Electro-Precipitation of Actinides on Boron-Doped Diamond Thin Films for Solid Sources Preparation for High-Resolution Alpha-Particle Spectrometry
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Products
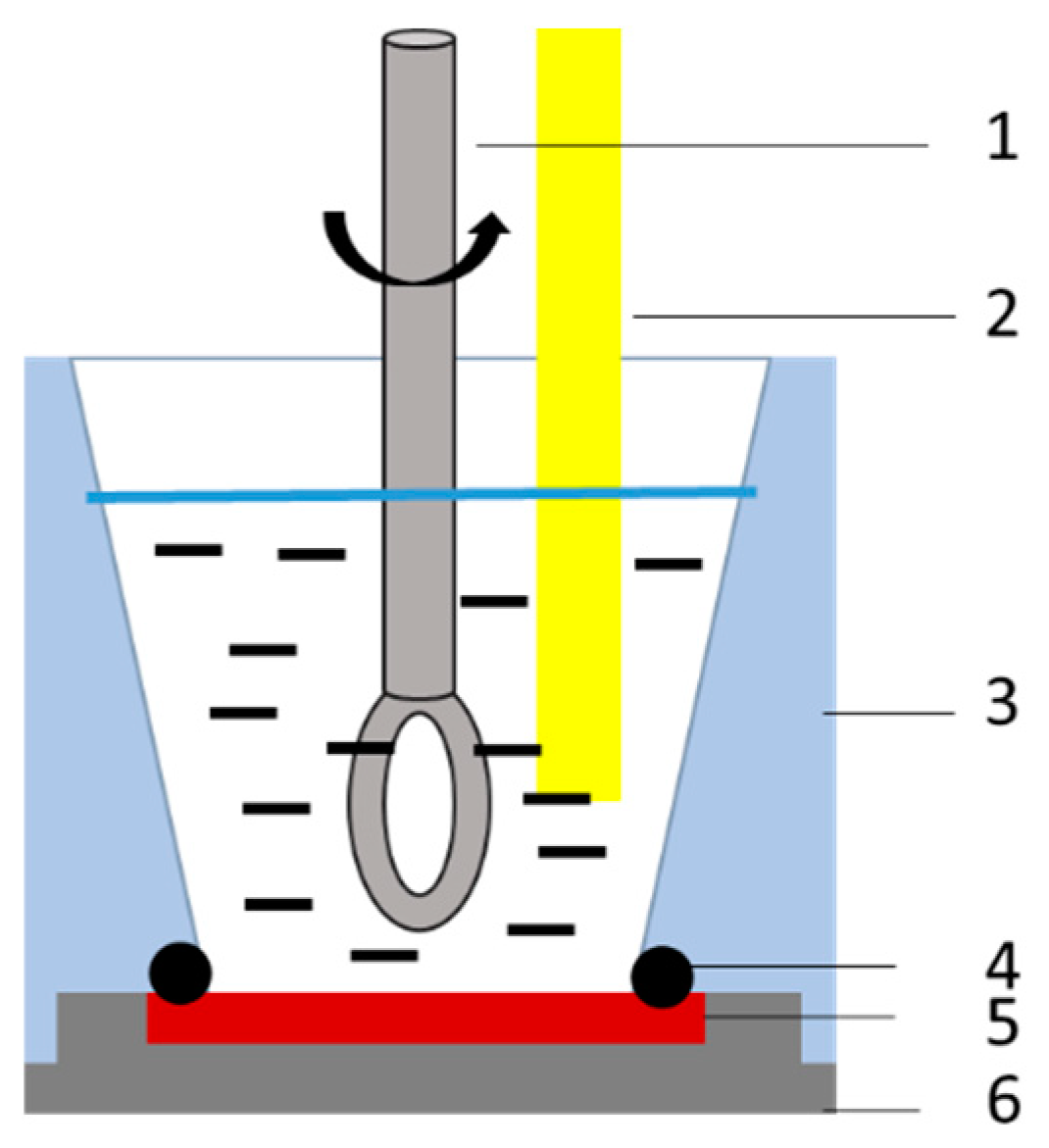
2.2. Electro-Precipitation Materials
2.3. Electro-Precipitation Process
2.4. Autoradiography
2.5. Alpha-Particle Spectrometry Measurement
2.6. Alpha-Particle Activity Measurements
3. Results and Discussion
3.1. Preparation of the Alpha-Particle Solid Source onto BDD Substrates
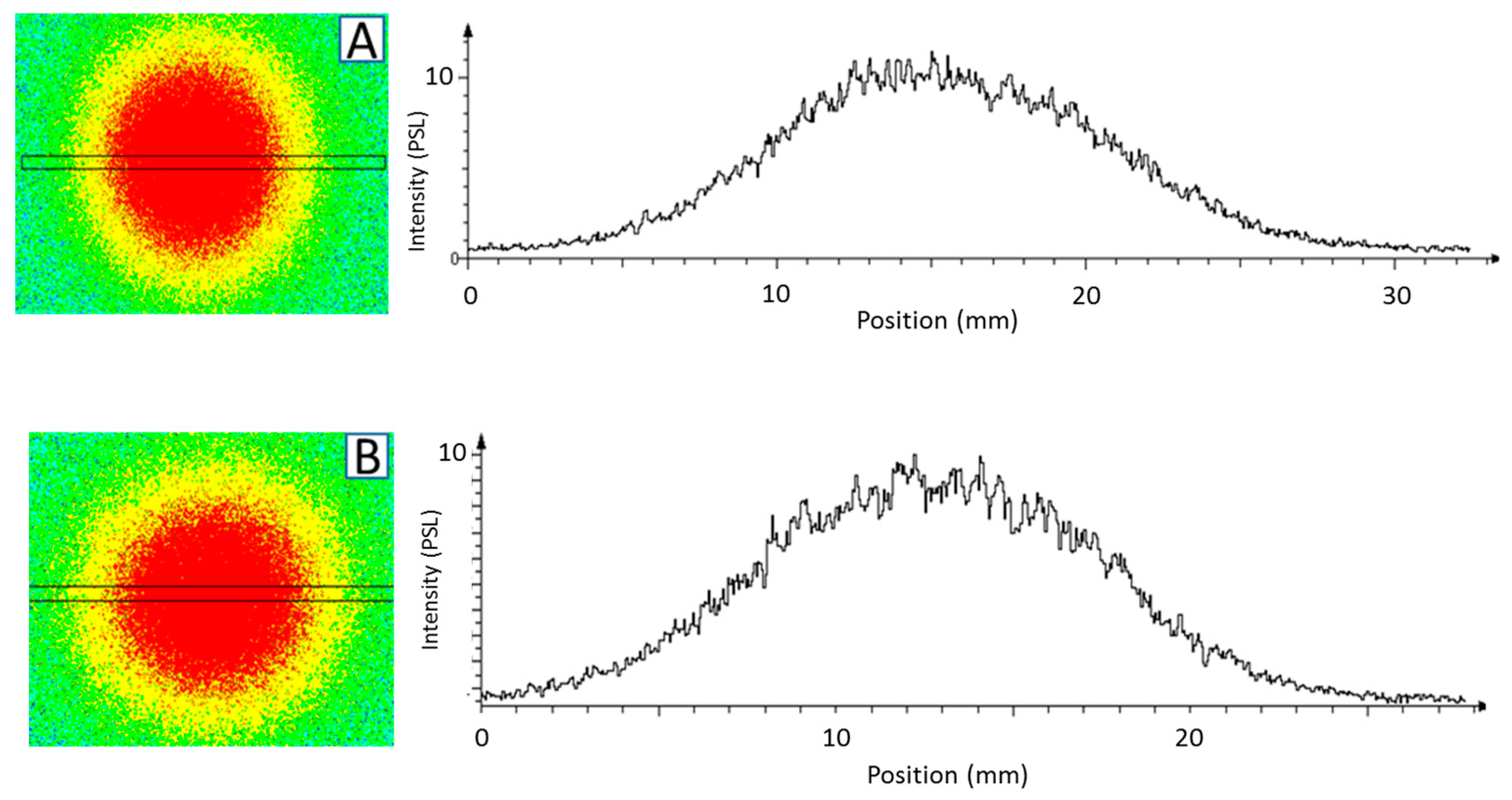
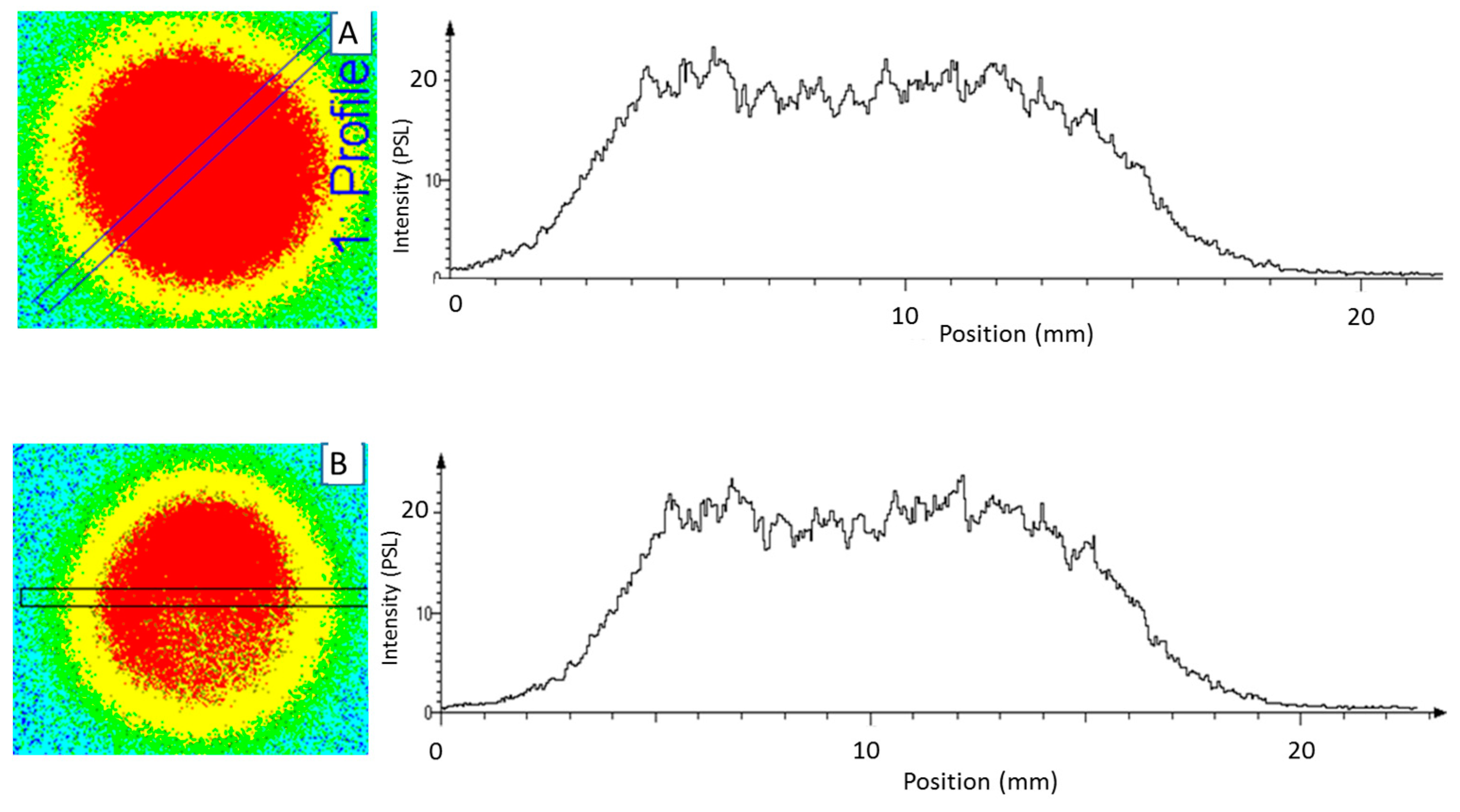
3.1.1. Uniformity of Solid Sources
3.1.2. Alpha-Particle Spectroscopy
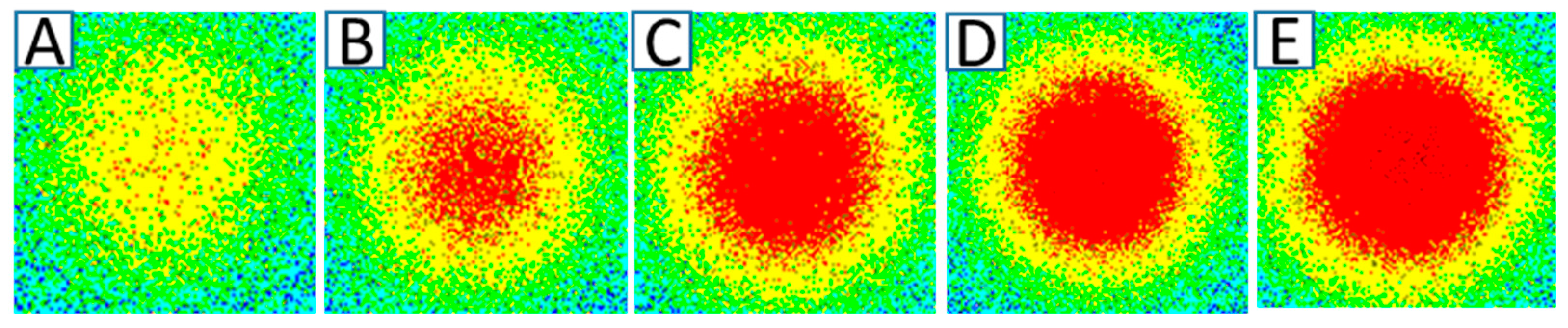
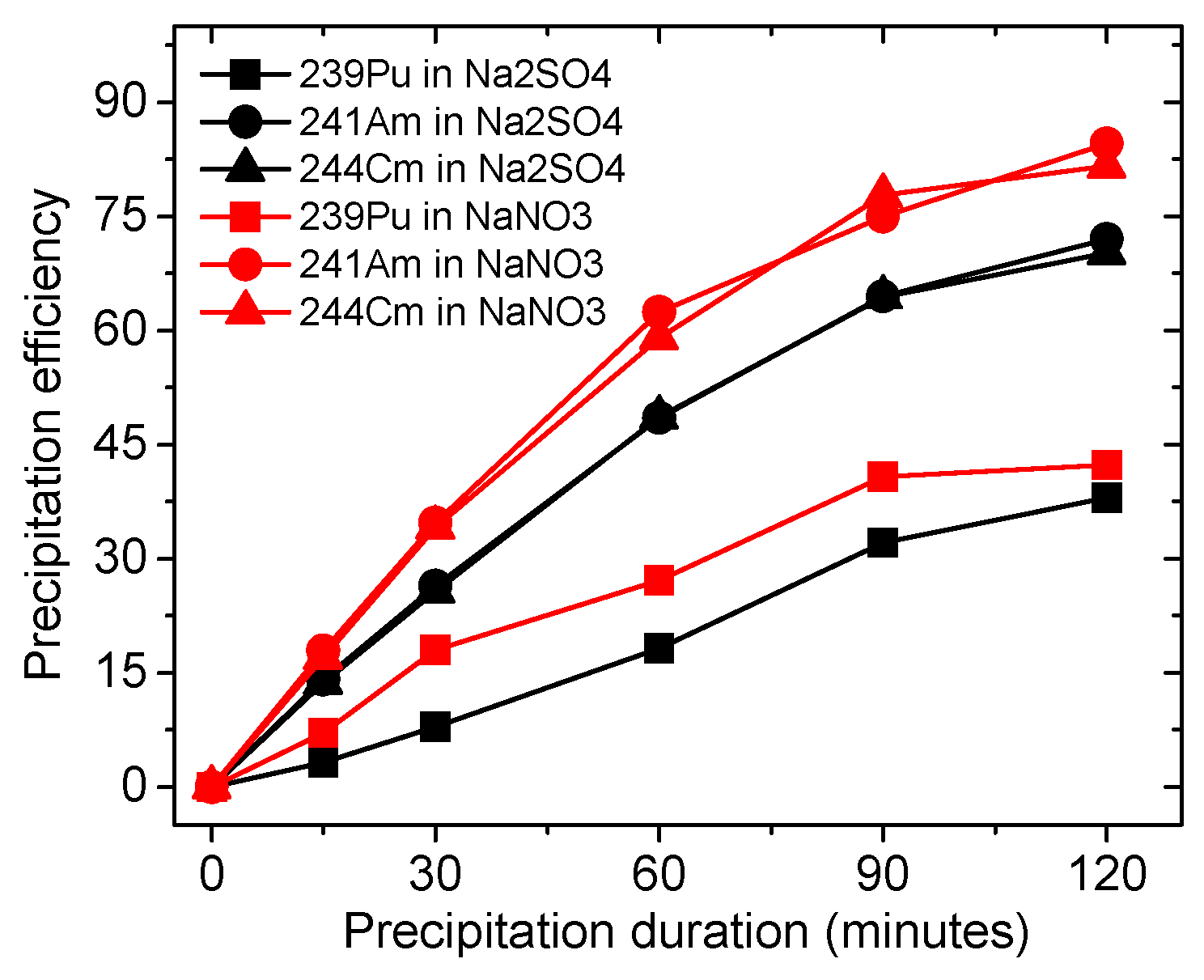
3.2. Electro-Precipitation Efficiency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wenzl, U.; Herz, D. Alpha-spectrometry in nuclear fuel analysis. J. Radioanal. Chem. 1974, 21, 473–483. [Google Scholar] [CrossRef]
- Wellum, R.; Molinet, R. The determination of 232U and 236Pu in solutions of irradiated reactor fuel by alphaspectrometry. Nucl. Instrum. Methods Phys. Res. 1984, 223, 523–527. [Google Scholar] [CrossRef]
- Bacrania, M.K.; Croce, M.P.; Bond, E.M.; Dry, D.E.; Moody, W.A.; LaMont, W.A.; Rabin, M.W.; Rim, J.H.; Smith, A.A.; Beall, J.A.; et al. Ultra-High-Resolution Alpha Spectrometry for Nuclear Forensics and Safeguards Applications. Int. Nucl. Inf. Syst. 2010, 42, 1–5. [Google Scholar]
- Jain, H.C.; Aggarwal, S.K. Role of Alpha Spectrometry in Nuclear Technology, in Artificial Radioactivity; Rao, K.N., Arnikar, H.J., Eds.; Tata McGraw-Hill: New Delhi, India, 1985; p. 263. [Google Scholar]
