Determination of Volatile Components from Live Water Lily Flowers by an Orthogonal-Array-Design-Assisted Trapping Cell
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Optimizing the Sampling Device by Orthogonal Array Design
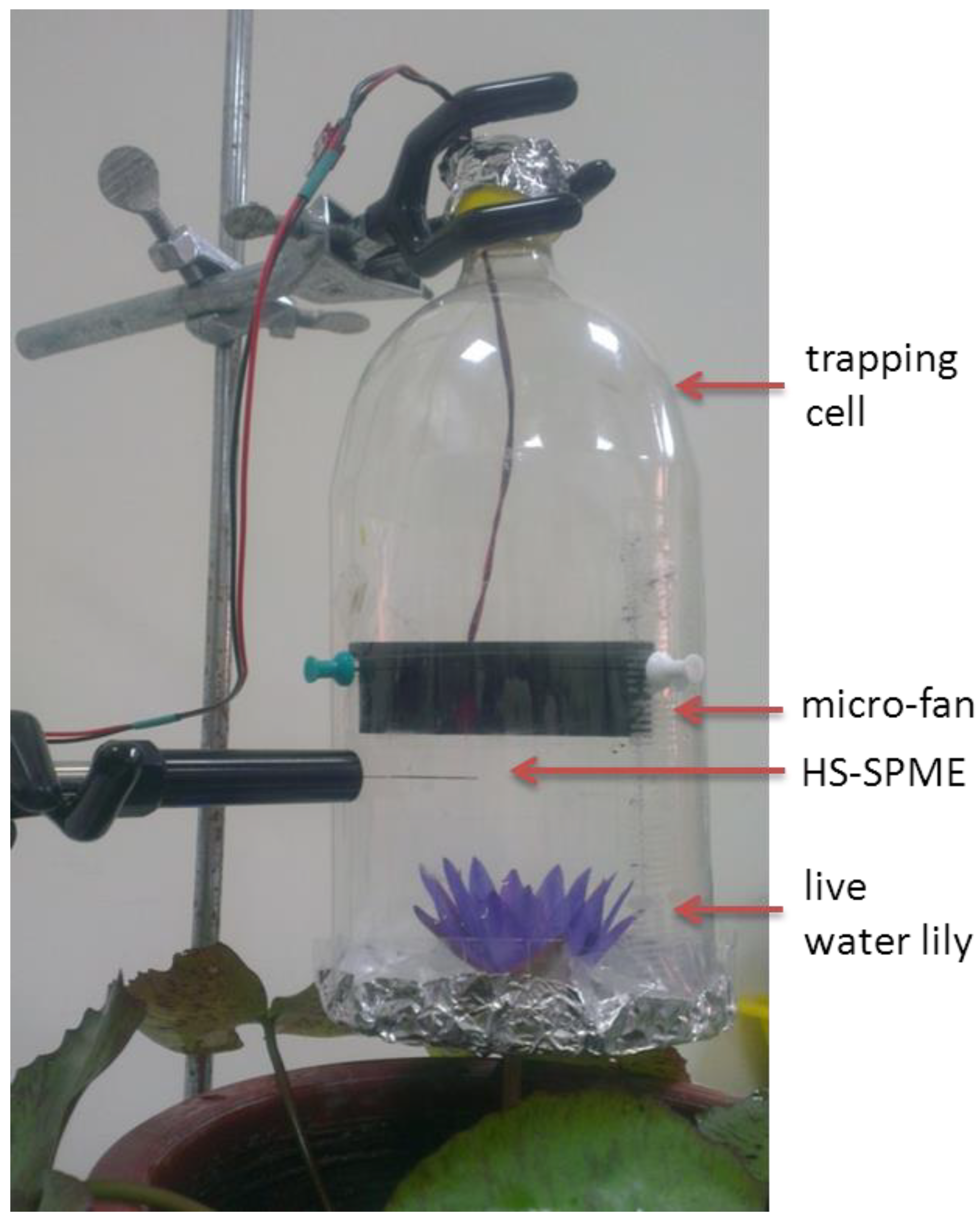
2.3. Sampling
2.4. Instrumentation
2.5. Identification of Components
2.6. Statistical Analysis
2.7. Standard Preparation and Method Validation
3. Results and Discussion
3.1. Optimization of the Device by Using OAD
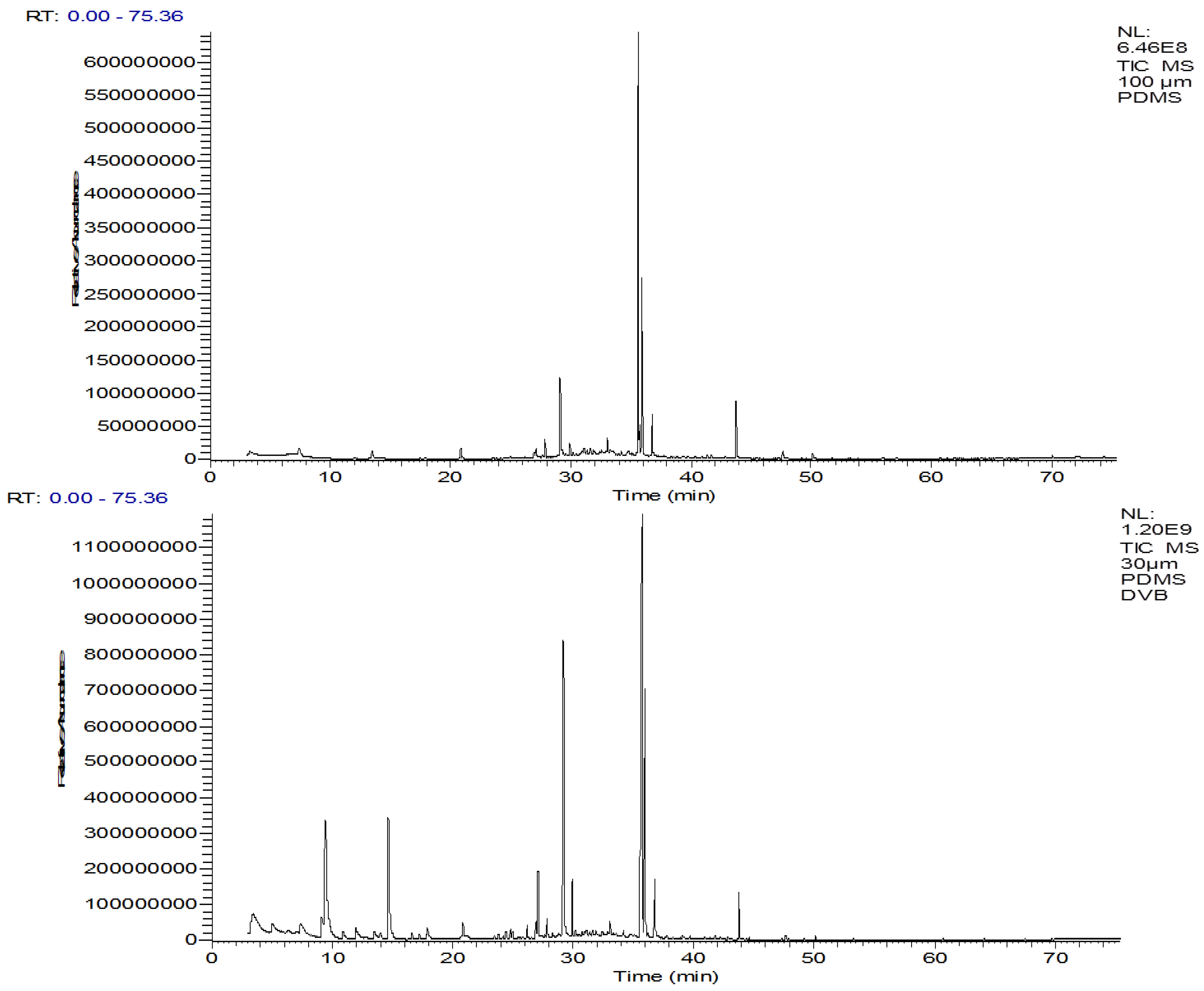
3.2. SPME Fiber Selection
3.3. Device Extraction Efficiency
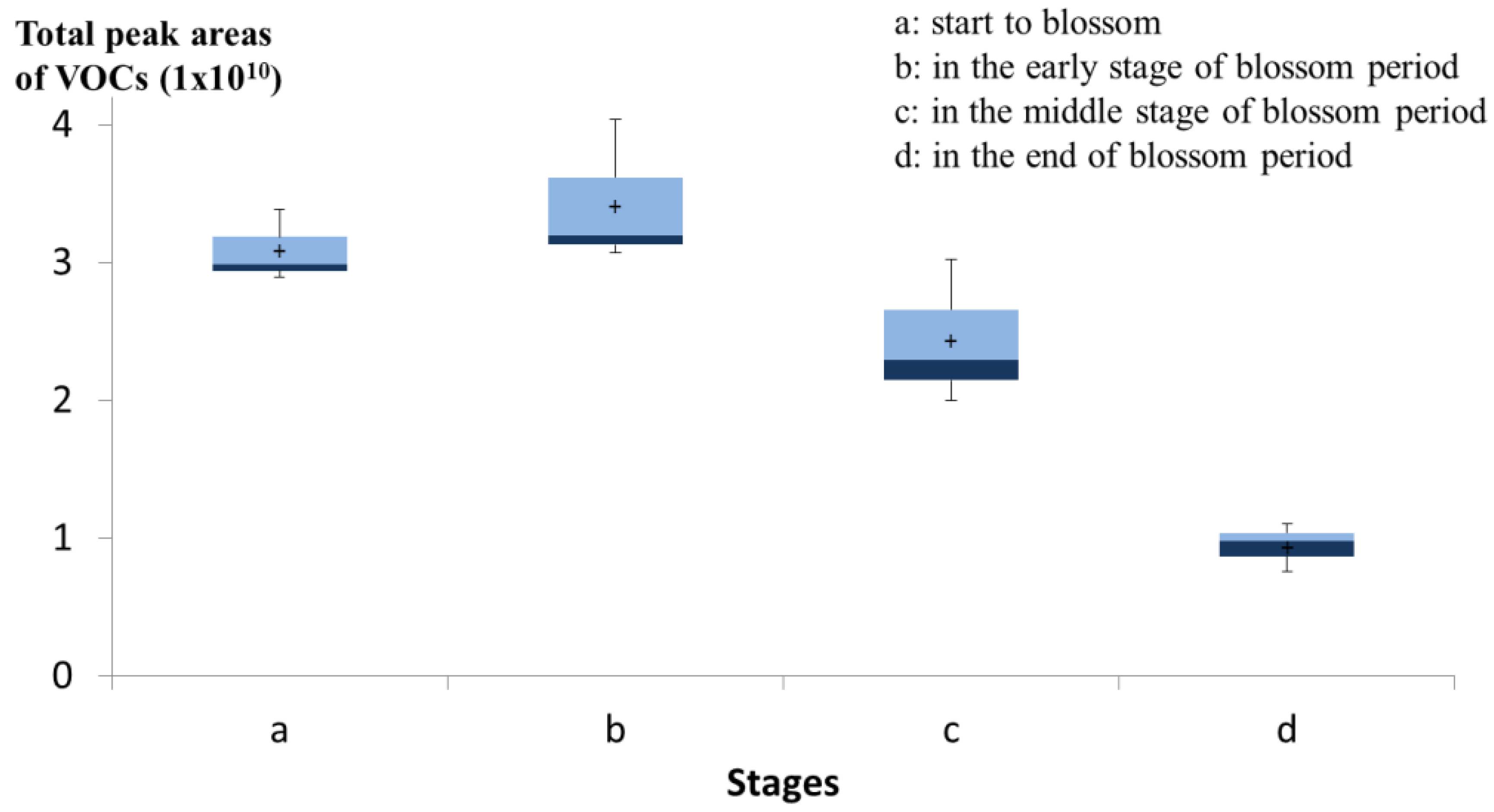
3.4. Main Volatiles from N. Caerulea Flower
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elshafie, H.S.; Camele, I. An overview of the biological effects of some mediterranean essential oils on human health. Biomed. Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef] [PubMed]
- Figueredo, G.; Nver, A.; Chalchat, J.C.; Arslan, D.; Zcan, M.M. A research on the composition of essential oil isolated from some aromatic plants by microwave and hydrodistillation. J. Food Biochem. 2011, 36, 334–343. [Google Scholar] [CrossRef]
- Ghelardini, C.; Galeotti, N.; Salvatore, G.; Mazzanti, G. Local anaesthetic activity of the essential oil of lavandula angustifolia. Planta Med. 1999, 65, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. A review of the composition of the essential oils and biological activities of angelica species. Sci. Pharm. 2017, 85, 33. [Google Scholar] [CrossRef]
- Chisvert, A.; Salvador, A. 6.1—Perfumes in cosmetics. Regulatory aspects and analytical methods for fragrance ingredients and other related chemicals in cosmetics. In Analysis of Cosmetic Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 243–256. [Google Scholar]
- Agnihotri, V.K.; Elsohly, H.N.; Khan, S.I.; Smillie, T.J.; Khan, I.A.; Walker, L.A. Antioxidant constituents of nymphaea caerulea flowers. Phytochemistry 2008, 69, 2061–2066. [Google Scholar] [CrossRef]
