Structural and Physicochemical Characteristics of Rice Bran Dietary Fiber by Cellulase and High-Pressure Homogenization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Rice bran Preparation
2.3. Purification of DF
2.4. Cellulase Enzymatic Treatments
2.5. High-Pressure Homogenization (HPH)
2.6. Scanning Electron Microscopy (SEM)
2.7. X-Ray Diffraction (XRD)
2.8. Fourier Transform Infrared (FT-IR) Spectroscopy
2.9. Thermogravimetric Analysis (TGA)
2.10. Physicochemical Properties
2.10.1. Water and Oil Holding Capacities
2.10.2. Swelling Capacity
2.10.3. Cation-Exchange Capacity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Structural Properties of RD-DF
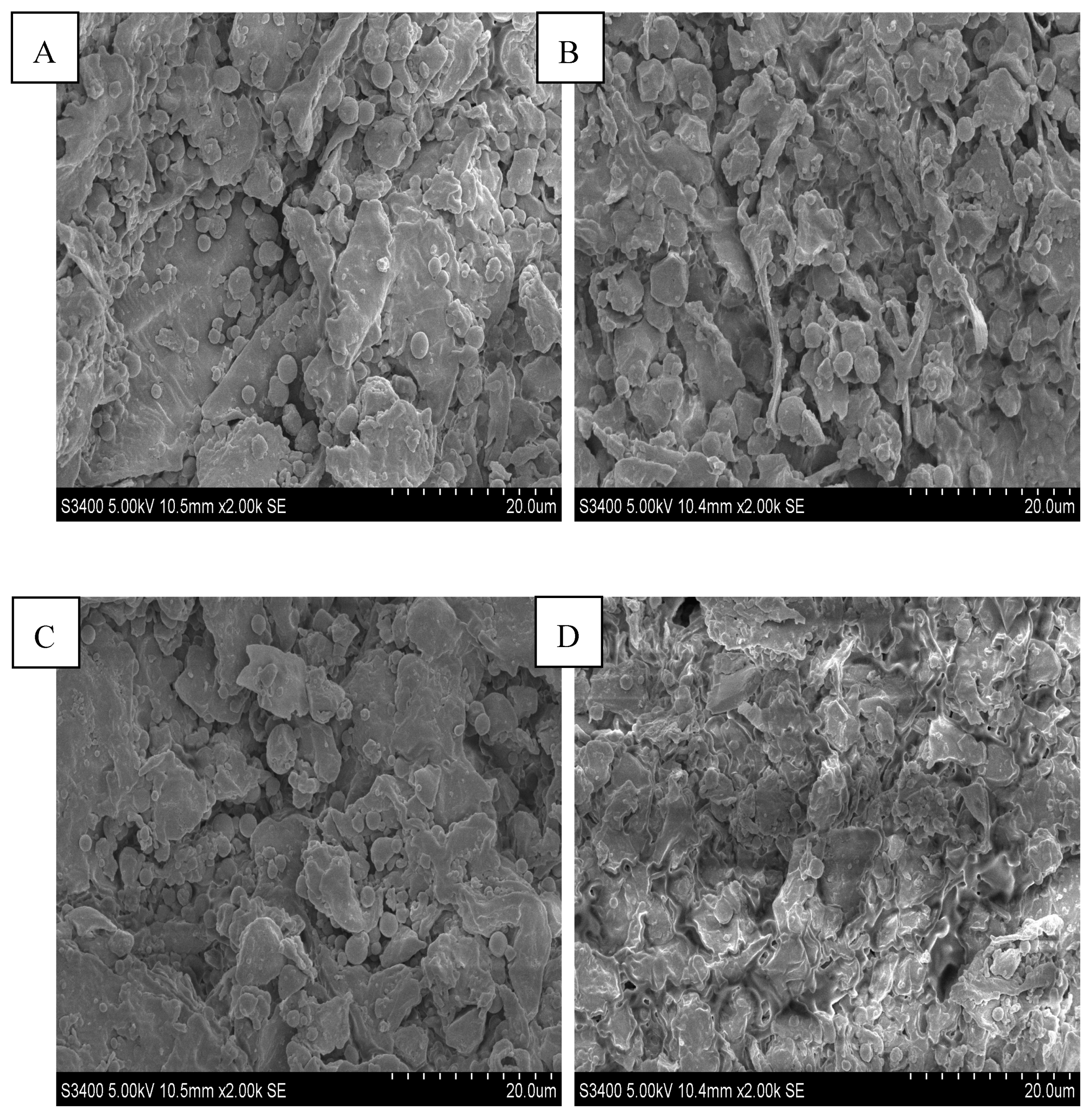
3.1.1. SEM
3.1.2. Crystalline and Molecular Structure
3.1.3. FTIR Spectra
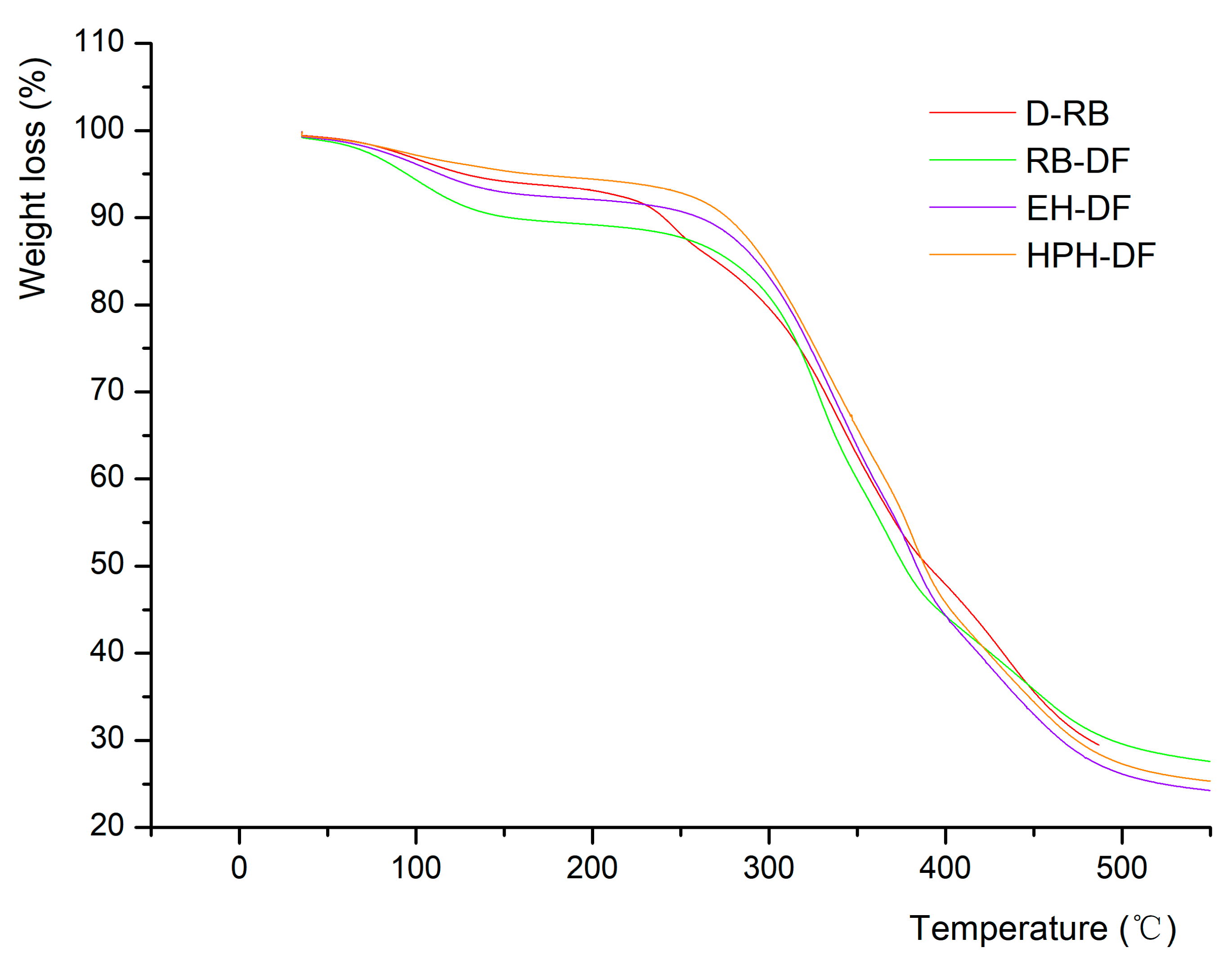
3.1.4. Thermal stability analysis
3.2. Physicochemical Properties of RB-DF
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Lattimer, J.M.; Haub, M.D. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Q.; Wang, L.; Zha, S.; Zhang, L.; Zhao, B. Physicochemical and functional properties of dietary fiber from maca (Lepidium meyenii Walp.) liquor residue. Carbohydr. Polym. 2015, 132, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Chater, P.I.; Wilcox, M.D.; Pearson, J.P.; Brownlee, I.A. The impact of dietary fibres on the physiological processes governing small intestinal digestive processes. Bioact. Carbohydr. Diet. Fibre 2015, 6, 117–132. [Google Scholar] [CrossRef]
- Ryan, E.P. Bioactive food components and health properties of rice bran. J. Am. Vet. Med. Assoc. 2011, 238, 593–600. [Google Scholar] [CrossRef]
- Kahlon, T.S.; Chow, F.I.; Knuckles, B.E.; Chiu, M.M. Cholesterol-lowering effects in hamsters of beta-gluca n-enriched barley fraction, dehulled whole barley, rice bran, and oat bran and their combinations. Cereal Chem. 1993, 70, 435–440. [Google Scholar]
- Huang, S.; He, Y.; Zou, Y.; Liu, Z. Modification of insoluble dietary fibres in soya bean okara and their physicochemical properties. Int. J. Food Sci. Technol. 2016, 50, 2606–2613. [Google Scholar] [CrossRef]
