Voltage-Sensitive Dye versus Intrinsic Signal Optical Imaging: Comparison of Tactile Responses in Primary and Secondary Somatosensory Cortices of Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgery for Optical Imaging
2.3. Whisker Stimulation
2.4. Optical Imaging
2.5. Analysis of Imaging Data
2.6. Histology
3. Results
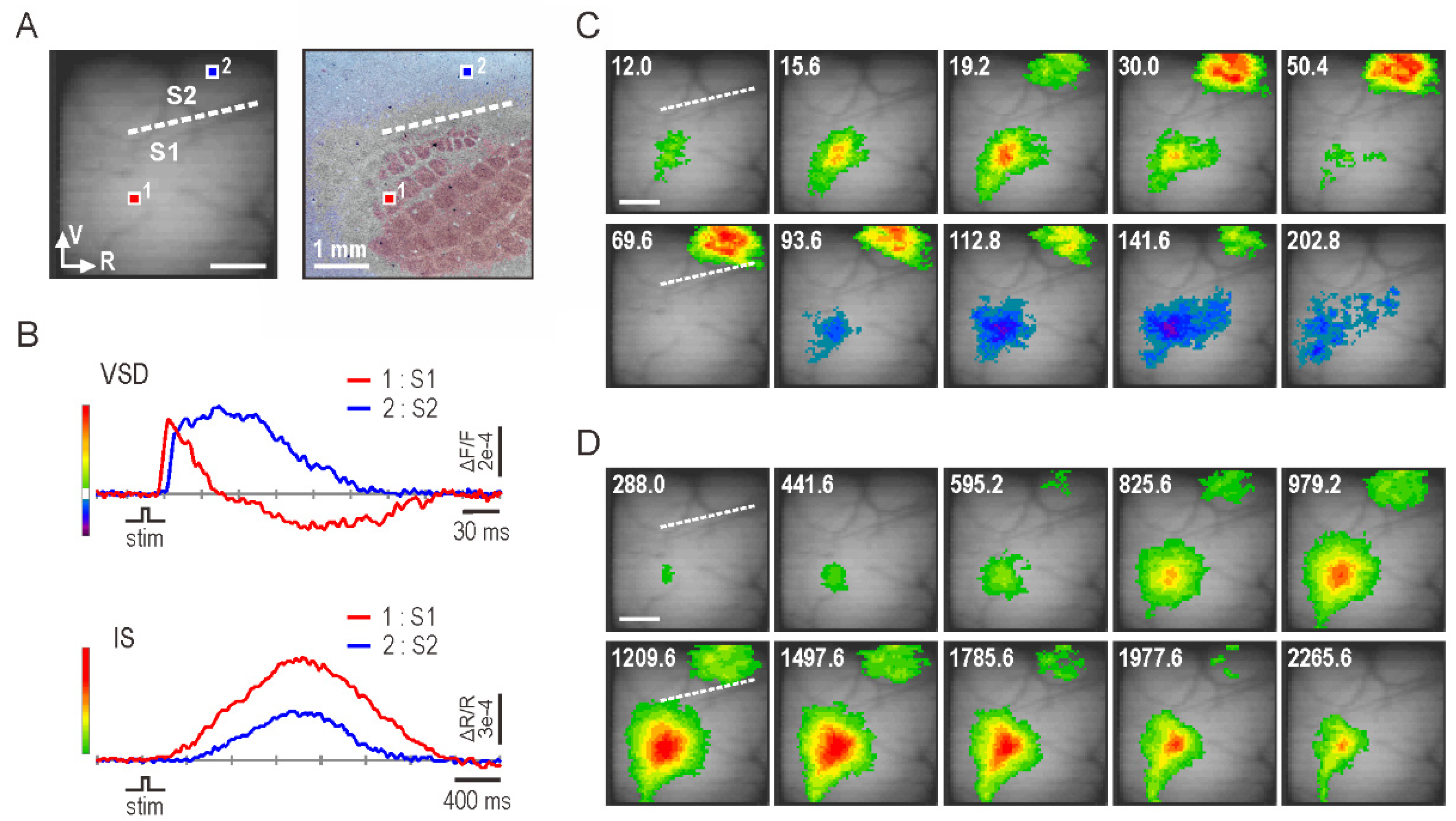
3.1. Optical Imaging of Whisker-Evoked Somatosensory Response
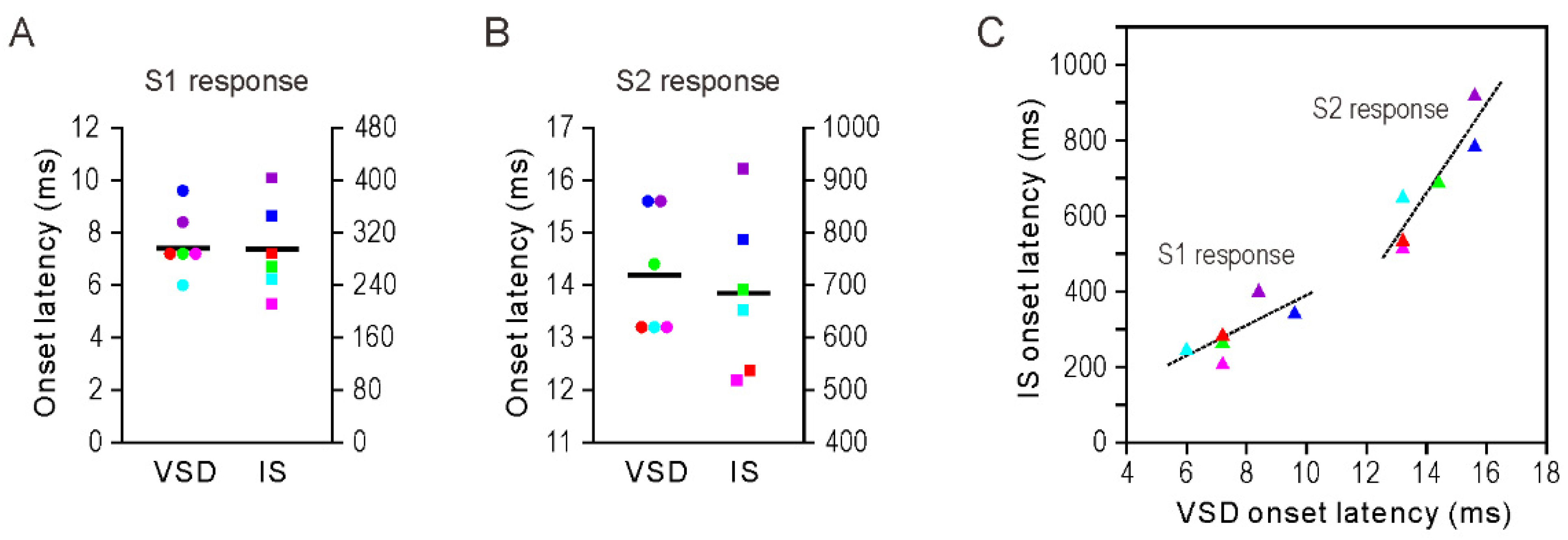
3.2. Onset Latency of Somatosensory Response
3.3. Amplitude and Extent of Somatosensory Response
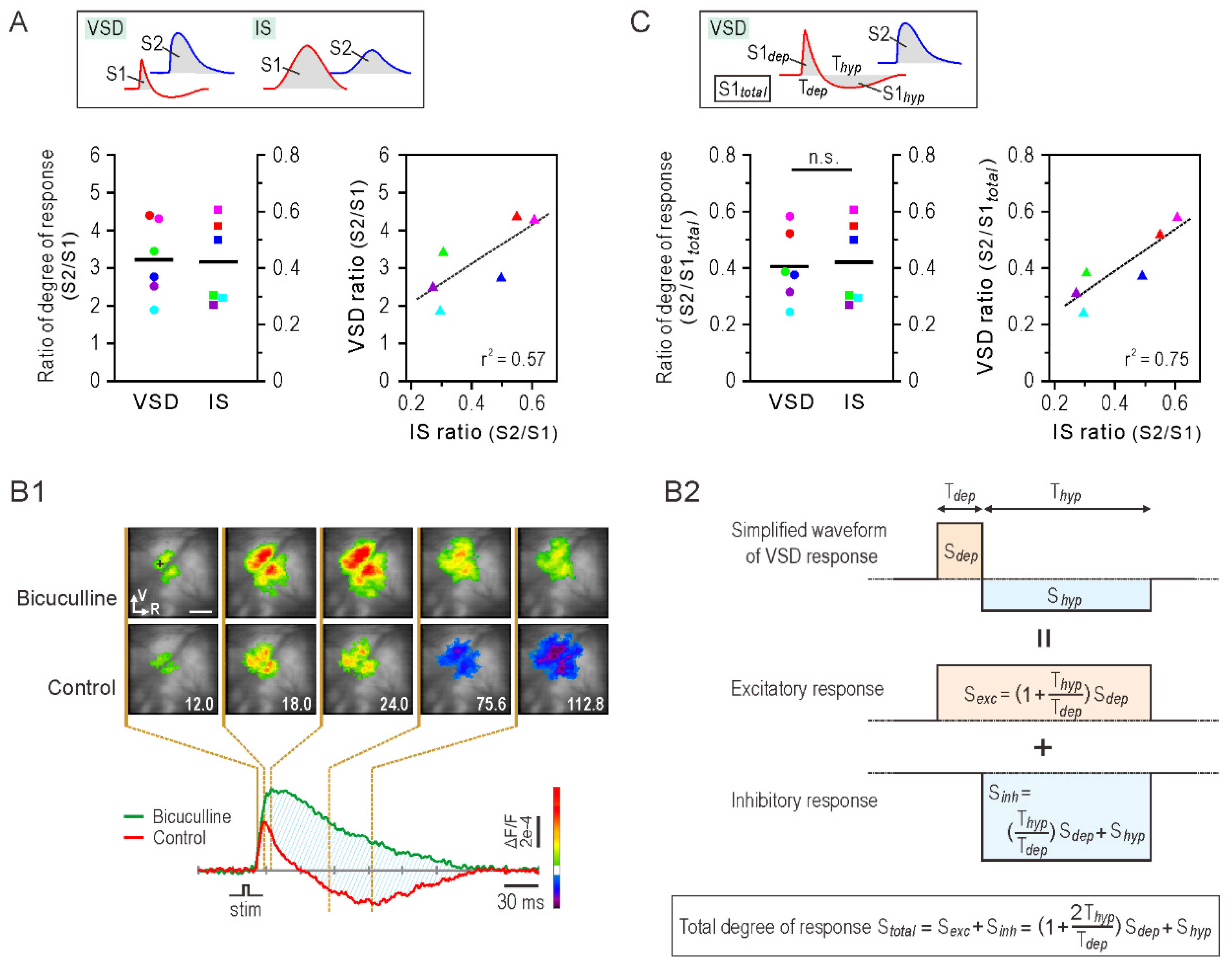
3.4. Degree of Somatosensory Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grinvald, A.; Omer, D.B.; Sharon, D.; Vanzetta, I.; Hildesheim, R. Voltage-sensitive-dye imaging of neocortical activity. Cold Spring Harb Protoc. 2016, 2016, pdb.top089367. [Google Scholar] [CrossRef] [PubMed]
- Grinvald, A.; Sharon, D.; Omer, D.; Vanzetta, I. Imaging the neocortex functional architecture using multiple intrinsic signals: Implications for hemodynamic-based functional imaging. Cold Spring Harb Protoc. 2016, 2016, pdb.top089375. [Google Scholar] [CrossRef] [PubMed]
- Takashima, I.; Kajiwara, R.; Iijima, T. Voltage-sensitive dye versus intrinsic signal optical imaging: Comparison of optically determined functional maps from rat barrel cortex. Neuroreport 2001, 12, 2889–2894. [Google Scholar] [CrossRef] [PubMed]
- Tsytsarev, V.; Premachandra, K.; Takeshita, D.; Bahar, S. Imaging cortical electrical stimulation in vivo: Fast intrinsic optical signal versus voltage-sensitive dyes. Opt. Lett. 2008, 33, 1032–1034. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, M.; Schwartz, T.H. Dynamic neurovascular coupling and uncoupling during ictal onset, propagation, and termination revealed by simultaneous in vivo optical imaging of neural activity and local blood volume. Cereb. Cortex. 2013, 23, 885–899. [Google Scholar] [CrossRef] [Green Version]
