Propranolol Relieves L-Dopa-Induced Dyskinesia in Parkinsonian Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
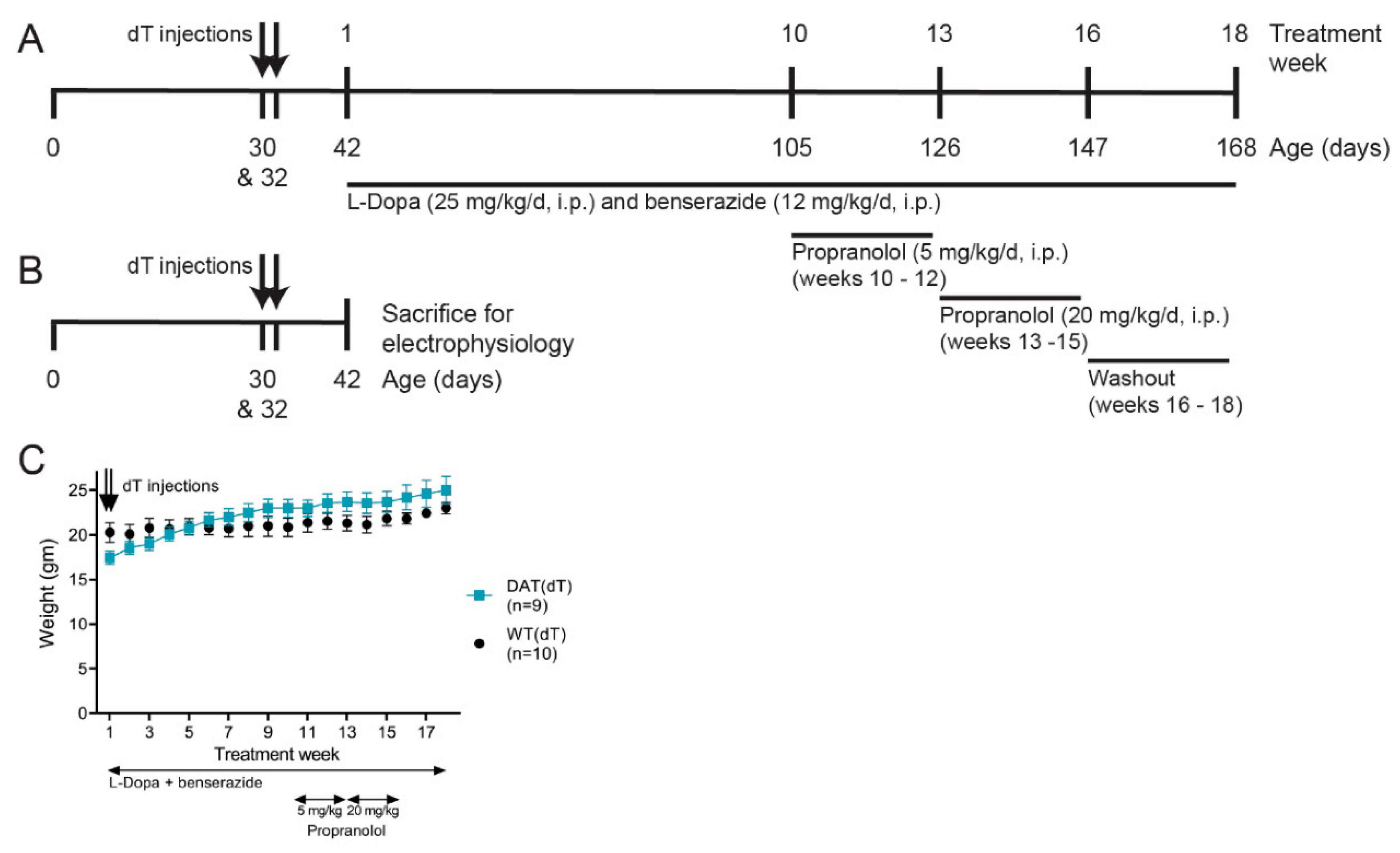
2.2. Experimental Design
2.3. Drug Administration
2.4. Behavior
2.4.1. Dyskinesia Scoring
2.4.2. Motor Skill Learning
2.4.3. Motor Coordination
2.4.4. Novelty Locomotion
2.5. Electrophysiology
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
3.1. L-Dopa Promotes Limb Dyskinesia in DAT(dT) Mice
3.2. Propranolol Reduces Limb Dyskinesia in DAT(dT) Mice
3.3. Propranolol Had No Effect on Rotarod Performance
3.4. High-Dose Propranolol Improves Balance-Beam Performance
3.5. Propranolol Improves Locomotor Hyperactivity
3.6. β-Adrenergic Receptors Modulate ChI Firing
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fahn, S. Description of Parkinson’s Disease as a Clinical Syndrome. Ann. N. Y. Acad. Sci. 2006, 991, 1–14. [Google Scholar] [CrossRef]
- Massano, J.; Bhatia, K.P. Clinical approach to parkinson’s disease: Features, diagnosis, and principles of management. Cold Spring Harb. Perspect. Med. 2012, 2, a008870. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, G.; Brotchie, J.M.; Grandas, F.; Nomoto, M.; Goetz, C.G. Levodopa-induced dyskinesias. Mov. Disord. 2007, 22, 1379–1389. [Google Scholar] [CrossRef]
- Turcano, P.; Mielke, M.M.; Bower, J.H.; Parisi, J.E.; Cutsforth-Gregory, J.K.; Ahlskog, J.E.; Savica, R. Levodopa-induced dyskinesia in Parkinson disease. Neurology 2018, 91, e2238–e2243. [Google Scholar] [CrossRef]
- Cenci, M.A. L-DOPA-induced dyskinesia: Cellular mechanisms and approaches to treatment. Park. Relat. Disord. 2007, 13, S263–S267. [Google Scholar] [CrossRef]
- Pons, R.; Pt, D.S.; Youroukos, S.; Orfanou, I.; Dinopoulos, A.; Cormand, B.; Ormazabal, A.; Garzía-Cazorla, A.; Serrano, M.; Artuch, R. Levodopa-induced dyskinesias in tyrosine hydroxylase deficiency. Mov. Disord. 2013, 28, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.; Calne, D.; Tsui, J.; De La Fuente-Fernández, R. The long-term response to levodopa in dopa-responsive dystonia. Park. Relat. Disord. 2001, 8, 1–5. [Google Scholar] [CrossRef]
- Aquino, C.C.; Fox, S.H. Clinical spectrum of levodopa-induced complications. Mov. Disord. 2014, 30, 80–89. [Google Scholar] [CrossRef]
- Cenci, M.A. Presynaptic Mechanisms of l-DOPA-Induced Dyskinesia: The Findings, the Debate, and the Therapeutic Implications. Front. Neurol. 2014, 5, 242. [Google Scholar] [CrossRef] [Green Version]
- Bamford, N.S.; Wightman, R.M.; Sulzer, D. Dopamine’s Effects on Corticostriatal Synapses during Reward-Based Behaviors. Neuron 2018, 97, 494–510. [Google Scholar] [CrossRef] [Green Version]
