vanillic acid grafted chitosan as a bio-functional wall material. Carbohydr. Polym. 2017, 174, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Kierulf, A.; Connie Lee, M.; Abbaspourrad, A. Improvement of physicochemical properties of encapsulated echium oil using nanostructured lipid carriers. Food Chem. 2018, 246, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Alamilla-Beltrán, L.; Chanona-Pérez, J.J.; Jiménez-Aparicio, A.R.; Gutiérrez-López, G.F. Description of morphological changes of particles along spray drying. J. Food Eng. 2005, 67, 179–184. [Google Scholar] [CrossRef]
- Gangurde, A.B.; Ali, M.T.; Pawar, J.N.; Amin, P.D. Encapsulation of vitamin E acetate to convert oil to powder microcapsule using different starch derivatives. J. Pharm. Investig. 2017, 47, 559–574. [Google Scholar] [CrossRef]





| Formular | SE (nm) | RE (nm) |
|---|---|---|
| P1 | 488.85 ± 30.8 | 600.85 ± 76.6 |
| P2 | 396.85 ± 3.0 | 483.95 ± 65.3 |
| P3 | 252.40 ± 11.5 | 469.55 ± 76.2 |
| P4 | 711.70 ± 168.1 | 660.85 ± 171.8 |
| P5 | 214.35 ± 3.5 | 473.50 ± 141.8 |
| P6 | 250.80 ± 8.9 | 360.40 ± 50.3 |
| P7 | 268.95 ± 13.4 | 374.75 ± 155.6 |
| P8 | 258.95 ± 7.3 | 293.35 ± 19.0 |
| P11 | 296.47 ± 22.8 | 472.73 ± 17.0 |
| P12 | 336.93 ± 37.8 | 472.97 ± 16.0 |
| P16 | 257.00 ± 8.3 | 216.57 ± 2.5 |
| P17 | 237.40 ± 10.1 | 330.20 ± 38.2 |
| P18 | 280.57 ± 17.8 | 246.20 ± 19.9 |
| P21 | 284.87 ± 5.1 | 342.13 ± 10.9 |
| P22 | 285.97 ± 2.0 | 405.60 ± 9.1 |
| P26 | 540.10 ± 39.5 | 847.70 ± 58.3 |
| P27 | 354.87 ± 3.3 | 695.83 ± 108.0 |
| P28 | 339.23 ± 24.1 | 712.20 ± 72.6 |
| Formular | Moisture (%) | Free Oil (g/100 g) | Encapsulated Oil (g/100 g) | Total Oil (g/100 g) |
|---|---|---|---|---|
| P1 | 5.10 ± 0.70 | 48.28 ± 1.42 | 10.85 ± 3.82 | 66.42 ± 1.33 |
| P2 | 3.50 ± 0.14 | 36.66 ± 3.26 | 10.30 ± 0.66 | 56.41 ± 2.47 |
| P3 | 5.05 ± 0.49 | 39.44 ± 3.48 | 5.92 ± 1.05 | 58.30 ± 0.30 |
| P4 | 8.30 ± 2.12 | 40.00 ± 2.99 | 7.79 ± 1.18 | 60.75 ± 0.83 |
| P5 | 6.20 ± 1.13 | 39.36 ± 0.90 | 9.24 ± 2.65 | 62.42 ± 0.23 |
| P6 | 3.55 ± 0.21 | 38.87 ± 2.21 | 13.74 ± 1.98 | 58.33 ± 3.01 |
| P7 | 4.10 ± 0.99 | 39.35 ± 1.60 | 9.66 ± 1.36 | 59.37 ± 2.29 |
| P8 | 4.98 ± 1.20 | 37.96 ± 2.97 | 8.66 ± 3.28 | 56.02 ± 5.89 |
| P11 | 2.90 ± 0.14 | 58.04 ± 1.53 | 17.33 ± 3.86 | 56.04 ± 2.90 |
| P12 | 2.85 ± 0.07 | 47.80 ± 0.37 | 11.36 ± 3.64 | 58.49 ± 7.90 |
| P16 | 3.75 ± 0.21 | 52.59 ± 0.97 | 10.27 ± 2.64 | 54.45 ± 1.79 |
| P17 | 3.75 ± 0.49 | 48.04 ± 1.44 | 10.86 ± 2.02 | 54.78 ± 2.93 |
| P18 | 4.00 ± 0.14 | 49.55 ± 0.63 | 10.18 ± 2.95 | 56.96 ± 4.26 |
| P21 | 5.90 ± 1.70 | 57.78 ± 0.42 | 11.77 ± 0.83 | 67.43 ± 4.52 |
| P22 | 4.75 ± 1.91 | 52.36 ± 0.44 | 10.22 ± 0.62 | 56.26 ± 1.94 |
| P26 | 6.45 ± 1.20 | 56.63 ± 5.43 | 9.18 ± 0.26 | 59.69 ± 3.84 |
| P27 | 3.80 ± 0.57 | 62.55 ± 2.06 | 8.57 ± 1.58 | 65.79 ± 6.57 |
| P28 | 5.50 ± 0.85 | 65.68 ± 1.69 | 7.73 ± 0.53 | 67.51 ± 2.03 |