- Roe, A.W.; Winberry, J.E.; Friedman, R.M. Study of single and multidigit activation in monkey somatosensory cortex using voltage-sensitive dye imaging. Neurophotonics 2017, 4, 031219. [Google Scholar] [CrossRef] [Green Version]
- Logothetis, N.K.; Wandell, B.A. Interpreting the BOLD signal. Annu. Rev. Physiol. 2004, 66, 735–769. [Google Scholar] [CrossRef]
- Devonshire, I.M.; Papadakis, N.G.; Port, M.; Berwick, J.; Kennerley, A.J.; Mayhew, J.E.W.; Overton, P.G. Neurovascular coupling is brain region-dependent. Neuroimage 2012, 59, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Sloan, H.L.; Austin, V.C.; Blamire, A.M.; Schnupp, J.W.H.; Lowe, A.S.; Allers, K.A.; Matthews, P.M.; Sibson, N.R. Regional differences in neurovascular coupling in rat brain as determined by fMRI and electrophysiology. Neuroimage 2010, 53, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Conner, C.R.; Ellmore, T.M.; Pieters, T.A.; DiSano, M.A.; Tandon, N. Variability of the relationship between electrophysiology and BOLD-fMRI across cortical regions in humans. J. Neurosci. 2011, 31, 12855–12865. [Google Scholar] [CrossRef] [Green Version]
- Devor, A.; Tian, P.; Nishimura, N.; Teng, I.C.; Hillman, E.M.; Narayanan, S.N.; Ulbert, I.; Boas, D.A.; Kleinfeld, D.; Dale, A.M. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J. Neurosci. 2007, 27, 4452–4459. [Google Scholar] [CrossRef] [Green Version]
- Takashima, I.; Kajiwara, R.; Iijima, T. Voltage-sensitive dye imaging of intervibrissal fur-evoked activity in the rat somatosensory cortex. Neurosci. Lett. 2005, 381, 258–263. [Google Scholar] [CrossRef]
- Ichikawa, M.; Iijima, T.; Matsumoto, G. Real-time optical imaging of neural activities in the brain. In Brain Mechanisms of Perception and Memory; Ono, T., Squire, L.R., Raichle, M.E., Perrett, D.I., Fukuda, M., Eds.; Oxford University Press: Oxford, UK, 1993; pp. 638–648. [Google Scholar]