- Bamford, N.S.; Cepeda, C. The Corticostriatal Pathway in Parkinson’s Disease. In Cortico-Subcortical Dynamics in Parkinson’s Disease; Tseng, K.Y., Ed.; Humana Press: New York, NY, USA, 2009; pp. 87–104. [Google Scholar]
- Ding, Y.; Won, L.; Britt, J.P.; Lim, S.A.O.; McGehee, D.S.; Kang, U.J. Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proc. Natl. Acad. Sci. USA 2011, 108, 840–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKinley, J.W.; Shi, Z.; Kawikova, I.; Hur, M.; Bamford, I.J.; Devi, S.P.S.; Vahedipour, A.; Darvas, M.; Bamford, N.S. Dopamine Deficiency Reduces Striatal Cholinergic Interneuron Function in Models of Parkinson’s Disease. Neuron 2019, 103, 1056–1072. [Google Scholar] [CrossRef] [PubMed]
- Bordia, T.; Perez, X.A.; Heiss, J.E.; Zhang, D.; Quik, M. Optogenetic activation of striatal cholinergic interneurons regulates L-dopa-induced dyskinesias. Neurobiol. Dis. 2016, 91, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Won, L.; Ding, Y.; Singh, P.; Kang, U.J. Striatal cholinergic cell ablation attenuates L-DOPA induced dyskinesia in Parkinsonian mice. J. Neurosci. 2014, 34, 3090–3094. [Google Scholar] [CrossRef] [Green Version]
- Ruberg, M.; Ploska, A.; Javoy-Agid, F.; Agid, Y. Muscarinic binding and choline acetyltransferase activity in Parkinsonian subjects with reference to dementia. Brain Res. 1982, 232, 129–139. [Google Scholar] [CrossRef]
- Doshay, L.J.; Constable, K. Treatment of paralysis agitans with orphenadrine (disipal) hydrochloride; results in one hundred seventy-six cases. J. Am. Med. Assoc. 1957, 163, 1352–1357. [Google Scholar] [CrossRef]
- Calne, D.B. Parkinsonism. Clinical and neuropharmacologic aspects. Postgrad. Med. 1978, 64, 82–88. [Google Scholar] [CrossRef]
- Lehmann, J.; Langer, S. The striatal cholinergic interneuron: Synaptic target of dopaminergic terminals? Neuroscience 1983, 10, 1105–1120. [Google Scholar] [CrossRef]
- Spehlmann, R.; Stahl, S.M. Dopamine acetylcholine imbalance in parkinson’s disease. Possible regenerative overgrowth of cholinergic axon terminals. Lancet 1976, 1, 724–726. [Google Scholar] [CrossRef]
- Bamford, I.J.; Bamford, N.S. The Striatum’s Role in Executing Rational and Irrational Economic Behaviors. Neuroscientist 2019, 25, 475–490. [Google Scholar] [CrossRef]
- Bamford, N.S.; McVicar, K. Localising movement disorders in childhood. Lancet Child Adolesc. Health 2019, 3, 917–928. [Google Scholar] [CrossRef]
