Recent Updates on the Use of Agro-Food Waste for Biogas Production
Abstract
1. Introduction
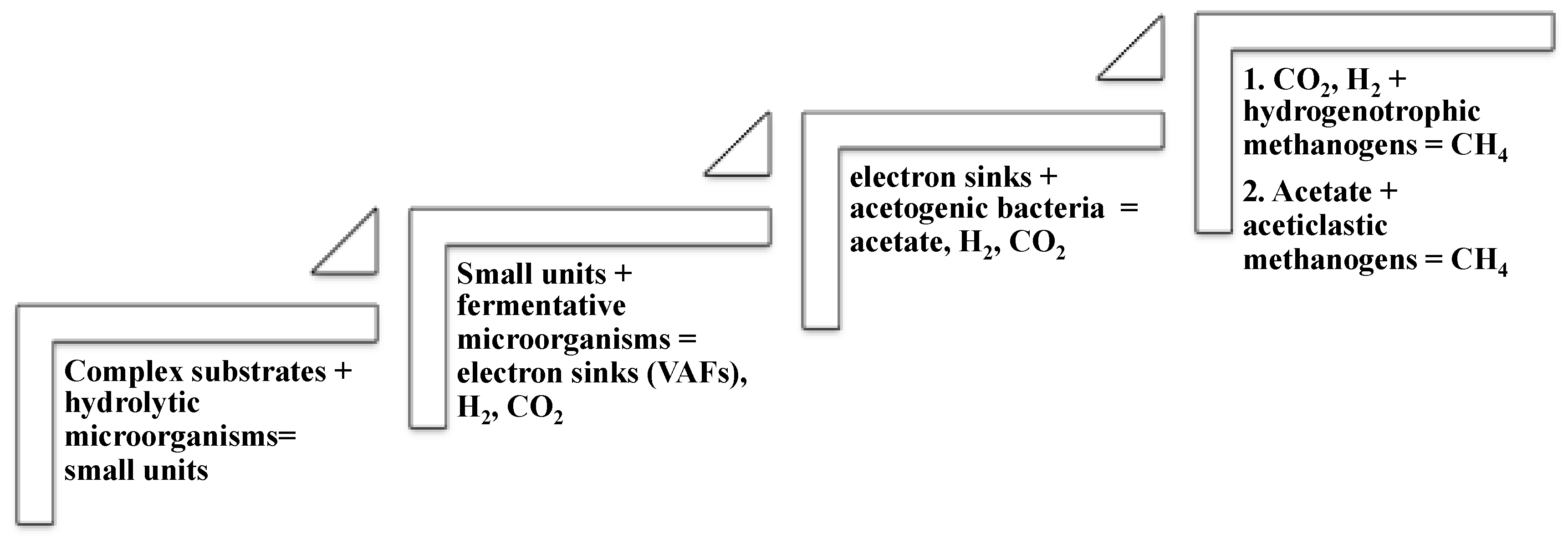
2. Microbial Processes of Anaerobic Digestion
2.1. Hydrolysis
2.2. Acidogenesis
2.3. Acetogenesis
2.4. Methanogenesis
- hydrogenotrophic methanogenesis, the most common metabolic pathway, where CO2 and H2 are transformed into methane;
- the aceticlastic methanogenesis, where acetate is directly converted to methane.
3. Anaerobic Digestion Plant Technologies
3.1. Monitoring and Control of Plant Efficiency
3.2. Economic Considerations about Biogas Plant
4. Agro-Food Substrates for Anaerobic Digestion
4.1. Livestock Manure
4.1.1. Cattle Manure
4.1.2. Horse Manure
4.1.3. Poultry Manure
4.1.4. Pig Manure
4.1.5. Inhibitory Substances in Livestock Manure
4.2. Fruits and Vegetables Waste
5. Substrate Composition and Microbial Communities
6. Strategies to Improve the Efficiency of the AD Process
6.1. Co-Digestion
6.2. Chemical Pre-Treatments
6.3. Microbiological Tools to Improve Biogas Production
7. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC; European Commission: Brussels, Belgium, 2009.
- Olivier, J.G.J.; Schure, K.M.; Peters, J.A.H.W. Trends in Global CO2 and Total Greenhouse Gas Emissions, Report (2017); PBL Publication Number: 2674; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2017. [Google Scholar]
- Akinbami, J.F.K.; Ilori, M.O.; Oyebisi, T.O.; Akinwumi, I.O.; Adeoti, I.O.O. Biogas Energy use in Nigeria: Current status. Future Prospects and Policy Implication. Renew. Sustain. Energy Rev. 2001, 5, 97–112. [Google Scholar] [CrossRef]
- Meyer-Aurich, A.; Schattauer, A.; Hellebrand, H.J.; Klauss, H.; Plochl, M.; Berg, W. Impact of uncertainties on greenhouse gas mitigation potential of biogas production from agricultural resources. Renew. Energy 2012, 37, 277–284. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. Urban Development Series; World Bank: Washington, DC, USA, 2018; License: Creative Commons Attribution CC BY 3.0 IGO. [Google Scholar] [CrossRef]
- Candolo, G. Biomasse vegetali: I possibili processi di conversione energetica. Agronomica 2005, 4, 31–38. [Google Scholar]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- Al Mamun, R.; Torii, S. Anaerobic co-digestion technology in solid wastes treatment for biomethane generation. Int. J. Sustain. Energy 2015, 36, 462–472. [Google Scholar] [CrossRef]
- Cirne, D.G.; Lehtomaki, A.; Bjornsson, L.; Blackall, L.L. Hydrolysis and microbial community analyses in two-stage anaerobic digestion of energy crops. J. Appl. Microbiol. 2007, 103, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pena, E.I.; Parameswaran, P.; Kang, D.W.; Canul-Chan, M.; Krajmalnik-Brown, R. Anaerobic digestion and co-digestion processes of vegetable and fruit residues: Process and microbial ecology. Bioresour. Technol. 2011, 102, 9447–9455. [Google Scholar] [CrossRef] [PubMed]
- Ros, M.; Frankle-Whittle, I.H.; Morales, A.B.; Insam, H.; Ayuso, M.; Pascual, J.A. Archaeal community dynamics and abiotic characteristics in a mesophilic anaerobic co-digestion process treating fruit and vegetable processing waste sludge with chopped fresh artichoke waste. Bioresour. Technol. 2013, 136, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Ou, Y.L.; Lin, J.G. Co-composting of green waste and food waste at low C/N ratio. Waste Manag. 2010, 30, 602–609. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Food Sci. Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef]
- Mane, A.B.; Rao, B.; Rao, A.B. Characterisation of fruit and vegetable waste for maximizing the biogas yield. Int. J. Adv. Technol. Eng. Sci. 2015, 3, 489–500. [Google Scholar]
- Braguglia, C.M.; Gallipoli, A.; Gianico, A.; Pagliaccia, A. Anaerobic bioconversion of food Wastes into energy: A critical review. Bioresour. Technol. 2018, 248, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Scarlat, N.; Dallemand, J.F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Deremince, B.; Königsberger, S. Statistical Report; European Biogas Association: Brussels, Belgium, 2017. [Google Scholar]
- André, L.; Pauss, A.; Ribeiro, T. Solid anaerobic digestion: State-of-art, scientific and technological hurdles. Bioresour. Technol. 2017, 247, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Chiumenti, A.; da Borso, F.; Limina, S. Dry anaerobic digestion of cow manure and agricultural products in a full-scale plant: Efficiency and comparison with wet fermentation. Waste Manag. 2018, 71, 704–710. [Google Scholar] [CrossRef]
- Nges, I.A.; Liu, J. Effects of solid retention time on anaerobic digestion of dewatered-sewage sludge in mesophiic and termophilic conditions. Renew. Energy 2010, 35, 2200–2206. [Google Scholar] [CrossRef]