- Ma, M.; Mu, T. Modification of deoiled cumin dietary fiber with laccase and cellulase under high hydrostatic pressure. Carbohydr. Polym. 2016, 136, 87–94. [Google Scholar] [CrossRef]
- Mateosaparicio, I.; Mateospeinado, C.; Rupérez, P. High hydrostatic pressure improves the functionality of dietary fibre in okara by-product from soybean. Innov. Food Sci. Emerg. Technol. 2010, 11, 445–450. [Google Scholar] [CrossRef]
- Sangnark, A.; Noomhorm, A. Effect of particle sizes on functional properties of dietary fibre prepared from sugarcane bagasse. Food Chem. 2003, 80, 221–229. [Google Scholar] [CrossRef]
- Alba, K.; Macnaughtan, W.; Laws, A.P.; Foster, T.J.; Campbell, G.M.; Kontogiorgos, V. Fractionation and characterisation of dietary fibre from blackcurrant pomace. Food Hydrocoll. 2018, 81, 398–408. [Google Scholar] [CrossRef]
- Chau, C.F.; Wang, Y.T.; Wen, Y.L. Different micronization methods significantly improve the functionality of carrot insoluble fibre. Food Chem. 2007, 100, 1402–1408. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, W.; Pang, X.; Liao, X.; Hu, X.; Wu, J. Emulsion stabilizing properties of pectins extracted by high hydrostatic pressure, high-speed shearing homogenization and traditional thermal methods: A comparative study. Food Hydrocoll. 2014, 35, 217–225. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Boil. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef]
- Palmero, P.; Colle, I.; Lemmens, L.; Panozzo, A.; Nguyen, T.T.; Hendrickx, M.; Van Loey, A. Enzymatic cell wall degradation of high-pressure-homogenized tomato puree and its effect on lycopene bioaccessibility. J. Sci. Food Agric. 2016, 96, 254–261. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, C. Extracting xylooligosaccharides in wheat bran by screening and cellulase assisted enzymatic hydrolysis. Int. J. Biol. Macromol. 2016, 92, 748–752. [Google Scholar] [CrossRef]