- Wang, W.; Darvas, M.; Storey, G.P.; Bamford, I.J.; Gibbs, J.T.; Palmiter, R.D.; Bamford, N.S. Acetylcholine encodes long-lasting presynaptic plasticity at glutamatergic synapses in the dorsal striatum after repeated amphetamine exposure. J. Neurosci. 2013, 33, 10405–10426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.A.O.; Kang, U.J.; McGehee, D.S. Striatal cholinergic interneuron regulation and circuit effects. Front. Synaptic Neurosci. 2014, 6, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meitzen, J.; Luoma, J.I.; Stern, C.M.; Mermelstein, P.G. Beta1-adrenergic receptors activate two distinct signaling pathways in striatal neurons. J. Neurochem. 2011, 116, 984–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisani, A.; Bonsi, P.; Centonze, D.; Martorana, A.; Fusco, F.; Sancesario, G.; De Persis, C.; Bernardi, G.; Calabresi, P. Activation of beta1-adrenoceptors excites striatal cholinergic interneurons through a camp-dependent, protein kinase-independent pathway. J. Neurosci. 2003, 23, 5272–5282. [Google Scholar] [CrossRef] [Green Version]
- Wainger, B.J.; DeGennaro, M.; Santoro, B.; Siegelbaum, S.A.; Tibbs, G.R. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nat. Cell Biol. 2001, 411, 805–810. [Google Scholar] [CrossRef]
- Mehvar, R.; Brocks, D.R. Stereospecific pharmacokinetics and pharmacodynamics of beta-adrenergic blockers in humans. J. Pharm. Pharm. Sci. 2001, 4, 185–200. [Google Scholar]
- Hatcher-Martin, J.M.; Armstrong, K.A.; Scorr, L.M.; Factor, S.A. Propranolol therapy for Tardive dyskinesia: A retrospective examination. Park. Relat. Disord. 2016, 32, 124–126. [Google Scholar] [CrossRef]
- Vasudevan, N.T.; Mohan, M.L.; Goswami, S.K.; Naga Prasad, S.V. Regulation of beta-adrenergic receptor function: An emphasis on receptor resensitization. Cell Cycle 2011, 10, 3684–3691. [Google Scholar] [CrossRef] [Green Version]
- Carpentier, A.F.; Bonnet, A.M.; Vidailhet, M.; Agid, Y. Improvement of levodopa-induced dyskinesia by propranolol in parkinson’s disease. Neurology 1996, 46, 1548–1551. [Google Scholar] [CrossRef]
- Manson, A.; Stirpe, P.; Schrag, A. Levodopa-Induced-Dyskinesias Clinical Features, Incidence, Risk Factors, Management and Impact on Quality of Life. J. Park. Dis. 2012, 2, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, R.G.; Gibbs, J.T.; Melief, E.J.; Postupna, N.O.; Sherfield, E.E.; Wilson, A.; Keene, D.; Montine, T.J.; Palmiter, R.D.; Darvas, M. Relative contributions of severe dopaminergic neuron ablation and dopamine depletion to cognitive impairment. Exp. Neurol. 2015, 271, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, M.; Iwawaki, T.; Taya, C.; Yonekawa, H.; Noda, M.; Inui, Y.; Mekada, E.; Kimata, Y.; Tsuru, A.; Kohno, K. Diphtheria toxin receptor–mediated conditional and targeted cell ablation in transgenic mice. Nat. Biotechnol. 