- Micolucci, F.; Gottardo, M.; Pavan, P.; Cavinato, C.; Bolzonella, D. Pilot scale comparison of single and double-stage thermophilic anaerobic digestion of food waste. J. Clean. Prod. 2018, 171, 1376–1385. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Horváth, I.S.; Tabatabaei, M.; Karimi, K.; Kumar, R. Recent updates on biogas production—A review. Biofuel Res. J. 2016, 10, 394–402. [Google Scholar] [CrossRef]
- Krzeminski, P.; Leverette, L.; Malamis, S.; Katsou, E. Membrane bioreactors—A review on recent developments in energy reduction, fouling control, novel configurations, LCA and market prospects. J. Membr. Sci. 2017, 527, 207–227. [Google Scholar] [CrossRef]
- Wandera, S.M.; Qiao, W.; Algapani, D.E.; Bi, S.; Yin, D.; Qi, X.; Dong, R. Searching for possibilities to improve the performance of full scale agricultural biogas plants. Renew. Energy 2018, 116, 720–727. [Google Scholar] [CrossRef]
- Spanjers, H.; van Lier, J.B. Instrumentation in anaerobic treatment–research and practice. Water Sci. Technol. 2006, 53, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.; Holm-Nielsen, J.B.; Esbensen, K.H. Monitoring of anaerobic digestion processes: A review perspective. Renew. Sustain. Energy Rev. 2011, 15, 3141–3155. [Google Scholar] [CrossRef]
- Li, L.; He, Q.; Wei, Y.; He, Q.; Peng, X. Early warning indicators for monitoring the process failure of anaerobic digestion system of food waste. Bioresour. Technol. 2014, 17, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, H.; Li, H.; Li, J. Study on indicators for on-line monitoring and diagnosis of anaerobic digestion process of piggery wastewater. Environ. Technol. Innov. 2017, 8, 423–430. [Google Scholar] [CrossRef]
- Stockl, A.; Lichti, F. Near-infrared spectroscopy (NIRS) for a real time monitoring of the biogas process. Bioresour. Technol. 2018, 247, 1249–1252. [Google Scholar] [CrossRef]
- Nguyen, D.; Gadhamshetty, V.; Nitayavardhana, S.; Khanal, S.K. Automatic process control in anaerobic digestion technology: A critical review. Bioresour. Technol. 2015, 193, 513–522. [Google Scholar] [CrossRef]
- Grando, R.L.; de Souza Antune, A.M.; da Fonseca, F.V.; Sánchez, A.; Barrena, R.; Font, X. Technology overview of biogas production in anaerobic digestion plants: A European evaluation of research and development. Renew. Sustain. Energy Rev. 2017, 80, 44–53. [Google Scholar] [CrossRef]
- Bauer, F.; Hulteberg, C.; Persson, T.; Tamm, D. Biogas Upgrading– Review of Commercial Technologies; SGC Rapport; SGC: Malmö, Sweden, 2013; p. 270. [Google Scholar]
- IRENA. Road Transport: The Cost of Renewable Solutions. Preliminay Findings; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2013. [Google Scholar]
- Asam, Z.U.Z.; Poulsen, T.G.; Nzami, A.-S.; Raxique, R.; Kiely, G.; Murphy, J.D. How Can We Improve Biomethane Production Per Unit of Feedstock in Biogas Plant. Appl. Energy 2013, 88, 2013–2018. [Google Scholar] [CrossRef]
- Aguirre-Villegas, H.A.; Larson, R. Evaluating greenhouse gas emissions from dairy manure management practices using survey data and lifecycle tools. J. Clean. Prod. 2017, 143, 169–179. [Google Scholar] [CrossRef]
- Sabia, E.; Napolitano, F.; Claps, S.; De Rosa, G.; Braghieri, A.; Pacelli, C. Dairy buffalo life cycle assessment as affected by heifer rearing system. J. Clean. Prod. 2018, 192, 647–655. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; de Haan, C. Livestock’s Long Shadow Environmental Issues and Options. In Proceedings of the Methane to Markets Partnership Expo, Beijing, China, 30 October–1 November 2007. [Google Scholar]
- Hristov, A.N.; Zaman, S.; Vander Pol, M.; Ndegwa, P.; Campbell, L.; Silva, S. Nitrogen losses from dairy manure estimated through nitrogen mass balance and chemical markers. J. Environ. Qual. 2001, 38, 2438–2448. [Google Scholar] [CrossRef]
- Amon, B.; Kryvoruchko, V.; Amon, T.; Zechmeister-Boltenstern, S. Methane, nitrous oxide and ammonia emissions during storage and after application of dairy cattle slurry and influence of slurry treatment. Agric. Ecosyst. Environ. 2006, 112, 153–162. [Google Scholar] [CrossRef]
- Aguirre-Villegas, H.A.; Larson, R.; Reinemann, D.J. Effects of management and co-digestion on life cycle emissions and energy from anaerobic digestion. Greenh. Gases 2015, 5, 603–621. [Google Scholar] [CrossRef]
- European Commission. Council Directive Concerning the Protection of Waters Against Pollution Caused by Nitrates from Agricultural Sources (91/676/EEC). 1991. Available online: http://ec.europa.eu/environment/water/water-nitrates/index_en.html (accessed on 21 March 2019).