- Paz, A.; Outeiriño, D.; Guerra, N.P.; Domínguez, J.M. Enzymatic hydrolysis of brewer’s spent grain to obtain fermentable sugars. Bioresour. Technol. 2019, 275, 402–409. [Google Scholar] [CrossRef]
- Wen, Y.; Niu, M.; Zhang, B.; Zhao, S.; Xiong, S. Structural characteristics and functional properties of rice bran dietary fiber modified by enzymatic and enzyme-micronization treatments: Food science + technology. Science + technologie alimentaire. LWT 2017, 75, 344–351. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, X.; Zhang, Z. Extrusion process improves the functionality of soluble dietary fiber in oat bran. J. Cereal Sci. 2011, 54, 98–103. [Google Scholar] [CrossRef]
- Ullah, I.; Yin, T.; Xiong, S.; Huang, Q.; Din, Z.U.; Zhang, J.; Javaid, A.B. Effects of thermal pre-treatment on physicochemical properties of nano-sized okara (soybean residue) insoluble dietary fiber prepared by wet media milling. J. Food Eng. 2018, 237, 18–26. [Google Scholar] [CrossRef]
- Shen, X.L.; Wu, J.M.; Chen, Y.; Zhao, G. Antimicrobial and physical properties of sweet potato starch films incorporated with potassium sorbate or chitosan. Food Hydrocoll. 2010, 24, 285–290. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Yuan, F.; Fan, R.; Gao, Y. Preparation and physicochemical properties of soluble dietary fiber from orange peel assisted by steam explosion and dilute acid soaking. Food Chem. 2015, 185, 90–98. [Google Scholar] [CrossRef]
- Sowbhagya, H.B.; Suma, P.F.; Mahadevamma, S.; Tharanathan, R.N. Spent residue from cumin: A potential source of dietary fiber. Food Chem. 2007, 104, 1220–1225. [Google Scholar] [CrossRef]
- Chau, C.F.; Cheung, C.K. Effects of the physico-chemical properties of three legume fibers on cholesterol absorption in hamsters. Nutr. Res. 1999, 19, 257–265. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.L.; Cheng, Y.K.; Jiang, Z.Y.; Jin, Y.; Zhang, H.S.; Liu, D.; Teng, L.R.; Zhang, G.R. Enzymo-chemical preparation, physico-chemical characterization and hypolipidemic activity of granular corn bran dietary fibre. J. Food Sci. Technol. 2015, 52, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Floury, J.; Desrumaux, A.; Legrand, J. Effect of Ultra-high-pressure Homogenization on Structure and on Rheological Properties of Soy Protein-stabilized Emulsions. J. Food Sci. 2010, 67, 3388–3395. [Google Scholar] [CrossRef]