2001, 19, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Madisen, L.; Zwingman, T.A.; Sunkin, S.M.; Oh, S.W.; Zariwala, H.A.; Gu, H.; Ng, L.L.; Palmiter, R.D.; Hawrylycz, M.J.; Jones, A.R.; et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010, 13, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamford, N.S.; Zhang, H.; Schmitz, Y.; Wu, N.-P.; Cepeda, C.; Levine, M.S.; Schmauss, C.; Zakharenko, S.S.; Zablow, L.; Sulzer, D. Heterosynaptic Dopamine Neurotransmission Selects Sets of Corticostriatal Terminals. Neuron 2004, 42, 653–663. [Google Scholar] [CrossRef] [Green Version]
- Bamford, N.S.; Zhang, H.; Joyce, J.A.; Scarlis, C.A.; Hanan, W.; Wu, N.-P.; André, V.M.; Cohen, R.; Cepeda, C.; Levine, M.S.; et al. Repeated Exposure to Methamphetamine Causes Long-Lasting Presynaptic Corticostriatal Depression that Is Renormalized with Drug Readministration. Neuron 2008, 58, 89–103. [Google Scholar] [CrossRef] [Green Version]
- Bamford, N.S.; Robinson, S.; Palmiter, R.D.; Joyce, J.A.; Moore, C.; Meshul, C.K. Dopamine Modulates Release from Corticostriatal Terminals. J. Neurosci. 2004, 24, 9541–9552. [Google Scholar] [CrossRef] [Green Version]
- Sebastianutto, I.; Maslava, N.; Hopkins, C.R.; Cenci, M.A. Validation of an improved scale for rating l-DOPA-induced dyskinesia in the mouse and effects of specific dopamine receptor antagonists. Neurobiol. Dis. 2016, 96, 156–170. [Google Scholar] [CrossRef]
- Girasole, A.E.; Lum, M.Y.; Nathaniel, D.; Bair-Marshall, C.J.; Guenthner, C.J.; Luo, L.; Kreitzer, A.C.; Nelson, A.B. A Subpopulation of Striatal Neurons Mediates Levodopa-Induced Dyskinesia. Neuron 2018, 97, 787–795. [Google Scholar] [CrossRef]
- Bennett, B.D.; Wilson, C.J. Spontaneous Activity of Neostriatal Cholinergic InterneuronsIn Vitro. J. Neurosci. 1999, 19, 5586–5596. [Google Scholar] [CrossRef] [Green Version]
- Joshi, P.R.; Wu, N.-P.; André, V.M.; Cummings, D.M.; Cepeda, C.; Joyce, J.A.; Carroll, J.B.; Leavitt, B.R.; Hayden, M.R.; Levine, M.S.; et al. Age-dependent alterations of corticostriatal activity in the YAC128 mouse model of Huntington disease. J. Neurosci. 2009, 29, 2414–2427. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, J. The Mouse Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: London, UK, 2005. [Google Scholar]
- Bennett, B.D.; Callaway, J.C.; Wilson, C.J. Intrinsic Membrane Properties Underlying Spontaneous Tonic Firing in Neostriatal Cholinergic Interneurons. J. Neurosci. 2000, 20, 8493–8503. [Google Scholar] [CrossRef] [PubMed]