- Bergamini, G.; Taddei, M.H.T.; Rosa, M.M.L.; Ferreira, M.T.; Cheberle, L.T.V.; dos Santos, S.M.C.; Mariano, N.A.; Ramos, E.C.T. Determination of 226Ra in drinking water samples by alpha spectrometry. J. Radioanal. Nucl. Chem. 2016, 307, 829–834. [Google Scholar] [CrossRef]
- Bojanowski, R.; Radecki, Z.; Burns, K. Determination of radium and uranium isotopes in natural waters by sorption on hydrous manganese dioxide followed by alpha-spectrometry. J. Radioanal. Nucl. Chem. 2005, 264, 437–443. [Google Scholar]
- Eikenberg, J.; Tricca, A.; Vezzu, G.; Bajo, S.; Ruethi, M.; Surbeck, H. Determination of 228Ra, 226Ra and 224Ra in natural water via adsorption on MnO2-coated discs. J. Environ. Radioact. 2001, 54, 109–131. [Google Scholar] [CrossRef]
- Vajda, N. Up-to-date radiochemical methods for the determination of long-lived radionuclides in the environment. J. Anal. Sci. Technol. 2011, 2, A108–A114. [Google Scholar] [CrossRef]
- Pappalardo, L.; Pappalardo, G.; Amorini, F.; Branciforti, M.G.; Romano, F.P.; de Sanoit, J.; Rizzo, F.; Scafiri, E.; Taormina, A.; Gatto Rotondo, G. The complementary use of PIXE-α and XRD non-destructive portable systems for the quantitative analysis of painted surfaces. X-Ray Spectrom. 2008, 37, 370–375. [Google Scholar] [CrossRef]
- Pappalardo, L.; de Sanoit, J.; Marchetta, C.; Pappalardo, G.; Romano, F.P.; Rizzo, F. A portable spectrometer for simultaneous PIXE and XRF analysis. X-Ray Spectrom. 2007, 36, 310–315. [Google Scholar] [CrossRef]