- Dehaut, A.; Himber, C.; Mulak, V.; Grard, T.; Krzewinski, F.; Le Fur, B.; Duflos, G. Evolution of volatile compounds and biogenic amines throughout the shelf life of marinated and salted anchovies (Engraulis encrasicolus). J. Agric. Food Chem. 2014, 62, 8014–8022. [Google Scholar] [CrossRef] [PubMed]
- Ozel, M.Z.; Kaymaz, H. Superheated water extraction, steam distillation and soxhlet extraction of essential oils of origanum onites. Anal. Bioanal. Chem. 2004, 379, 1127–1133. [Google Scholar] [CrossRef]
- Cai, J.; Liu, B.; Su, Q. Comparison of simultaneous distillation extraction and solid-phase microextraction for the determination of volatile flavor components. J. Chromatogr. A 2001, 930, 1–7. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Mi, J.; Zhang, M.; Wang, Y.; Jiang, Z.; Hu, P. Gc-ms profiling of volatile components in different fermentation products of cordyceps sinensis mycelia. Molecules 2017, 22, 1800. [Google Scholar] [CrossRef]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Augusto, F.; Leite e Lopes, A.; Zini, C.A. Sampling and sample preparation for analysis of aromas and fragrances. Trac Trends Anal. Chem. 2003, 22, 160–169. [Google Scholar] [CrossRef]
- Semenov, S.N.; Koziel, J.A.; Pawliszyn, J. Kinetics of solid-phase extraction and solid-phase microextraction in thin adsorbent layer with saturation sorption isotherm. J. Chromatogr. A 2000, 873, 39–51. [Google Scholar] [CrossRef]
- Rubiolo, P.; Belliardo, F.; Cordero, C.; Liberto, E.; Sgorbini, B.; Bicchi, C. Headspace-solid-phase microextraction fast GC in combination with principal component analysis as a tool to classify different chemotypes of chamomile flower-heads (Matricaria recutita L.). Phytochem. Anal. PCA 2006, 17, 217–225. [Google Scholar] [CrossRef]