- Maurice, N.; Mercer, J.; Chan, C.S.; Hernandez-Lopez, S.; Held, J.; Tkatch, T.; Surmeier, D.J. D2 Dopamine Receptor-Mediated Modulation of Voltage-Dependent Na+ Channels Reduces Autonomous Activity in Striatal Cholinergic Interneurons. J. Neurosci. 2004, 24, 10289–10301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundblad, M.; Picconi, B.; Lindgren, H.; Cenci, M. A model of l-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: Relation to motor and cellular parameters of nigrostriatal function. Neurobiol. Dis. 2004, 16, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Bhide, N.; Lindenbach, D.; Barnum, C.J.; George, J.A.; Surrena, M.A.; Bishop, C. Effects of the beta-adrenergic receptor antagonist Propranolol on dyskinesia and L-DOPA-induced striatal DA efflux in the hemi-parkinsonian rat. J. Neurochem. 2015, 134, 222–232. [Google Scholar] [CrossRef]
- Kreitzer, A.C.; Malenka, R.C. Endocannabinoid-mediated rescue of striatal ltd and motor deficits in parkinson’s disease models. Nature 2007, 445, 643–647. [Google Scholar] [CrossRef]
- Weiss, S.; Kemp, D.E.; Lenox, R.H.; Ellis, J. Alpha 2-adrenergic receptors mediate inhibition of cyclic amp production in neurons in primary culture. Brain Res. 1987, 414, 390–394. [Google Scholar] [CrossRef]
- Burke, R.E. Recent Advances in Research on Parkinson Disease: Synuclein and Parkin. Neurology 2004, 10, 75–81. [Google Scholar] [CrossRef]
- Lindenbach, D.; Ostock, C.Y.; Jaunarajs, K.L.E.; Dupre, K.B.; Barnum, C.J.; Bhide, N.; Bishop, C. Behavioral and cellular modulation of L-DOPA-induced dyskinesia by beta-adrenoceptor blockade in the 6-hydroxydopamine-lesioned rat. J. Pharmacol. Exp. Ther. 2011, 337, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Mancilla, B.; Bédard, P.J. Effect of Nondopaminergic Drugs on L-DOPA-Induced Dyskinesias in MPTP-Treated Monkeys. Clin. Neuropharmacol. 1993, 16, 418–427. [Google Scholar] [CrossRef]
- Wong, M.Y.; Borgkvist, A.; Choi, S.J.; Mosharov, E.V.; Bamford, N.S.; Sulzer, D. Dopamine-dependent corticostriatal synaptic filtering regulates sensorimotor behavior. Neuroscience 2015, 290, 594–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulzer, D.; Cragg, S.J.; Rice, M.E. Striatal dopamine neurotransmission: Regulation of release and uptake. Basal Ganglia 2016, 6, 123–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bankston, J.R.; DeBerg, H.A.; Stoll, S.; Zagotta, W.N. Mechanism for the inhibition of the cAMP dependence of HCN ion channels by the auxiliary subunit TRIP8b. J. Biol. Chem. 2017, 292, 17794–17803. [Google Scholar] [CrossRef] [PubMed]
- Threlfell, S.; Lalic, T.; Platt, N.J.; Jennings, K.A.; Deisseroth, K.; Cragg, S.J. Striatal Dopamine Release Is Triggered by Synchronized Activity in Cholinergic Interneurons. Neuron 2012, 75, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanpied, T.A.; Clarke, R.J.; Johnson, J.W. Amantadine Inhibits NMDA Receptors by Accelerating Channel Closure during Channel Block. J. Neurosci. 2005, 25, 3312–3322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubayashi, H.; Swanson, K.L.; Albuquerque, E.X. Amantadine inhibits nicotinic acetylcholine receptor function in hippocampal neurons. J. Pharmacol. Exp. Ther. 1997, 281, 834–844. [Google Scholar]