- Al Seadi, T.; Rutz, D.; Prassl, H.; Köttner, M.; Finsterwalder, T.; Volk, S.; Janssen, R. Biogas Handbook; University of Southern Denmark Esbjerg: Esbjerg, Denmark, 2008. [Google Scholar]
- Sommer, S.G.; Christensen, K.V.; Jensen, L.S. Environmental Technology for Treatment and Management of Bio-Waste; Sommer, S.G., Christensen, K.V., Eds.; University of Southern Denmark, Faculty of Engineering, Institute of Chemical Engineering, Biotechnology and Environmental Engineering & Lars Stoumann Jensen, University of Copenhagen, Faculty of Life Science, Plant and Soil Science Laboratory, Department of Agricultural Sciences, Thorvaldsensvej 40, 1871 Frederiksberg C, DENMARK; Syddansk Universitet: Odense, Denmark, 2008. [Google Scholar]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Zollitsch, W.; Mayer, K.; Gruber, L. Biogas production from maize and dairy cattle manure: Influence of biomass composition on the methane yield. Bioresour. Technol. 2007, 100, 5777–5782. [Google Scholar] [CrossRef]
- Alzate, M.; Muñoz, R.; Rogalla, F.; Fdz-Polanco, F.; Pérez-Elvira, S. Biochemical methane potential of microalgae: Influence of substrate to inoculum ratio, biomass concentration and pretreatment. Bioresour. Technol. 2012, 123, 488–494. [Google Scholar] [CrossRef]
- Li, K.; Liu, R.; Chen, S. Comparison of anaerobic digestion characteristics and kinetics of four livestock manures with different substrate concentrations. Bioresour. Technol. 2015, 198, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, X.; He, C.; Chen, C.; Bai, J.; Ren, N.; Wang, J. Transformation of dissolved organic matters in swine, cow and chicken manures during composting. Bioresour. Technol. 2014, 168, 222–228. [Google Scholar] [CrossRef]
- Olowoyeye, J. Comparative studies on biogas production using six different animal dungs. J. Biol. Agric. Health 2013, 3, 7–12. [Google Scholar]
- Triolo, J.L.; Ward, A.J.; Pedersen, L.; Sommer, S.G. Characteristics of Animal Slurry as a Key Biomass for Biogas Production in Denmark. In Biomass Now—Sustainable Growth and Use; Matovic, M.D., Ed.; InTech—Open Access Publisher: London, UK, 2013. [Google Scholar] [CrossRef]
- Chen, F.; Yu, G.; Li, W.; Liu, F.W.; Zhang, W.P.; Bu, Y.S.; Li, X. Maximal methane potential of different animal manures collected in northwest region of China. Int. J. Agric. Biol. Eng. 2017, 10, 202–208. [Google Scholar]
- Fantozzi, C. Buratti, Biogas production from different substrates in an experimental Continuously Stirred Tank Reactor anaerobic digester. Bioresour. Technol. 2009, 100, 5783–5789. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.H.; Triolo, J.M.; Cu, T.T.T.; Pedersen, L.; Sommer, S.G. Validation and recommendation of methods to measure biogas production potential of animal manure. Asian Australas. J. Anim. 2013, 26, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Andrade, W.R.; Xavier, C.A.N.; Coca, F.O.C.G.; Arruda, L.D.O.; Santos, T.M.B. Biogas production from ruminant and monogastric animal manure co-digested with manipueira. Arch. Zootec. 2016, 65, 251–380. [Google Scholar]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef]
- Budiyono, B.; Widiasa, I.N.; Johari, S.; Sunarso, S. Increasing Biogas Production Rate from Cattle Manure Using Rumen Fluid as Inoculums. Int. J. Sci. Eng. 2014, 6, 31–38. [Google Scholar] [CrossRef]
- Osman, G.A.M.; Elhasan, H.E.; Hassan, A.B. Effect of cow rumen fluid concentration on biogas production from goat manure. Sudan. J. Agric. Sci. 2015, 2, 1–7. [Google Scholar]
- Lawal, A.A.; Dzivama, A.U.; Wasinda, M.K. Effect of inoculum to substrate ratio on biogas production of sheep paunch manure. Res. Agric. Eng. 2016, 62, 8–14. [Google Scholar] [CrossRef]
- Mönch-Tegeder, M.; Lemmer, A.; Oechsner, H.; Jungbluth, T. Investigation of the methane potential of horse manure. Agric. Eng. Int. CIGR J. 2013, 15, 161–172. [Google Scholar]
- Yohaness, M.T. Biogas Potential from Cow Manure: Influence of Diet. Second Cycle, A2E; SLU, Department of Microbiology: Uppsala, Sweden, 2010. [Google Scholar]
- Rico, J.L.; Garcia, H.; Rico, C.; Tejero, I. Characterisation of solid and liquid fractions of dairy manure with regard to their component distribution and methane production. Bioresour. Technol. 2007, 98, 971–979. [Google Scholar] [CrossRef]
- Monteiro, E.; Mantha, V.; Rouboa, A. Prospective application of farm cattle manure for bioenergy production in Portugal. Renew. Energy 2011, 36, 627–631. [Google Scholar] [CrossRef]
- Costa, M.S.D.M.; Costa, L.A.D.M.; Lucas, J.D., Jr.; Pivetta, L.A. Potentials of biogas production from super young bulls manure fed with different diets. Eng. Agric. 2013, 33, 1090–1098. [Google Scholar]
- Orrico, M.A.P., Jr.; Orrico, A.C.A.; Lucas, J.D., Jr.; Sampaio, A.A.M.; Fernandes, A.R.M.; Oliveira, E.A.D. Biodigestão anaeróbia dos dejetos da bovinocultura de corte: Influência do período, do genótipo e da dieta. Rev. Bras. Zoot. 2012, 41, 1533–1538. [Google Scholar] [CrossRef]
- De Mendonça Costa, M.S.S.; de Lucas, J., Jr.; de Mendonça Costa, L.A.; Orrico, A.C.A. A highly concentrated diet increases biogas production and the agronomic value of young bull’s manure. Waste Manag. 2016, 48, 521–527. [Google Scholar]
- Matos, C.F.; Paes, J.L.; Pinheiro, E.F.M.; De Campos, D.V.B. Biogas production from dairy cattle manure, under organic and conventional production systems. Eng. Agríc. Jaboticabal 2017, 37, 1081–1090. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ellegaard, L. Co-Digestion of Manure and Organic Wastes in Centralized Biogas Plant: Status and Future Trend; Environment and Resources, Technical University of Denmark: Lyngby, Denmark, 2003. [Google Scholar]
- Tufaner, F.; Avsar, Y. Effects of co-substrate on biogas production from cattle manure: A review. Int. J. Environ. Sci. Technol. 2016, 13, 2303–2312. [Google Scholar] [CrossRef]
- Abbasi, T.; Tauseef, S.; Abbasi, S.A. Biogas Energy, Vol 2; Springer Science & Business Media: New York, NY, USA, 2011. [Google Scholar]