- Ciucanu, I. Per-o-methylation reaction for structural analysis of carbohydrates by mass spectrometry. Anal. Chim. Acta 2006, 576, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Augusto, F.; Luiz Pires Valente, A. Applications of solid-phase microextraction to chemical analysis of live biological samples. TrAC Trends Anal. Chem. 2002, 21, 428–438. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Q.; Li, J.; Luo, J.; Chen, W.; Li, X. Comparative study of volatile compounds in the fruit of two banana cultivars at different ripening stages. Molecules 2018, 23, 2456. [Google Scholar] [CrossRef] [PubMed]
- Vercammen, J.; Pham-Tuan, H.; Sandra, P. Automated dynamic sampling system for the on-line monitoring of biogenic emissions from living organisms. J. Chromatogr. A 2001, 930, 39–51. [Google Scholar] [CrossRef]
- Mookherjee, B.D.; Trenkle, R.W.; Wilson, R.A. The chemistry of flowers, fruits and spices: Live vs. Dead—A new dimension in fragrance research. Pure Appl. Chem. 1990, 62, 1357. [Google Scholar] [CrossRef]
- Tahmasebi, E.; Yamini, Y.; Saleh, A. Extraction of trace amounts of pioglitazone as an anti-diabetic drug with hollow fiber liquid phase microextraction and determination by high-performance liquid chromatography-ultraviolet detection in biological fluids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Sobhi, H.R.; Yamini, Y.; Esrafili, A.; Abadi, R.H. Suitable conditions for liquid-phase microextraction using solidification of a floating drop for extraction of fat-soluble vitamins established using an orthogonal array experimental design. J. Chromatogr. A 2008, 1196–1197, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.d.S.; da Rocha, G.O.; de Andrade, J.B. A SDME/GC–MS methodology for determination of organophosphate and pyrethroid pesticides in water. Microchem. J. 2011, 99, 303–308. [Google Scholar] [CrossRef]