- Chen, D.; Lawton, D.; Thompson, M.R.; Liu, Q. Biocomposites reinforced with cellulose nanocrystals derived from potato peel waste. Carbohydr. Polym. 2012, 90, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Karaman, E.; Yılmaz, E.; Tuncel, N.B. Physicochemical, microstructural and functional characterization of dietary fibers extracted from lemon, orange and grapefruit seeds press meals. Bioact. Carbohydr. Diet. Fibre 2017, 11, 9–17. [Google Scholar] [CrossRef]
- Xu, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan–starch composite film: Preparation and characterization. Ind. Crops Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Chylińska, M.; Szymańska-Chargot, M.; Kruk, B.; Zdunek, A. Study on dietary fibre by Fourier transform-infrared spectroscopy and chemometric methods. Food Chem. 2016, 196, 114–122. [Google Scholar] [CrossRef]
- SzymaåńSka-Chargot, M.; Cybulska, J.; Zdunek, A. Sensing the structural differences in cellulose from apple and bacterial cell wall materials by Raman and FT-IR spectroscopy. Sensors 2011, 11, 5543–5560. [Google Scholar] [CrossRef]
- Vanderghem, C.; Brostaux, Y.; Jacquet, N.; Blecker, C.; Paquot, M. Optimization of formic/acetic acid delignification of Miscanthus×giganteus for enzymatic hydrolysis using response surface methodology. Ind. Crops Prod. 2012, 35, 280–286. [Google Scholar] [CrossRef]
- Ma, S.; Han, W.; Li, L.; Zheng, X.; Wang, X. The thermal stability, structural changeability, and aggregability of glutenin and gliadin proteins induced by wheat bran dietary fiber. Food Funct. 2019, 10, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, M.; Lan, X.; Zhang, W.; Gong, S.; Wu, J.; Wang, Z. Modification of dietary fibers from purple-fleshed potatoes (Heimeiren) with high hydrostatic pressure and high pressure homogenization processing: A comparative study. Innov. Food Sci. Emerg. Technol. 2017, 42, 157–164. [Google Scholar]