- Zhu, N. Effect of low initial C/N ratio on aerobic composting of swine manure with rice straw. Bioresour. Technol. 2007, 98, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Böske, J.; Wirth, B.; Garlipp, F.; Mumme, J.; Van den Weghe, H. Anaerobic digestion of horse dung mixed with different bedding materials in an upflow solid-state (UASS) reactor at mesophilic conditions. Bioresour. Technol. 2014, 158, 111–118. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Shi, J.; Li, Y. Solid-state anaerobic digestion of spent wheat straw from horse stall. Bioresour. Technol. 2011, 102, 9432–9437. [Google Scholar] [CrossRef] [PubMed]
- Wartell, B.A.; Krumins, V.; Alt, J.; Kang, K.; Schwab, B.J.; Fennell, D.E. Methane production from horse manure and stall waste with softwood bedding. Bioresour. Technol. 2012, 112, 42–50. [Google Scholar] [CrossRef]
- Lopes, M.; Baptista, P.; Duarte, E.; Moreira, A.L.N. Enhanced biogas production from anaerobic co-digestion of pig slurry and horse manure with mechanical pre-treatment. Environ. Technol. 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Bujoczek, G.; Oleszkiewicz, J.; Sparling, R.; Cenkowski, S. High solid anaerobic digestion of chicken manure. J. Agric. Eng. Res. 2000, 76, 51–60. [Google Scholar] [CrossRef]
- Gangagni Rao, A.; Sasi Kanth Reddy, T.; Surya Prakash, S.; Vanajakshi, J.; Joseph, J.; Jetty, A.; Rajashekhara Reddy, A.; Sarma, P.N. Biomethanation of poultry litter leachate in UASB reactor coupled with ammonia stripper for enhancement of overall performance. Bioresour. Technol. 2008, 99, 8679–8684. [Google Scholar] [CrossRef]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, Q.; Lu, Y. Inhibitory effects of ammonia on methanogen mcrA transcripts in anaerobic digester sludge. EMS Microbiol. Ecol. 2014, 87, 368–377. [Google Scholar] [CrossRef]
- Niu, Q.; Qiao, W.; Qiang, H.; Li, Y.Y. Microbial community shifts and biogas conversion computation during steady, inhibited and recovered stages of thermophilic methane fermentation on chicken manure with a wide variation of ammonia. Bioresour. Technol. 2013, 146, 223–233. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Borowski, S.; Domanski, J.; Weatherley, L. Anaerobic co-digestion of swine and poultry manure with municipal sewage sludge. Waste Manag. 2014, 34, 513–521. [Google Scholar] [CrossRef]
- Ali, S.; Ali Shah, T.; Afzal, A.; Tabbassum, R. Evaluating the co-digestion effects on chicken manure and rotten potatoes in batch experiments. Int. J. Biosci. 2017, 10, 150–159. [Google Scholar]
- Nie, H.; Jacobi, H.F.; Strach, K.; Xu, C.; Zhou, H.; Liebetrau, J. Mono-fermentation of chicken manure: Ammonia inhibition and recirculation of the digestate. Bioresour. Technol. 2015, 178, 238–246. [Google Scholar] [CrossRef]
- Praes, M.F.M.; de Lucas, J., Jr.; Hermes, R.; Sorbara, J.O.B.; Ferreira, M.S.; Cardoso, P.B.C.S. Effect of a broiler diet containing probiotic and exogenous enzymes on the manure used for biogas production. In Proceedings of the Conference on Sustainable Agriculture through ICT Innovation, Torino, Italy, 23–27 June 2013. [Google Scholar]
- Gaworski, M.; Jabłoński, S.; Pawlaczyk-Graja, I.; Ziewiecki, R.; Rutkowski, P.; Wieczyńska, A.; Gancarz, R.; Łukaszewicz, M. Enhancing biogas plant production using pig manure and corn silage by adding wheat straw processed with liquid hot water and steam explosion. Biotechnol. Biofuels 2017, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Cuetos, M.J.; Fernández, C.; Gómez, X.; Morán, A. Anaerobic Co-digestion of Swine Manure with Energy Crop Residues. Biotechnol. Bioprocess Eng. 2011, 16, 1044–1052. [Google Scholar] [CrossRef]
- Hamilton, D.W. Anaerobic Digestion of Animal Manure: Understanding the Basic Processes. Oklahoma Cooperative Extension Service BAE-1747; Division of Agricultural Sciences and Natural Resources, Oklahoma State University: Stillwater, OK, USA, 2014. [Google Scholar]
- Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition; European Commission: Brussels, Belgium, 2003.
- Masse, D.I.; Lu, D.; Masse, L.; Droste, R.L. Effect of antibiotics on psychrophilic anaerobic digestion of swine manure slurry in sequencing batch reactors. Bioresour. Technol. 2000, 75, 205–211. [Google Scholar] [CrossRef]
- Beneragama, N.; Moriya, Y.; Yamashiro, T.; Iwasaki, M.; Lateef, S.A.; Ying, C.; Umetsu, K. The survival of cefazolin resistant bacteria in mesophilic co-digestion of dairy manure and waste milk. Waste Manag. Res. 2013, 31, 843–848. [Google Scholar] [CrossRef]
- Álvarez, J.A.; Otero, L.; Lema, J.M.; Omil, F. The effect and fate of antibiotics during the anaerobic digestion of pig manure. Bioresour. Technol. 2010, 101, 8581–8586. [Google Scholar] [CrossRef]
- Shi, J.C.; Liao, X.D.; Wu, Y.B.; Liang, J.B. Effect of antibiotics on methane arising from anaerobic digestion of pig manure. Anim. Feed Sci. Technol. 2011, 166–167, 457–463. [Google Scholar] [CrossRef]
- Beneragama, N.; Iwasaki, M.; Lateef, S.A.; Umetsu, K. The effect of cefazolin on biogas production from thermophilic and mesophilic anaerobic co-digestion of dairy manure and waste milk. J. Natl. Sci. Found. Sri Lanka 2015, 43, 369–376. [Google Scholar] [CrossRef]
- Mitchell, S.M.; Ullman, J.L.; Teel, A.L.; Watts, R.J. The effects of the antibiotics ampicillin, florfenicol, sulfamethazine, and tylosin on biogas production and their degradation efficiency during anaerobic digestion. Bioresour. Technol. 2013, 149, 244–252. [Google Scholar] [CrossRef]
- Ke, X.; Zhao, X.; Li, R.D. Effect of copper ions on pig manure anaerobic digestion. Renew. Environ. Resour. 2013, 31, 60–63. [Google Scholar]
- Sun, J.P.; Zheng, P.; Hu, B.L.; Yu, Y. Cumulative inhibition of heavy metals to anaerobic digestion of piggery wastewater. Acta Sci. Circumstantiae 2009, 29, 1643–1648. [Google Scholar]