- Takashima, I.; Ichikawa, M.; Iijima, T. High-speed CCD imaging system for monitoring neural activity in vivo and in vitro, using a voltage-sensitive dye. J. Neurosci. Methods 1999, 91, 147–159. [Google Scholar] [CrossRef]
- Wong-Riley, M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979, 171, 11–28. [Google Scholar] [CrossRef]
- Takashima, I.; Kajiwara, R.; Iijima, T. Functional mapping of optically detected neural activity onto a standardized cortical structure of rodent barrels. IEICE Trans. Inf. Syst. 2002, E85D, 143–151. [Google Scholar]
- Liang, M.; Mouraux, A.; Iannetti, G.D. Parallel processing of nociceptive and non-nociceptive somatosensory information in the human primary and secondary somatosensory cortices: Evidence from dynamic causal modeling of functional magnetic resonance imaging data. J. Neurosci. 2011, 31, 8976–8985. [Google Scholar] [CrossRef]
- Chung, Y.G.; Han, S.W.; Kim, H.S.; Chung, S.C.; Park, J.Y.; Wallraven, C.; Kim, S.P. Intra- and inter-hemispheric effective connectivity in the human somatosensory cortex during pressure stimulation. BMC Neurosci. 2014, 15, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalberlah, C.; Villringer, A.; Pleger, B. Dynamic causal modeling suggests serial processing of tactile vibratory stimuli in the human somatosensory cortex—An fMRI study. Neuroimage 2013, 74, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Khoshnejad, M.; Piché, M.; Saleh, S.; Duncan, G.; Rainville, P. Serial processing in primary and secondary somatosensory cortex: A DCM analysis of human fMRI data in response to innocuous and noxious electrical stimulation. Neurosci. Lett. 2014, 577, 83–88. [Google Scholar] [CrossRef]
- Rowe, M.J.; Turman, A.B.; Murray, G.M.; Zhang, H.Q. Parallel organization of somatosensory cortical areas I and II for tactile processing. Clin. Exp. Pharmacol. Physiol. 1996, 23, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Kwegyir-Afful, E.E.; Keller, A. Response properties of whisker-related neurons in rat second somatosensory cortex. J. Neurophysiol. 2004, 92, 2083–2092. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.E.; Yang, H.; Minamisawa, G.; O’Connor, D.H. Sensory and decision-related activity propagate in a cortical feedback loop during touch perception. Nat. Neurosci. 2016, 19, 1243–1249. [Google Scholar] [CrossRef]