- Manickam, E.; Sdraulig, S.; Tinker, R.A. Method design and validation for the determination of uranium levels in human urine using high resolution alpha spectrometry. J. Environ. Radioact. 2008, 99, 491–501. [Google Scholar] [CrossRef]
- Kehagia, K.; Potiriadis, C.; Bratakos, S.; Koukouliou, V.; Drikos, G. Determination of 226Ra in urine samples by alpha spectrometry. Radiat. Prot. Dosim. 2007, 127, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Kramer-Tranblay, S.; Li, C. Rapid determination of 226Ra in urine samples. Radiat. Prot. Dosim. 2012, 151, 30–35. [Google Scholar] [CrossRef]
- Salih, N.F.; Jafri, Z.M.; Aswood, M.S. Measurement of radon concentration in blood and urine samples collected from female cancer patients using RAD7. J. Radiat. Res. Appl. Sci. 2016, 9, 332–336. [Google Scholar] [CrossRef]
- DeRegge, P.; Boden, R. Review of chemical separation techniques applicable to alpha spectrometric measurements. Nucl. Instrum. Methods Phys. Res. 1984, 223, 181–187. [Google Scholar] [CrossRef]
- Horwitz, E.P.; Chiarizia, R.; Dietz, M.L.; Diamond, H. Separation and pre-concentration of actinides from acidic media by extraction chromatography. Anal. Chim. Acta 1993, 281, 361–372. [Google Scholar] [CrossRef]
- Crespo, M.T. A review of electrodeposition methods for the preparation of alpha-radiation sources. Appl. Radiat. Isot. 2012, 70, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.K.; Chourasiya, G.; Duggal, R.K.; Singh, C.P.; Rawat, A.S.; Jain, H.C. A comparative study of different methods of preparation of sources for alpha spectrometry of plutonium. Nucl. Instrum. Methods Phys. Res. Sect. A 1985, 238, 463–468. [Google Scholar] [CrossRef]