- Ji, C.; Kong, C.; Mei, Z.L.; Li, J. A Review of the Anaerobic Digestion of Fruit and Vegetable Waste. Appl. Biochem. Biotechnol. 2017, 183, 906–922. [Google Scholar] [CrossRef]
- FAO, Food and Agriculture Organization of the United Nations. FAO Statistical Yearbook 2014: Latin America and the Carubbean Food and Agriculture; FAO: Roma, Italy, 2014. [Google Scholar]
- Boullagui, H.; Touhami, Y.; Cheikh, R.B.; Hamndi, M. Bioreactor performance in anaerobic digestion of fruit and vegetable waste. Process Biochem. 2005, 40, 989–995. [Google Scholar] [CrossRef]
- Scano, E.A.; Asquer, C.; Pistis, A.; Ortu, L.; Demontis, V.; Cocco, D. Biogas from anaerobic digestion of fruit and vegetable wastes: Experimental results on pilot-scale and preliminary performance evaluation of a full-scale power plant. Energy Convers. Manag. 2014, 77, 22–30. [Google Scholar] [CrossRef]
- Wang, C.; Zuo, J.; Chen, X.; Xing, W.; Xing, L.; Li, P.; Lu, X.; Li, C. Microbial community structures in an integrated two-phase anaerobic bioreactor fed by fruit vegetable wastes and wheat straw. J. Environ. Sci. 2014, 26, 2484–2492. [Google Scholar] [CrossRef]
- Almonani, F.; Shawaqfah, M.; Bhosale, R.R.; Kumar, A.; Khraisheh, M.A.M. Intermediate ozonization to enhance biogas production in batch and continuous systems using animal dung and agricultural waste. Int. Biodeterior. Biodegrad. 2016, 30, 1–12. [Google Scholar]
- Favaro, L.; Basaglia, M.; Casella, S. Processing wheat bran into ethanol using mild treatments and highly fermentative yeasts. Biomass Bioenergy 2012, 46, 605–617. [Google Scholar] [CrossRef]
- Dinuccio, E.; Balsari, P.; Gioelli, F.; Menardo, S. Evaluation of the biogas productivity potential of some Italian agro-industrial biomasses. Bioresour. Technol. 2010, 101, 3780–3783. [Google Scholar] [CrossRef]
- Nzila, C.; Dewulf, J.; Spanjers, H.; Kiriamiti, H.; van Lagenhove, H. Biowaste energy potential in Kenya. Renew. Energy 2010, 35, 2698–2704. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, J.; Yu, Q.; Yong, X.; Xie, X.; Zhang, L.; Wei, P.; Jia, H. Different organic loading rates on the biogas production during the anaerobic digestion of rice straw: A pilot study. Bioresour. Technol. 2017, 244, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.K.; Park, S.C. Biochemical methane potential and solid state anaerobic digestion of Korean food wastes. Bioresour. Technol. 1995, 52, 245–253. [Google Scholar] [CrossRef]
- Ciuta, S.; Antognoni, S.; Rada, E.C.; Ragazzi, M.; Badea, A.; Cioca, L.I. Respirometrix Index and biogas potential of different foods and Agricultural discarded biomass. Sustainability 2016, 8, 1311. [Google Scholar] [CrossRef]
- Aliyu, S.; Bala, M. Brewer’s spent grain: A review of its potential applications. Afr. J. Biotechnol. 2011, 10, 324–331. [Google Scholar]
- Okoye, B.O.; Igbokwe, P.K.; Ude, C.N. Comparative study of biogas production from cow dung and brewer’s spent grain. Int. J. Res. Adv. Eng. Technol. 2016, 2, 19–21. [Google Scholar]
- Luz, F.C.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Anaerobic digestion of coffee grounds soluble fraction at laboratory scale: Evaluation of the biomethane potential. Appl. Energy 2017, 207, 166–175. [Google Scholar] [CrossRef]
- García, C.A.; Peňa, A.; Betancourt, R.; Cardona, C.A. Energetic and environmental assessment of thermochemical and biochemical ways for producing energy from agricultural solid residues: Coffee cut-stems case. J. Environ. Manag. 2018, 216, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Abdul Aziz, N.I.H.; Hanafiah, M.M.; Yasreen, M.; Ali, M. Sustainable biogas production from agrowaste and effluents—A promising step for small-scale industry income. Renew. Energy 2019, 132, 363–369. [Google Scholar] [CrossRef]
- Kafle, G.K.; Bhattarai, S.; Kim, S.H.; Chen, L. Anaerobic digestion of Chinese cabbage waste silage with swine manure for biogas production: Batch and continuous study. Environ. Technol. 2014, 35, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Abubaker, J.; Risberg, K.; Pell, M. Biogas Residues as Fertilizers effects on wheat growth and soil microbial activities. Appl. Energy 2012, 99, 126–134. [Google Scholar] [CrossRef]
- Shen, F.; Yuan, H.; Pang, Y.; Chen, S.; Zhu, B.; Zou, D.; Liu, Y.; Ma, J.; Yu, L.; Li, X. Performances of anaerobic co-digestion of fruit and vegetable waste (FVW) and food waste (FW): Single-phase vs. two-phase. Bioresour. Technol. 2013, 144, 80–85. [Google Scholar] [CrossRef]
- Liu, X.; Gao, X.; Wang, W.; Zheng, L.; Zhou, Y.; Sun, Y. Pilot-scale anaerobic co-digestion of municipal biomass waste: Focusing on biogas production and GHG reduction. Renew. Energy 2012, 44, 463–468. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, Y.W.; Jahng, D. Anaerobic co-digestion of food waste and piggery wastewater: Focusing on the role of trace elements. Bioresour. Technol. 2011, 102, 5048–5059. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Wu, S.; Zhang, W.; Dong, R. Effects of organic loading rate and effluent recirculation on the performance of two-stage anaerobic digestion of vegetable waste. Bioresour. Technol. 2013, 146, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Pavi, S.; Kramer, L.E.; Gomes, L.P.; Miranda, L.A.S. Biogas production from co-digestion of organic fraction of municipal solid waste and fruit and vegetable waste. Bioresour. Technol. 2017, 228, 362–367. [Google Scholar] [CrossRef]
- Ziganshin, A.M.; Liebetrau, J.; Pröter, J.; Kleinsteuber, S. Microbial community structure and dynamics during anaerobic digestion of various agricultural waste materials. Appl. Microbiol. Biotechnol. 2013, 97, 5161–5174. [Google Scholar] [CrossRef] [PubMed]
- Zverlov, V.V.; Hiegl, W.; Köck, D.E.; Kellermann, J.; Köllmeier, T.; Schwarz, W.H. Hydrolytic bacteria in mesophilic and thermophilic degradation of plant biomass. Eng. Life Sci. 2010, 10, 528–536. [Google Scholar] [CrossRef]