- Condylis, C.; Lowet, E.; Ni, J.; Bistrong, K.; Ouellette, T.; Josephs, N.; Chen, J.L. Context-dependent sensory processing across primary and secondary somatosensory cortex. Neuron 2020, 106, 515–525. [Google Scholar] [CrossRef]
- Theyel, B.B.; Llano, D.A.; Sherman, S.M. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat. Neurosci. 2010, 13, 84–88. [Google Scholar] [CrossRef]
- Hubatz, S.; Hucher, G.; Shulz, D.E.; Férézou, I. Spatiotemporal properties of whisker-evoked tactile responses in the mouse secondary somatosensory cortex. Sci. Rep. 2020, 10, 763. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.C.; Yen, C.T. Functional connectivity of the secondary somatosensory cortex of the rat. Anat. Rec. 2008, 291, 960–973. [Google Scholar] [CrossRef]
- Hooks, B.M.; Hires, S.A.; Zhand, Y.X.; Huber, D.; Petreanu, L.; Svoboda, K.; Shepherd, G.M.G. Laminar analysis of excitatory local circuits in vibrissal motor and sensory cortical areas. PLoS Biol. 2011, 9, e1000572. [Google Scholar] [CrossRef]
- Grinvald, A.; Hildesheim, R. VSDI: A new era in functional imaging of cortical dynamics. Nat. Rev. Neurosci. 2004, 5, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, R.N.S.; Krause, M.R.; Mazer, J.A. Surround suppression and sparse coding in visual and barrel cortices. Front. Neural Circuits 2012, 6, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvell, G.E.; Simons, D.J. Somatotopic organization of the second somatosensory area (SII) in the cerebral cortex of the mouse. Somatosens. Res. 1986, 3, 213–237. [Google Scholar] [CrossRef] [PubMed]
- Goldin, M.A.; Harrell, E.R.; Estebanez, L.; Shulz, D.E. Rich spatio-temporal stimulus dynamics unveil sensory specialization in cortical area S2. Nat. Commun. 2018, 9, 4053. [Google Scholar] [CrossRef] [PubMed]