| Formular | Inlet Temp. (°C) | Outlet Temp. (°C) | Atomization Pressure | Olive Oil (g) | DW (mL) | γ-CD (g) | Hydrogenated Lecithin (g) | Total (g) |
|---|---|---|---|---|---|---|---|---|
| P18-1 | 180 | 85–90 | 15 | 9.3 | 200 | 4.8 | 4.5 | 18.6 |
| P18-2 | 180 | 85–90 | 15 | 9.3 | 200 | 5.8 | 3.5 | 18.6 |
| P18-3 | 180 | 85–90 | 15 | 9.3 | 200 | 6.8 | 2.5 | 18.6 |
| P18-4 | 180 | 85–90 | 15 | 9.3 | 200 | 7.8 | 1.5 | 18.6 |
| P18-5 | 180 | 85–90 | 15 | 9.3 | 200 | 8.8 | 0.5 | 18.6 |
| P18-6 | 180 | 85–90 | 15 | 9.3 | 400 | 7.8 | 1.5 | 18.6 |
| P18-7 | 150 | 70–75 | 15 | 9.3 | 400 | 7.8 | 1.5 | 18.6 |
| P18-8 | 150 | 70–75 | 10 | 9.3 | 400 | 7.8 | 1.5 | 18.6 |
| Formular | Moisture (%) | SE (nm) | RE (nm) | Free Oil (g/100 g) | Encapsulated Oil (g/100 g) | Total Oil (g/100 g) |
|---|---|---|---|---|---|---|
| P18-1 | 4.17 ± 0.50 | 337.17 ± 8.32 | 351.77 ± 9.13 | 42.23 ± 0.03 | 10.20 ± 0.64 | 56.95 ± 0.70 |
| P18-2 | 3.67 ± 0.40 | 281.70 ± 1.93 | 388.13 ± 13.88 | 39.30 ± 4.60 | 11.14 ± 0.19 | 54.63 ± 2.11 |
| P18-3 | 3.87 ± 0.31 | 305.13 ± 3.45 | 323.20 ± 7.37 | 37.98 ± 4.67 | 12.35 ± 1.74 | 52.84 ± 3.52 |
| P18-4 | 3.40 ± 0.30 | 341.80 ± 4.95 | 344.73 ± 11.86 | 35.33 ± 1.21 | 14.12 ± 0.88 | 52.10 ± 1.52 |
| P18-5 | 3.67 ± 0.25 | 352.77 ± 24.8 | 378.33 ± 10.82 | 33.67 ± 0.07 | 14.22 ± 2.78 | 51.58 ± 1.85 |
| P18-6 | 2.03 ± 0.15 | 355.83 ± 21.1 | 366.80 ± 16.51 | 32.53 ± 2.20 | 18.55 ± 0.67 | 52.77 ± 0.94 |
| P18-7 | 2.33 ± 0.06 | 354.93 ± 6.21 | 337.37 ± 15.46 | 30.33 ± 0.83 | 19.14 ± 1.07 | 51.89 ± 0.93 |
| P18-8 | 2.43 ± 0.15 | 328.37 ± 19.5 | 355.97 ± 14.28 | 29.21 ± 0.66 | 19.38 ± 0.81 | 52.44 ± 1.30 |
| Formular | Moisture (%) | SE (nm) | RE (nm) | Free Oil (g/100 g) | Encapsulated Oil (g/100 g) | Total Oil (g/100 g) |
|---|---|---|---|---|---|---|
| P-O | 2.63 ± 0.21 | 309.37 ± 10.17 | 326.70 ± 9.53 | 33.46 ± 1.22 | 18.57 ± 0.69 | 53.29 ± 1.50 |
| EPA | DHA | |
|---|---|---|
| Concentration (mg/mg powder) | 0.19 ± 0.02 | 0.22 ± 0.01 |
| Remaining percent (%) | 78.68 ± 7.39 | 98.23 ± 4.38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, C.-J.; Na, Y.-G.; Huh, H.W.; Kim, M.; Lee, H.-K.; Cho, C.-W. The Effect of Pharmaceutical Excipients for Applying to Spray-Dried Omega-3 Powder. Appl. Sci. 2019, 9, 1177. https://doi.org/10.3390/app9061177
Hwang C-J, Na Y-G, Huh HW, Kim M, Lee H-K, Cho C-W. The Effect of Pharmaceutical Excipients for Applying to Spray-Dried Omega-3 Powder. Applied Sciences. 2019; 9(6):1177. https://doi.org/10.3390/app9061177
Chicago/Turabian StyleHwang, Chan-Joo, Young-Guk Na, Hyun Wook Huh, MinKi Kim, Hong-Ki Lee, and Cheong-Weon Cho. 2019. "The Effect of Pharmaceutical Excipients for Applying to Spray-Dried Omega-3 Powder" Applied Sciences 9, no. 6: 1177. https://doi.org/10.3390/app9061177
APA StyleHwang, C.-J., Na, Y.-G., Huh, H. W., Kim, M., Lee, H.-K., & Cho, C.-W. (2019). The Effect of Pharmaceutical Excipients for Applying to Spray-Dried Omega-3 Powder. Applied Sciences, 9(6), 1177. https://doi.org/10.3390/app9061177