- Svensson, T.H.; Strömberg, U. Potentiation by amantadine hydrochloride of L-dopa-induced effects in mice. J. Pharm. Pharmacol. 1970, 22, 639–640. [Google Scholar] [CrossRef]
- Dragašević-Mišković, N.T.; Petrović, I.; Stanković, I.; Kostić, V.S. Chemical management of levodopa-induced dyskinesia in Parkinson’s disease patients. Expert Opin. Pharmacother. 2018, 20, 219–230. [Google Scholar] [CrossRef]






| General Dyskinesia | |
| 0 | Normal behavior |
| 1 | Twitching with low or no movement |
| 2 | Shaking arms coupled with low or no movement |
| 3 | Perched on hind legs while craning to one side or abnormal arm posture |
| 4 | (3) but for longer than three seconds |
| Limb Dyskinesia | |
| 0 | Normal behavior |
| 1 | Moving a limb repeatedly |
| 2 | ≥2 limbs move slowly |
| 3 | ≥2 limbs move quickly |
| 4 | Waving limbs while standing |
| Monday | Treatment |
| Tuesday | Treatment |
| Wednesday | Pre-treatment habituation and monitoring (90 min); treatment; locomotor and behavioral monitoring (120 min) |
| Thursday | Pre-treatment habituation and monitoring (90 min); treatment; locomotor and behavioral monitoring (120 min) |
| Friday | Rotarod and balance beam on treatment weeks 1, 10, 13, 16, and 18; pre-treatment habituation (90 min); treatment; locomotor and behavioral monitoring (120 min) |
| Saturday | Treatment |
| Sunday | Treatment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Bamford, I.J.; McKinley, J.W.; Devi, S.P.S.; Vahedipour, A.; Bamford, N.S. Propranolol Relieves L-Dopa-Induced Dyskinesia in Parkinsonian Mice. Brain Sci. 2020, 10, 903. https://doi.org/10.3390/brainsci10120903
Shi Z, Bamford IJ, McKinley JW, Devi SPS, Vahedipour A, Bamford NS. Propranolol Relieves L-Dopa-Induced Dyskinesia in Parkinsonian Mice. Brain Sciences. 2020; 10(12):903. https://doi.org/10.3390/brainsci10120903
Chicago/Turabian StyleShi, Ziqing, Ian J. Bamford, Jonathan W. McKinley, Suma Priya Sudarsana Devi, Annie Vahedipour, and Nigel S. Bamford. 2020. "Propranolol Relieves L-Dopa-Induced Dyskinesia in Parkinsonian Mice" Brain Sciences 10, no. 12: 903. https://doi.org/10.3390/brainsci10120903
APA StyleShi, Z., Bamford, I. J., McKinley, J. W., Devi, S. P. S., Vahedipour, A., & Bamford, N. S. (2020). Propranolol Relieves L-Dopa-Induced Dyskinesia in Parkinsonian Mice. Brain Sciences, 10(12), 903. https://doi.org/10.3390/brainsci10120903