- Melzer, P.; Champney, G.C.; Maguire, M.J.; Ebner, F. Rate code and temporal code for frequency of whisker stimulation in rat primary and secondary somatic sensory cortex. Exp. Brain Res. 2006, 172, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Handwerker, D.A.; Ollinger, J.M.; D’Esposito, M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage 2004, 21, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.L.; Gheres, K.W.; Proctor, E.A.; Drew, P.J. Neurovascular coupling and bilateral connectivity during NREM and REM sleep. Elife 2020, 9, e62071. [Google Scholar] [CrossRef] [PubMed]
- Anenberg, E.; Chan, A.W.; Xie, Y.; LeDue, J.M.; Murphy, T.H. Optogenetic stimulation of GABA neurons can decrease local neuronal activity while increasing cortical blood flow. J. Cereb. Blood Flow Metab. 2015, 35, 1579–1586. [Google Scholar] [CrossRef] [Green Version]
- Uhlirova, H.; Kılıç, K.; Tian, P.; Thunemann, M.; Desjardins, M.; Saisan, P.A.; Sakadžić, S.; Ness, T.V.; Mateo, C.; Cheng, Q.; et al. Cell type specificity of neurovascular coupling in cerebral cortex. Elife 2016, 5, e14315. [Google Scholar] [CrossRef]
- Vazquez, A.; Fukuda, M.; Kim, S.G. Inhibitory neuron activity contributions to hemodynamic responses and metabolic load examined using an inhibitory optogenetic mouse model. Cereb. Cortex 2018, 28, 4105–4119. [Google Scholar] [CrossRef] [Green Version]
- Havlicek, M.; Ivanov, D.; Roebroeck, A.; Uludağ, K. Determining excitatory and inhibitory neuronal activity from multimodal fMRI data using a generative hemodynamic model. Front. Neurosci. 2017, 11, 616. [Google Scholar] [CrossRef] [Green Version]
- Otsu, Y.; Maru, E.; Ohata, H.; Takashima, I.; Kajiwara, R.; Iijima, T. Optical recording study of granule cell activities in the hippocampal dentate gyrus of kainate-treated rats. J. Neurophysiol. 2000, 83, 2421–2430. [Google Scholar] [CrossRef] [Green Version]