- Sibbens, G.; Altzitzoglou, T. Preparation of radioactive sources for radionuclide metrology. Metrologia 2007, 44, S71–S78. [Google Scholar] [CrossRef]
- Garcia-Torano, E. Current status of alpha-particle spectrometry. Appl. Radiat. Isot. 2006, 64, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.K. Alpha-particle spectrometry for the determination of alpha emitting isotopes in nuclear, environmental and biological samples: Past, present and future. Anal. Methods 2016, 8, 5353–5371. [Google Scholar] [CrossRef]
- Vajda, N.; Martin, P.; Kim, C.K. Alpha spectrometry. In Handbook of Radioactivity Analysis; L’Annunziata, M.F., Ed.; Academic Press: Cambridge, MA, USA, Capter 6; 2012; pp. 363–422. [Google Scholar] [CrossRef]
- Vajda, N.; Kim, C.K. Determination of transuranium isotopes (Pu, Np, Am) by radiometric techniques: A review of analytical methodology. Anal. Chem. 2011, 83, 4688–4719. [Google Scholar] [CrossRef]
- Vajda, N.; Kim, C.K. Determination of Pu isotopes by alpha spectrometry: A review of analytical methodology. J. Radioanal. Nucl. Chem. 2010, 283, 203–223. [Google Scholar] [CrossRef]
- Van Ammel, R.; Eykens, S.; Eykens, R.; Pomme, S. Preparation of drop-deposited quantitative uranium sources with low self-absorption. Nucl. Instrum. Methods Phys. Res. Sect. A 2011, 652, 76–78. [Google Scholar] [CrossRef]
- Sill, C.W.; Williams, R.L. Preparation of actinides for alpha spectrometry without electrodeposition. Anal. Chem. 1981, 53, 415–421. [Google Scholar] [CrossRef]
- Broda, E. Advances in Radiochemistry and in the Method of Producing Radioelements by Neutron Irradiation; Cambrigde University Press: Cambrigde, UK, 1950; 152p. [Google Scholar]