- Canosa, P.; Rodriguez, I.; Rubi, E.; Cela, R. Optimization of solid-phase microextraction conditions for the determination of triclosan and possible related compounds in water samples. J. Chromatogr. A 2005, 1072, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Vandendool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Strehmel, N.; Hummel, J.; Erban, A.; Strassburg, K.; Kopka, J. Retention index thresholds for compound matching in gc-ms metabolite profiling. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 871, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Flamini, G.; Cioni, P.L.; Maccioni, S.; Baldini, R. Essential oil composition and in vivo volatiles emission by different parts of coleostephus myconis capitula. Nat. Prod. Commun. 2010, 5, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Custodio, L.; Serra, H.; Nogueira, J.M.; Goncalves, S.; Romano, A. Analysis of the volatiles emitted by whole flowers and isolated flower organs of the carob tree using HS-SPME-GC/MS. J. Chem. Ecol. 2006, 32, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Steffen, A.; Pawliszyn, J. Analysis of flavor volatiles using headspace solid-phase microextraction. J. Agric. Food Chem. 1996, 44, 2187–2193. [Google Scholar] [CrossRef]
- Yin, D.D.; Yuan, R.Y.; Wu, Q.; Li, S.S.; Shao, S.; Xu, Y.J.; Hao, X.H.; Wang, L.S. Assessment of flavonoids and volatile compounds in tea infusions of water lily flowers and their antioxidant activities. Food Chem. 2015, 187, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lyu, S.; Chen, D.; Lin, Y.; Chen, J.; Chen, G.; Ye, N. Volatiles emitted at different flowering stages of jasminum sambac and expression of genes related to alpha-farnesene biosynthesis. Molecules 2017, 22, 546. [Google Scholar] [CrossRef] [PubMed]
- Ohkado, Y.; Kawamura, Y.; Mutsuga, M.; Tamura, H.O.; Tanamoto, K. Analysis of residual volatiles in recycled polyethylene terephthalate. Shokuhin Eiseigaku Zasshi J. Food Hyg. Soc. Jpn. 2005, 46, 13–20. [Google Scholar] [CrossRef]