- Morrison, M. Miron Adhesion to cellulose by Ruminococcus albus: A combination of cellulosomes and Pil-proteins? FEMS Microbiol. Lett. 2000, 185, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Wirth, R.; Kovács, E.; Maróti, G.; Bagi, Z.; Rákhely, G.; Kovács, K. Characterization of a biogas-producing microbial community by short-read next generation DNA sequencing. Biotechnol. Biofuels 2012, 5, 41. [Google Scholar] [CrossRef]
- Karakashev, D.; Batstone, D.J.; Trably, E.; Angelidaki, I. Acetate oxidation is the dominant methanogenic pathway from acetate in the absence of Methanosaetaceae. Appl. Environ. Microbiol. 2006, 72, 5138–5141. [Google Scholar] [CrossRef]
- Anderson, I.; Ulrich, L.E.; Lupa, B.; Susanti, D.; Porat, I.; Hooper, S.D.; Lykidis, A.; Sieprawska-Lupa, M.; Dharmarajan, L.; Goltsman, E.; et al. Genomic characterization of methanomicrobiales reveals three classes of methanogens. PLoS ONE 2009, 4, 5797. [Google Scholar] [CrossRef]
- Rao, P.V.; Baral, S.S. Experimental design of mixture for the anaerobic co-digestion of sewage sludge. Chem. Eng. J. 2011, 172, 977–986. [Google Scholar] [CrossRef]
- El-Mashad, H.M.; Zhang, R.H. Biogas production from co-digestion of dairy manure and food waste. Bioresour. Technol. 2010, 101, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Li, L.Q.; Zheng, M.X.; Fu, G.Z.; Lar, J.S. Anaerobic co-digestion of cattle manure with corn stover pretreated by sodium hydroxide for efficient biogas production. Energy Fuels 2009, 23, 4635–4639. [Google Scholar] [CrossRef]
- Ferrer, P.; Cambra-Lopez, M.; Cerisuelo, A.; Penaranda, D.S.; Moset, V. The use of agricultural substrates to improve methane yield in anaerobic co-digestion with pig slurry: Effect of substrate type and inclusion level. Waste Manag. 2014, 34, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Misi, S.N.; Forster, C.F. Semi-continuous anaerobic co-digestion of agro-wastes. Environ. Technol. 2002, 23, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Zhang, D.; Li, G.; Lu, J.; Li, S. Solid state anaerobic co-digestion of tomato residues with dairy manure and corn stover for biogas production. Bioresour. Technol. 2016, 217, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.V.; Alves, M.M.; Costa, J.C. Design of experiments to asses pre-treatment and co-digestion strategies that optimize biogas production from macroalgae Gracilaria vermiculophylla. Bioresour. Technol. 2014, 162, 323–330. [Google Scholar] [CrossRef]
- Yoroklu, H.C.; Korkmaz, E.; Demir, N.M.; Ozkaya, B.; Demir, A. The impact of pretreatment and inoculums to substrate ratio on methane potential of organic wastes from various origins. J. Mat. Cycles Waste Manag. 2018, 20, 800–809. [Google Scholar] [CrossRef]
- Poulsen, T.G.; Adelard, L. Improving biogas quality and methane yield via co-digestion of agricultural and urban biomass wastes. Waste Manag. 2016, 54, 118–125. [Google Scholar] [CrossRef]
- Otun, T.F.; Ojo, O.M.; Ajibade, F.O.; Babatola, J.O. Evaluation of biogas production from the digestion and co-digestion of animal waste, food waste and fruit waste. Int. J. Environ. Res. 2015, 3, 12–24. [Google Scholar]
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The anaerobic co-digestion of food waste and cattle manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef]
- Cabbai, V.; Ballico, M.; Aneggi, E.; Goi, D. BMP tests of source selected OFMSW to evaluate anaerobic codigestion with sewage sludge. Waste Manag. 2013, 33, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Alatriste-Mondragon, F.; Samar, P.; Cox, H.H.J.; Ahring, B.K.; Iranpour, R. Anaerobic codigestion of municipal, farm, and industrial organic wastes: A survey of recent literature. Water Environ. Res. 2006, 78, 607–636. [Google Scholar] [CrossRef]
- Gil, J.A.; Márquez, P.; Gutiérrez, M.C.; Martin, M.A. Optimizing the selection of organic waste for biomethanization. Environ. Technol. 2017, 10, 1–13. [Google Scholar] [CrossRef]
- Deressa, L.; Libsu, S.; Chavan, R.B.; Manaye, D.; Debassa, A. Production of biogas from fruit and vegetable wastes mixed with different wastes. Environ. Ecol. Res. 2015, 3, 65–71. [Google Scholar]
- Nagarajan, G.; Rajakumar, S.; Ayyasamy, P.M. Vegetable wastes: An alternative resource for biogas and bio compost production through lab scale process. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 379–387. [Google Scholar]
- Hubenov, V.N.; Mihaylova, S.N.; Simeonov, I.S. Anaerobic co-digestion of waste fruits and vegetables and swine manure in a pilot-scale bioreactor. Bulgarian Chem. Commun. 2015, 47, 788–792. [Google Scholar]
- Di Maria, F.; Baratta, M. Boosting methane generation by co-digestion of sludge with fruit and vegetable waste: International environment of digester and methanogenic pathway. Waste Manag. 2015, 43, 130–136. [Google Scholar] [CrossRef]
- Pandit, P.D.; Gulhane, M.K.; Khardenavis, A.A.; Purohit, H.J. Mining of hemicelluloses and lignin degrading genes from differentially enriched methane producing microbial community. Bioresour. Technol. 2016, 216, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luo, L.; Li, W.; Wang, X.; Sun, Y.; Sun, Y.; Gong, W. Optimization of mixing ratio of ammoniated rice straw and food waste co-digestion and impact of trace element supplementation on biogas production. J. Mater. Cycles Waste Manag. 2018, 20, 745–753. [Google Scholar] [CrossRef]
- Niasar, H.S.; Karimi, K.; Zilouei, H.; Salehian, P.; Jeihanipour, A. Effects of lime pretreatment on biogas production from dry dairy cattle manure. Minerva Biotecnol. 2011, 23, 77–82. [Google Scholar]
- Ayala-Parra, P.; Liu, Y.; Sierra-Alvarez, R.; Field, J.A. Pretreatment to enhance the anaerobic biodegradability of Chlorella protothecoides algas biomass. Environ. Prog. Sustain. Energy 2018, 37, 418–424. [Google Scholar] [CrossRef]
- Aslanzadeh, S.; Taherzadeh, M.J.; Horváth, I.S. Pretreatment of straw fraction of manure for improved biogas production. BioResources 2011, 6, 5193–5205. [Google Scholar]