- Robbens, C.J. Analytical Chemistry of Manhattan Project; McGraw-Hill Book Company: New York, NY, USA, 1950; p. 449. [Google Scholar]
- Puphal, K.W.; Olsen, D.R. Electrodeposition of alpha-emitting nuclides from a mixture oxalate-chloride electrolyte. Anal. Chem. 1972, 44, 284–289. [Google Scholar] [CrossRef]
- Talvitie, N.A. Electrodeposition of Actinides for alpha spectrometric determination. Anal. Chem. 1972, 44, 280–283. [Google Scholar] [CrossRef]
- Mohapatra, P.K.; Khopkar, P.K. Hydrolysis of actinides and lanthanides: Hydrolysis of some trivalent actinide and lanthanide ions studied by extraction with thenoyltrifluoroacetone. Polyhydron 1989, 8, 2071–2076. [Google Scholar] [CrossRef]
- Neck, V.; Kim, J.I. Solubility and hydrolysis of tetravalent actinides. Radiochim. Acta 2001, 89, 1–16. [Google Scholar] [CrossRef]
- Kressin, I.K. Electrodeposition o plutonium and Americium for high resolution alpha spectrometry. Anal. Chem. 1977, 49, 842–846. [Google Scholar] [CrossRef]
- Hallstadius, L. A method for the electrodeposition of actinides. Nucl. Instrum. Meth. Phys. Res. A 1984, 223, 266–267. [Google Scholar] [CrossRef]
- De Sanoit, J.; Tran, Q.T.; Pomorski, M.; Pierre, S.; Mer-calfati, C.; Bergonzo, P. Design of an electrochemically assisted radiation sensor for α-spectrometry of actinides traces in water. Appl. Radiat. Isot. 2013, 80, 32–41. [Google Scholar] [CrossRef]
- Tran, Q.T.; Pomorski, M.; de Sanoit, J.; Scorsone, E.; Bergonzo, P. Optimization of Actinides Trace Precipitation on Diamond/Si PIN Sensor for Alpha-Spectrometry in Aqueous Solution. Trans. Nucl. Sci. 2014, 61, 2082–2089. [Google Scholar] [CrossRef]