- Lan, G.; Chen, H.; Chen, S.; Tian, J. Chemical composition and physicochemical properties of dietary fiber from Polygonatum odoratum as affected by different processing methods. Food Res. Int. 2012, 49, 406–410. [Google Scholar] [CrossRef]
- Tejada-ortigoza, V.; Garcia-Amezquita, L.E.; Serna-Saldívar, S.O.; Welti-Chanes, J. Advances in the Functional Characterization and Extraction Processes of Dietary Fiber. Food Eng. Rev. 2015, 8, 1–21. [Google Scholar] [CrossRef]


| Sample. | WHC (g/g) | OHC (g/g) | SC (mL/g) | CEC (mmol/g) |
|---|---|---|---|---|
| D-RB | 3.02 ± 0.38 a | 3.13 ± 0.14 a | 1.00 ± 0.02 a | 0.21 ± 0.02 a |
| RB-DF | 3.20 ± 0.13 ab | 4.23 ± 0.13 b | 1.93 ± 0.09 b | 0.27 ± 0.01 b |
| EH-DF | 3.60 ± 0.27 b | 3.92 ± 0.11 c | 3.875 ± 0.01 c | 0.32 ± 0.01 c |
| HPH-DF | 5.81 ± 0.24 c | 3.43 ± 0.17 d | 5.26 ± 0.01 d | 0.38 ± 0.02 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, F.; Zhao, T.; Wan, H.; Li, M.; Sun, L.; Wang, Z.; Zhang, S. Structural and Physicochemical Characteristics of Rice Bran Dietary Fiber by Cellulase and High-Pressure Homogenization. Appl. Sci. 2019, 9, 1270. https://doi.org/10.3390/app9071270
Xie F, Zhao T, Wan H, Li M, Sun L, Wang Z, Zhang S. Structural and Physicochemical Characteristics of Rice Bran Dietary Fiber by Cellulase and High-Pressure Homogenization. Applied Sciences. 2019; 9(7):1270. https://doi.org/10.3390/app9071270
Chicago/Turabian StyleXie, Fengying, Tian Zhao, Hongchen Wan, Miao Li, Lina Sun, Zhongjiang Wang, and Shuang Zhang. 2019. "Structural and Physicochemical Characteristics of Rice Bran Dietary Fiber by Cellulase and High-Pressure Homogenization" Applied Sciences 9, no. 7: 1270. https://doi.org/10.3390/app9071270
APA StyleXie, F., Zhao, T., Wan, H., Li, M., Sun, L., Wang, Z., & Zhang, S. (2019). Structural and Physicochemical Characteristics of Rice Bran Dietary Fiber by Cellulase and High-Pressure Homogenization. Applied Sciences, 9(7), 1270. https://doi.org/10.3390/app9071270