- Panico, A.; d’Antonio, G.; Esposito, G.; Frunzo, L.; Iodice, P.; Pirozzi, F. The Effect of Substrate-Bulk Interaction on Hydrolysis Modeling in Anaerobic Digestion Process. Sustainability 2014, 6, 8348–8363. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V. Pretreatment methods to improve anaerobic biodegradability of organic municipal solid waste fractions. Chem. Eng. J. 2014, 240, 24–37. [Google Scholar] [CrossRef]
- Azman, S.; Khadem, A.F.; Van Lier, J.B.; Zeeman, G.; Plugge, C.M. Presence and role of anaerobic hydrolytic microbes in conversion of lignocellulosic biomass for biogas production. Crit. Rev. Environ. Sci. Technol. 2015, 25, 2523–2564. [Google Scholar] [CrossRef]
- Mason, P.M.; Stuckey, D.C. Biofilms, bubbles and boundary layers—A new approach to understanding cellulolysis in anaerobic and ruminant digestion. Water Res. 2016, 104, 93–100. [Google Scholar] [CrossRef]
- Ferraro, A.; Dottorini, G.; Massini, G.; Mazzurco, V.; Signorini, A.; Lembob, G.; Fabbricino, M. Combined bioaugmentation with anaerobic ruminal fungi and fermentative bacteria to enhance biogas production from wheat straw and mushroom spent straw. Bioresour. Technol. 2018, 260, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Căter, M.; Fanedl, L.; Malovrh, S.; Marinšek Logar, R. Biogas production from brewery spent grain enhanced by bioaugmentation with hydrolytic anaerobic bacteria. Bioresour. Technol. 2015, 186, 261–269. [Google Scholar] [CrossRef]
- Eze, J.I.; Agbo, K.E. Studies on the microbial spectrum in anaerobic biomethannization of cow dung in 10 m3 fixed dome biogas digester. Int. J. Phys. Sci. 2010, 5, 1331–1337. [Google Scholar]
- Sunarso, J.S.; Budiyono, W. The effect of feed to inoculums ratio on biogas production rate from cattle manure using rumen fluid as inoculums. Int. J. Sci. Eng. 2010, 1, 41–45. [Google Scholar] [CrossRef]
- Gomathi, V.; Ramasamy, K.; Reddy, M.R.V.P.; Ramalakshmi, A.; Ramanathan, A. Methane emission by gut symbionts of Termites. Acad. J. Plant Sci. 2009, 2, 189–194. [Google Scholar]
- Iyagba, E.T.; Mangibo, I.A.; Muhammad, Y.S. The study of cow dung as co-substrate with rice husk in biogas production. Sci. Res. 2009, 4, 861–866. [Google Scholar]
- Budiyono, I.N.; Widiasa, S.; Johari, S. The kinetic of biogas production rate from cattle manure in batch mode. Int. J. Chem. Biol. Eng. 2010, 3, 39–44. [Google Scholar]
- Mirdamadian, S.H.; Khayam-Nekoui, S.M.; Ghanavati, H. Reduce of fermentation time in composting process by using a special microbial consortium. World Acad. Sci. Eng. Technol. 2011, 76, 533–537. [Google Scholar]
- Rother, M.; Metcalf, W.W. Genetic technologies for Archaea. Curr. Opin. Microbiol. 2005, 8, 745–751. [Google Scholar] [CrossRef]
- Senthilkumar, V.; Gunasekaran, P. Bioethanol production from cellulosic substrates: Engineered bacteria and process integration challenges. J. Sci. Ind. Res. 2005, 64, 845–853. [Google Scholar]
| Advantages | Drawbacks | |
|---|---|---|
| Mesophilic process |
|
|
| Thermophilic process |
|
|
| (a) unstructured reactor versus conventional anaerobic reactor [22] | |||
| Description | Advantages | Drawbacks | |
| Unstructured reactor | Lagoons covered by flexible polymeric membranes |
|
|
| Anaerobic sequencing batch reactor—ASBR | Single tank fill-and-draw unit, used for treatment and fermentation OLR is variable |
|
|
| Complete stirred tank reactor—CSTR | Intermittent or continuous complete mixing in one or more high-rate reactors, generally heated. Used for wet process. |
|
|
| Anaerobic plug- flow reactor—APFR | Linear horizontal reactors, with no internal agitation, for dry or semi-dry process. |
|
|
| (b) sludge retention reactor [22] | |||
| Description | Advantages | Drawbacks | Description |
| Anaerobic contact reactor—ACR | Agitated reactor and a solid settling tank for microorganism recycling, generally in mesophilic condition. |
| ▪ Dilute nature of the digestate with limited organic loading rate |
| Up-flow anaerobic sludge bed reactor—UASB | Dense sludge bed in the bottom for the wastewater-biomass contact |
|
|
| Up-flow anaerobic solid-state reactor—UASS | Quadruple two-phase, two-stage reactor, with AF section, used for solid biomass, with dry process. |
|
|
| Anaerobic baffled reactor—ABR | Compartments in one reactor, baffled to force incoming wastewater up through a series of blanked sludge |
|
|
| Internal circulation reactor—IC | It is similar to two UASB reactors working together, it can separate gas, liquid and biomass simultaneously |
| |
| (c) membrane reactor [22] | |||
| Description | Advantages | Drawbacks | Description |
| Anaerobic filter reactor—AF | Biofilm to separate biomass from effluent, at up-flow or down-flow condition |
|
|
| Anaerobic fluidized bed reactor—AFBR | Small-inert particles used as the medium for bacterial attachment |
|
|
| Expanded granular sludge blanket—EGSB | Modification of UASB reactor, used when volumetric gas production rate is low and mixing is insufficient It can separate gas, liquid and solid biomass simultaneously |
| ▪ Suspended solids cannot be substantially removed |
| Parameter | Unit | Description, Range and Measurement Method |
|---|---|---|
| Process stability | ||
| Temperature T | °C |
Variations of only 2–3 °C within the optimal range can affect performance. Measurement during process by i.e., thermocouples |
| pH | - | Neutral environment, between 6.5–7.5, acceptability 6–8. Measured by pH meter or pH/redox electrode sensor |
| Volatile Fatty Acids VFAs | mgAc/L | A significant increase highlights a malfunction; it can lead to a drop in pH (acidosis), which also leads to an irreversible block of the process. Typical range 200–2000 mgAc/L. Measured by UV/FTIR-IR spectrometer, gas chromatography or indirect by COD. |
| Alkalinity TA | mg CaCO3/L | Buffer capacity of the system which contributes to guarantee neutral pH. A stable system has values in the range of 2500–5000 mg CaCO3/L. Direct measure with NIRS or titration, indirect through redox potential sensor and electrical conductivity |