- De Sanoit, J.; Vanhove, E.; Mailley, P.; Bergonzo, P. Electrochemical diamond sensors for TNT detection in water. Electrochim. Acta 2009, 54, 5688–5693. [Google Scholar] [CrossRef]
- Maybeck, V.; Edgington, R.; Bongrain, A.; Welch, J.O.; Scorsone, E.; Bergonzo, P.; Jackman, R.B.; Offenhäusser, A. Boron- Doped Nanocrystalline Diamond Microelectrode Arrays Monitor Cardiac Action Potentials. Adv. Healthc. Mater. 2014, 3, 283–289. [Google Scholar] [CrossRef]
- Kiran, R.; Scorsone, E.; de Sanoit, J.; Arnault, J.C.; Mailley, P.; Bergonzo, P. Boron doped diamond electrodes for direct measurement in biological fluids: An in situ regeneration approach. J. Electrochem. Soc. 2013, 160. [Google Scholar] [CrossRef]
- Scorsone, E.; Belghiti, D.; Habchi, M.; Bergonzo, P. Boron doped diamond/metal nanocatalyst hybrid electrode arrays for analytical applications. In Proceedings of the 2017 ISOCS/IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Montreal, Canada, 28–31 May 2017. [Google Scholar] [CrossRef]
- Belghiti, D.K.; Zadeh-Habchi, M.; Scorsone, E.; Bergonzo, P. Boron Doped Diamond/Metal Nanoparticle Catalysts Hybrid Electrode Array for the Detection of Pesticides in Tap Water. Procedia Eng. 2016, 168, 428–431. [Google Scholar] [CrossRef]
- Belghiti, D.K.; Scorsone, E.; de Sanoit, J.; Bergonzo, P. Simultaneous detection of indole and 3-methylindole using boron-doped diamond electrodes. Phys. Stat. Solidi (A) Appl. Mater. Sci. 2016, 213, 2662–2671. [Google Scholar] [CrossRef]