- Gorji, S.; Biparva, P.; Bahram, M.; Nematzadeh, G. Development of magnetic solid phase microextraction method for determination of the endocrine disrupting chemicals leached from reused plastic bottles. Talanta 2019, 194, 859–869. [Google Scholar] [CrossRef] [PubMed]

| Variables | Level | |||||||
|---|---|---|---|---|---|---|---|---|
| Low (−1) | High (1) | |||||||
| The angle of fiber to fan (A) | Vertical | Parallel | ||||||
| The airstream (B) | Positive pressure | Negative pressure | ||||||
| The distance of fan from flower (C) | Low | High | ||||||
| Run | A | B | A*B | C | A*C | B*C | -- | |
| 1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 814,672 |
| 2 | −1 | −1 | −1 | 1 | 1 | 1 | 1 | 472,099 |
| 3 | −1 | 1 | 1 | −1 | −1 | 1 | 1 | 2,149,132 |
| 4 | −1 | 1 | 1 | 1 | 1 | −1 | −1 | 1,016,438 |
| 5 | 1 | −1 | 1 | −1 | 1 | −1 | 1 | 917,401 |
| 6 | 1 | −1 | 1 | 1 | −1 | 1 | −1 | 663,680 |
| 7 | 1 | 1 | −1 | −1 | 1 | 1 | −1 | 2,386,180 |
| 8 | 1 | 1 | −1 | 1 | −1 | −1 | 1 | 1,931,771 |
| K1 | 4,452,341 | 2,867,852 | 5,604,722 | 6,267,385 | 5,559,255 | 4,680,282 | 4,880,969 | |
| K2 | 5,899,032 | 7,483,521 | 4,746,651 | 4,083,988 | 4,792,118 | 5,671,091 | 5,470,403 | |
| Range | 1,446,691 | 4,615,669 | 858,071 | 2,183,397 | 767,137 | 990,809 | 589,433 | |
| Variables | Level | |||||||
|---|---|---|---|---|---|---|---|---|
| Low (−1) | High (1) | |||||||
| The location of fiber to fan (A) | Up | Down | ||||||
| The airstream (B) | Positive pressure | Negative pressure | ||||||
| The distance of fan from flower (C) | Low | High | ||||||
| Run | A | B | A*B | C | A*C | B*C | -- | |
| 1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 899,329 |
| 2 | −1 | −1 | −1 | 1 | 1 | 1 | 1 | 723,738 |
| 3 | −1 | 1 | 1 | −1 | −1 | 1 | 1 | 2,147,733 |
| 4 | −1 | 1 | 1 | 1 | 1 | −1 | −1 | 1,816,269 |