| VFA/TA | - | Ratio value < 0.3 indicates the stable operation of the digester. |
| Reactor management | ||
| Operational pressure P | mbar | Measurement during process |
| Capacity V | m3/d or t/d | Measurement of mass or volume per day |
| Reactor volume Vr | m3 | Determined by design/construction |
| Hydraulic retention time HRT | d | Ratio between the volume of the reactor considered and the flow rate to the reactor. Calculation from process data, indirect by measuring the incoming flow. |
| Sludge Retention Time SRT | d | Ratio between the total mass of volatile solids in the reactor and the flow of solids extracted from the reactor. Indirect by measuring concentration and flow rate SV. |
| Organic Load rate OLR | Kg or TS/m3 d | Quantity of input substrate (influent flow rate), calculated by concentration of organic substance and referred to the volume unit of the reactor. Indirect measurement by flow measure and organic substance concentration (sample weight for ST, SV, or COD and BOD measurement) |
| Substrate removal efficiency | % | Function of the SV concentration in the influent flow rate (kg/m3) and SV concentration in the effluent flow rate (kg/m3) |
| Biogas flow rate | Nm3/d or Nm3/y | Output biogas flow. If connected to the flow and concentration of the incoming substrate, it is called a specific production (m3/m3). Measurement during operation by flowmeter. |
| Methane concentration CH4 and biogas composition | % | % CH4 - %CO2 - %H2S - O2 – other Measurement during operation by electrochemical or IR spectroscopic method |
| Plant efficiency | ||
| Gross Energy | kWh | Calculated from biogas flow and methane concentration using Low Heating Value |
| Electricity production | kWh | Measurement at power unit |
| Output to grid | kWh | Measurement after power unit |
| Efficiency of power unit | % | Calculation from operating data |
| Thermal/electric station supply | kWh | Calculation from operating data |
| Thermal/electric specific station supply | kWh/m3 | Calculation from operating data |
| Energy production | kWh | Sum of energy that can be used |
| Plant efficiency | % | Net energy drawn from gross energy |
| Plant availability | % | Percentage of hours a year in which it is fully functioning |
| Use | % | Ratio of the real input to the designed capacity |
| Total investment | € | All expenses |
| Funding | € | Pre-determined |
| Funding percentage | % | Percentage of all subsidies in relation to the total investment |
| Specific investment | €/m3 | Calculation from operating data… |
| Specific treatment cost | €/m3 | Calculation from operating data… |
| pH | TS% | VS% | C/N | BIOGAS YIELD | |
|---|---|---|---|---|---|
| Cattle manure [47,50,51,52,53,54,55,56] | 5.33 ÷ 8.30 | 9.4 ÷ 22.75 | 10.25 ÷ 93.11 | 10.5 ÷ 26.64 | 169 ÷ 270 mL/gVS |
| Pig manure [47,50,51,52,53,54,55] | 6.9 ÷ 7.87 | 5.4 ÷ 92.10 | 26.93 ÷ 80.2 | 6 ÷ 18.91 | 318 ÷ 409 mL/gVS |
| Goat manure [55,57] | 8.13 | 81.63 ÷ 97.1 | 64.01 | 16 ÷ 20 | - |
| Sheep manure [54,58] | 7.8 | 17.4 ÷ 37.03 | 16.3 ÷ 76.49 | - | 0.572 ÷ 1.468 Nm3/kg VS |
| Poultry manure [47,51,52,53,54,55] | 6.63 ÷ 7.95 | 42.9 ÷ 90.15 | 47.5 ÷ 84.46 | 3.8 ÷ 14.44 | 192 ÷ 377 mL/gVS |
| Horse manure [55,59] | 8.25 | 33.49 ÷ 53.38 | 30.40 ÷ 46.65 | 22.63 ÷ 40.12 | 0.164 ÷ 0.212 Nm3/kg VS |
| Rabbit manure [47] | - | 27.84 | 87.94 | 17.9 | 211 ÷ 323 mL/gVS |
| pH | TS% | VS (%TS) | C/N | BIOGAS YIELD | |
|---|---|---|---|---|---|
| Wheat waste (straw, bran) [103,104] | - | 88.7÷91.0 | 88 | 87 | - |
| Barley straw [105,106] | 7.87 | 89.17÷90.5 | 94.04÷94.3 | 417 L/kg VS, 229 L methane/kg VS | |
| Corn residue [105,106,107] | 5.05 | 81.8÷93.02 | 94.65÷97.50 | 51÷57 | 641 L/kg VS, 317 L methane/kg VS |
| Rice straw [105,107] | 6.2÷8.14 | 88.7÷93.49 | 76.02÷91.9 | 50÷70.88 | 416 L/kg VS, 195 L methane/kg VS |
| Boiled rice [108] | - | 35 | 99 | 25.56 | 0.29 m3 methane/kg VS |
| Apple scraps [109] | - | 25 | 90 | 50.5 | - |
| Fresh cabbage residue [108] | - | 5 | 84 | 9.73 | 0.28 m3 methane/kg VS |
| Tomato seeds and skin [105] | 4.7 | 32 | 97.8 | 424 L/kg VS, 218 L methane/kg VS | |
| Sugar cane bagasse [106] | - | 93.51 | 83.91÷89.72 | 20.37 | 363 m3/ton VS |
| Tea residue [106] | - | 90.96 | 86.64 | - | 385 m3/ton VS |
| Grape marc [105,109] | 3.58÷4.40 | 20.44÷60.90 | 88.66÷98 | 18.57÷29.23 | 225–360 L/kg VS, 98–116 L methane/kg VS |
| Brewery spent grain [13,110,111] | 5.08 | 21.1÷30 | 84÷96.5 | 11.25÷25 | 392–491 L/kg VS, 187–273 L methane/kg VS |
| Spent coffee grounds [112,113] | - | 32.37÷95.88 | 92.76÷93.84 | 35.3 | - |
| Coffee cut-stems [113] | - | 88.56÷95.88 | 92.76 | - | - |
| Palm oil mill effluent [114] | 7.74 | 6.73 | - | - | - |
| FVW (from supermarket) [100] | 4.66 | 19.54 | 96.21 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, M.C.; Braghieri, A.; Capece, A.; Napolitano, F.; Romano, P.; Galgano, F.; Altieri, G.; Genovese, F. Recent Updates on the Use of Agro-Food Waste for Biogas Production. Appl. Sci. 2019, 9, 1217. https://doi.org/10.3390/app9061217
Caruso MC, Braghieri A, Capece A, Napolitano F, Romano P, Galgano F, Altieri G, Genovese F. Recent Updates on the Use of Agro-Food Waste for Biogas Production. Applied Sciences. 2019; 9(6):1217. https://doi.org/10.3390/app9061217
Chicago/Turabian StyleCaruso, Marisa Carmela, Ada Braghieri, Angela Capece, Fabio Napolitano, Patrizia Romano, Fernanda Galgano, Giuseppe Altieri, and Francesco Genovese. 2019. "Recent Updates on the Use of Agro-Food Waste for Biogas Production" Applied Sciences 9, no. 6: 1217. https://doi.org/10.3390/app9061217
APA StyleCaruso, M. C., Braghieri, A., Capece, A., Napolitano, F., Romano, P., Galgano, F., Altieri, G., & Genovese, F. (2019). Recent Updates on the Use of Agro-Food Waste for Biogas Production. Applied Sciences, 9(6), 1217. https://doi.org/10.3390/app9061217