- Piret, G.; Hébert, C.; Mazellier, J.-P.; Rousseau, L.; Scorsone, E.; Cottance, M.; Lissorgues, G.; Heuschkel, M.O.; Picaud, S.; Bergonzo, P.; et al. 3D-nanostructured boron-doped diamond for microelectrode array neural interfacing. Biomaterials 2015, 53, 173–183. [Google Scholar] [CrossRef]
- Tran, Q.T.; de Sanoit, J.; Pierre, S.; Arnault, J.-C.; Bergonzo, P. Diamond electrodes for trace alpha pollutant sequestration via covalent grafting of nitrilotriacetic acid (NTA) ligand. Electrochim. Acta 2014, 136, 430–434. [Google Scholar] [CrossRef]
- Vanhove, E.J.; Mailley, P.; Pinault, M.A.; Jomard, F.; Bergonzo, P. High Reactivity and stability of diamond electrode: The influence of the B-doping concentration. Phys. Stat. Sol. 2009, 2006, 2063–2069. [Google Scholar] [CrossRef]
- Kiran, R.; de Sanoit, J.; Scorsone, E. (WO2012110600) Method for activating a doped diamond electrode. Patent application no: 1151341, 18 February 2011. [Google Scholar]
- Kiran, R.; Scorsone, E.; Mailley, P.; Bergonzo, P. Quasi-real time quantification of uric acid in urine using boron doped diamond microelectrode with in situ cleaning. Anal. Chem. 2012, 84, 10207–10213. [Google Scholar] [CrossRef]
- Tsoupko-Sitnikov, V.; Dayras, F.; de Sanoit, J.; Filossofov, D. Ap- plication of rotating disk electrode technique for the preparation ofNp, Pu and Am –sources. J. Appl. Radiat. Isot. 2012, 52, 357–364. [Google Scholar] [CrossRef]
- Bergonzo, P.; Foulon, F.; Brambilla, A.; Tromson, D.; Jany, C.; Haan, S. Corrosion hard CVD diamond alpha particle detectors for nuclear liquid source monitoring. Diam. Relat. Mater. 2000, 9, 1003–1007. [Google Scholar] [CrossRef]