| 5 | 1 | −1 | 1 | −1 | 1 | −1 | 1 | 3,103,363 |
| 6 | 1 | −1 | 1 | 1 | −1 | 1 | −1 | 2,416,830 |
| 7 | 1 | 1 | −1 | −1 | 1 | 1 | −1 | 1,411,773 |
| 8 | 1 | 1 | −1 | 1 | −1 | −1 | 1 | 1,585,760 |
| K1 | 5,587,070 | 7,143,260 | 4,620,600 | 7,562,198 | 7,049,652 | 7,404,721 | 6,544,201 | |
| K2 | 8,517,725 | 6,961,535 | 9,484,195 | 6,542,597 | 7,055,143 | 6,700,074 | 7,560,593 | |
| Range | 2,930,655 | 181,725 | 4,863,596 | 1,019,600 | 5490 | 704,646 | 1,016,392 | |
| Peak | Rt | Compound | Formula | RI | Area (%) | Area (%) (No Fan) |
|---|---|---|---|---|---|---|
| 1 | 7.42 | Benzaldehyde | C7H6O | 970 | 0.05 | 1.14 |
| 2 | 9.13 | 2-ethyl-1-hexanol | C8H18O | 1017 | 0.96 | 3.66 |
| 3 | 9.46 | benzyl alcohol | C7H8O | 1025 | 4.42 | 6.70 |
| 4 | 13.99 | 1-Undecene | C11H22 | 1136 | 0.24 | 0.48 |
| 5 | 14.65 | Benzyl acetate | C9H10O2 | 1152 | 10.42 | 69.56 |
| 6 | 15.3 | m-Methylphenyl acetate | C9H10O2 | 1168 | 0.03 | 0.34 |
| 7 | 16.64 | decanal | C10H20O | 1200 | 0.17 | 0.68 |
| 8 | 20.74 | Tridecane | C13H28 | 1300 | 0.05 | 0.24 |
| 9 | 21.12 | Undecanal | C11H22O | 1309 | 0.11 | 0.19 |
| 10 | 24.82 | decanoic acid, ethyl ester | C12H24O2 | 1400 | 0.12 | 0.20 |
| 11 | 25.01 | Tetradecane | C14H30 | 1404 | 0.07 | 0.88 |
| 12 | 25.75 | α-Ionone | C13H20O | 1422 | 0.16 | 0.12 |
| 13 | 26.2 | trans-α-Bergamotene | C15H24 | 1433 | 0.26 | 0.22 |
| 14 | 26.93 | trans-Geranylacetone | C13H22O | 1451 | 0.26 | 0.32 |
| 15 | 27.11 | (E)-β-Farnesene | C15H24 | 1455 | 1.55 | 0.24 |
| 16 | 28.08 | β-Ionone | C13H20O | 1479 | 0.24 | -- |
| 17 | 28.27 | 1-Pentadecene | C15H30 | 1484 | 0.23 | -- |
| 18 | 28.68 | 1,3,6,10-Dodecatetraene, 3,7,11-trimethyl-, (Z,E)- | C15H24 | 1494 | 0.09 | -- |
| 19 | 28.79 | 1,3-Cyclohexadiene, 5-(1,5-dimethyl-4-hexenyl)-2-methyl-, [S-(R*,S*)]- | C15H24 | 1496 | 0.26 | -- |
| 20 | 29.13 | Pentadecane | C15H32 | 1505 | 15.49 | 2.58 |
| 21 | 29.22 | α-farnesene | C15H24 | 1507 | 0.47 | -- |