- Mennerick, S.; Chisari, M.; Shu, H.J.; Taylor, A.; Vasek, M.; Eisenman, L.N.; Zorumski, C.F. Diverse voltage-sensitive dyes modulate GABAA receptor function. J. Neurosci. 2010, 30, 2871–2879. [Google Scholar] [CrossRef] [Green Version]
- Grandy, T.H.; Greenfield, S.A.; Devonshire, I.M. An evaluation of in vivo voltage-sensitive dyes: Pharmacological side effects and signal-to-noise ratios after effective removal of brain-pulsation artifacts. J. Neurophysiol. 2012, 108, 2931–2945. [Google Scholar] [CrossRef] [PubMed]
- Kozberg, M.; Hillman, E. Neurovascular coupling and energy metabolism in the developing brain. Prog. Brain Res. 2016, 225, 213–242. [Google Scholar] [PubMed] [Green Version]
- Mutoh, H.; Mishina, Y.; Gallero-Salas, Y.; Knöpfel, T. Comparative performance of a genetically-encoded voltage indicator and a blue voltage-sensitive dye for large scale cortical voltage imaging. Front. Cell Neurosci. 2015, 9, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knöpfel, T.; Song, C. Optical voltage imaging in neurons: Moving from technology development to practical tool. Nat. Rev. Neurosci. 2019, 20, 719–727. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takashima, I.; Kajiwara, R. Voltage-Sensitive Dye versus Intrinsic Signal Optical Imaging: Comparison of Tactile Responses in Primary and Secondary Somatosensory Cortices of Rats. Brain Sci. 2021, 11, 1294. https://doi.org/10.3390/brainsci11101294
Takashima I, Kajiwara R. Voltage-Sensitive Dye versus Intrinsic Signal Optical Imaging: Comparison of Tactile Responses in Primary and Secondary Somatosensory Cortices of Rats. Brain Sciences. 2021; 11(10):1294. https://doi.org/10.3390/brainsci11101294
Chicago/Turabian StyleTakashima, Ichiro, and Riichi Kajiwara. 2021. "Voltage-Sensitive Dye versus Intrinsic Signal Optical Imaging: Comparison of Tactile Responses in Primary and Secondary Somatosensory Cortices of Rats" Brain Sciences 11, no. 10: 1294. https://doi.org/10.3390/brainsci11101294