- Mer, C.; Tromson, D.; de Sanoit, J.; Pomorski, M.; Bergonzo, P. Diamond detectors for alpha monitoring in corrosive media for nuclear waste activity monitoring. In Proceedings of the 2009 1st International Conference on Advancements in Nuclear Instrumentation, Measurement Methods and their Applications, Marseille, France, 7–10 June 2009. [Google Scholar] [CrossRef]


| Experiment | Electrolyte | FWHM (keV) | ||
|---|---|---|---|---|
| Stainless Steel | BDD | Standard | ||
| 1 | Na2SO4 | 13.8 | 13.2 | 12 |
| 2 | NaNO3 | 12.5 | 14.5 | 12 |
| Experiment | Electrolyte | Precipitation Yields (%) | |
|---|---|---|---|
| Stainless Steel | Diamond | ||
| a | 0.3 M Na2SO4 | 49.7 | 58.7 |
| b | 0.3 M NaNO3 | 71 | 70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, Q.-T.; Pierre, S.; de Sanoit, J.; Pomorski, M.; Bergonzo, P. Electro-Precipitation of Actinides on Boron-Doped Diamond Thin Films for Solid Sources Preparation for High-Resolution Alpha-Particle Spectrometry. Appl. Sci. 2019, 9, 1473. https://doi.org/10.3390/app9071473
Tran Q-T, Pierre S, de Sanoit J, Pomorski M, Bergonzo P. Electro-Precipitation of Actinides on Boron-Doped Diamond Thin Films for Solid Sources Preparation for High-Resolution Alpha-Particle Spectrometry. Applied Sciences. 2019; 9(7):1473. https://doi.org/10.3390/app9071473
Chicago/Turabian StyleTran, Quang-Thuan, Sylvie Pierre, Jacques de Sanoit, Michal Pomorski, and Philippe Bergonzo. 2019. "Electro-Precipitation of Actinides on Boron-Doped Diamond Thin Films for Solid Sources Preparation for High-Resolution Alpha-Particle Spectrometry" Applied Sciences 9, no. 7: 1473. https://doi.org/10.3390/app9071473