| 22 | 29.92 | β-Sesquiphellandrene | C15H24 | 1524 | 1.30 | 0.22 |
| 23 | 32.4 | Propanoic acid, 2-methtyl-1-(1,1-dimethylethylethyl)-2-methyl-1,3-propanediyl ester | C16H30O4 | 1584 | 0.11 | 0.20 |
| 24 | 33.05 | hexadecane | C16H34 | 1600 | 0.18 | 0.17 |
| 25 | 35.59 | 6,9-heptadecadiene | C14H28O | 1662 | 40.10 | 5.49 |
| 26 | 35.71 | 9-Tetradecen-1-ol, (E)- | C14H28O | 1665 | 0.83 | -- |
| 27 | 35.89 | 8-heptadecene | C14H30O | 1670 | 15.27 | 1.80 |
| 28 | 36.71 | 2-pentadecanone | C15H30O | 1690 | 0.51 | -- |
| 29 | 36.78 | heptadecne | C17H36 | 1691 | 2.23 | 1.75 |
| 30 | 37.64 | E,E-10,12-hexadecadienal | C16H28O | 1712 | 0.77 | -- |
| 31 | 37.77 | Z,Z-10,13-hexadecadienal | C16H28O | 1715 | 0.63 | -- |
| 32 | 43.72 | 2-heptadecanone | C17H34O | 1861 | 2.21 | 2.32 |
| 33 | 50.09 | Eicosane | C20H42 | 2016 | 0.21 | 0.49 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, F.-J.; Liu, H.-J.; Lee, M.-Y.; Lin, C.-C. Determination of Volatile Components from Live Water Lily Flowers by an Orthogonal-Array-Design-Assisted Trapping Cell. Appl. Sci. 2019, 9, 1269. https://doi.org/10.3390/app9071269
Tsai F-J, Liu H-J, Lee M-Y, Lin C-C. Determination of Volatile Components from Live Water Lily Flowers by an Orthogonal-Array-Design-Assisted Trapping Cell. Applied Sciences. 2019; 9(7):1269. https://doi.org/10.3390/app9071269
Chicago/Turabian StyleTsai, Feng-Jie, Hsiao-Ju Liu, Ming-Yung Lee, and Chih-Chien Lin. 2019. "Determination of Volatile Components from Live Water Lily Flowers by an Orthogonal-Array-Design-Assisted Trapping Cell" Applied Sciences 9, no. 7: 1269. https://doi.org/10.3390/app9071269
APA StyleTsai, F.-J., Liu, H.-J., Lee, M.-Y., & Lin, C.-C. (2019). Determination of Volatile Components from Live Water Lily Flowers by an Orthogonal-Array-Design-Assisted Trapping Cell. Applied Sciences, 9(7), 1269. https://doi.org/10.3390/app9071269






