Odor Identification and Regional Gray Matter Atrophy in Patients with Alzheimer’s Disease, Parkinson’s Disease, and the Healthy Elderly: A Cross-Sectional Structural MRI Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical Testing
2.3. Odor Identification Testing
2.4. Magnetic Resonance Imaging
2.4.1. Acquisition
2.4.2. Analysis
2.5. Statistical Analysis
3. Results
3.1. Clinical and Demographic Characteristics
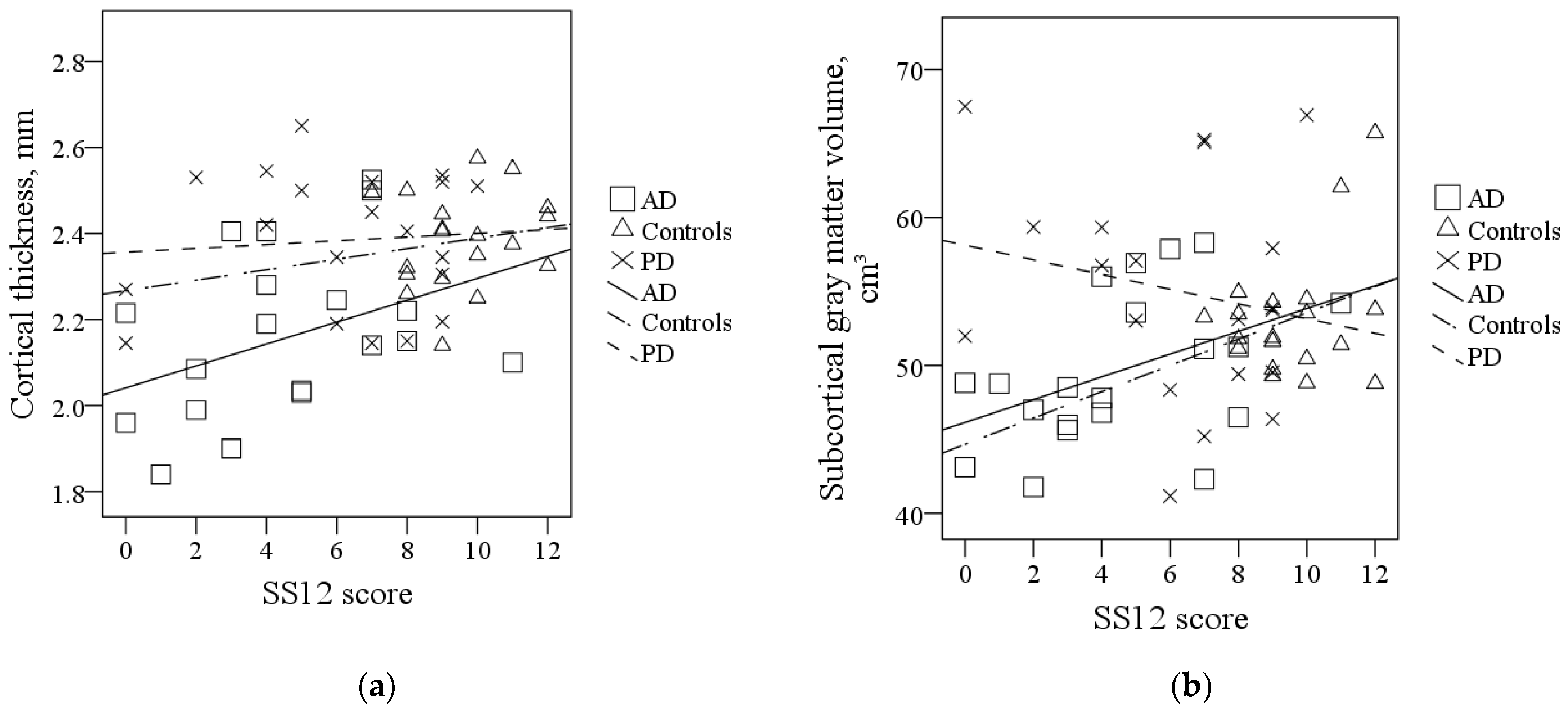
3.2. Global Gray Matter Morphometric Measures
3.3. Regional Gray Matter Morphometric Measures
3.3.1. AD Group
3.3.2. PD Group
3.3.3. Control Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hedner, M.; Larsson, M.; Arnold, N.; Zucco, G.M.; Hummel, T. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J. Clin. Exp. Neuropsychol. 2010, 32, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Kovács, T. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res. Rev. 2004, 3, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Schubert, C.R.; Cruickshanks, K.J.; Klein, B.E.; Klein, R.; Nondahl, D.M. Prevalence of olfactory impairment in older adults. JAMA 2002, 288, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
- Sollai, G.; Crnjar, R. Age-Related Olfactory Decline Is Associated with Levels of Exercise and Non-exercise Physical Activities. Front. Aging Neurosci. 2021, 13, 695115. [Google Scholar] [CrossRef]
- Mazzatenta, A.; Cellerino, A.; Origlia, N.; Barloscio, D.; Sartucci, F.; Di Giulio, C.; Domenici, L. Olfactory phenotypic expression unveils human aging. Oncotarget 2016, 7, 19131–19200. [Google Scholar] [CrossRef] [Green Version]
- Rahayel, S.; Frasnelli, J.; Joubert, S. The effect of Alzheimer’s disease and Parkinson’s disease on olfaction: A meta-analysis. Behav. Brain Res. 2012, 231, 60–74. [Google Scholar] [CrossRef]
- Silva, M.M.E.; Mercer, P.B.S.; Witt, M.C.Z.; Pessoa, R.R. Olfactory dysfunction in Alzheimer’s disease Systematic review and meta-analysis. Dement. Neuropsychol. 2018, 12, 123–132. [Google Scholar] [CrossRef]
- Marin, C.; Vilas, D.; Langdon, C.; Alobid, I.; López-Chacón, M.; Haehner, A.; Hummel, T.; Mullol, J. Olfactory Dysfunction in Neurodegenerative Diseases. Curr. Allergy Asthma Rep. 2018, 18, 42. [Google Scholar] [CrossRef]
- Fullard, M.E.; Morley, J.F.; Duda, J.E. Olfactory Dysfunction as an Early Biomarker in Parkinson’s Disease. Neurosci. Bull. 2017, 33, 515–525. [Google Scholar] [CrossRef]
- Lötsch, J.; Ultsch, A.; Hummel, T. A Unifying Data-Driven Model of Human Olfactory Pathology Representing Known Etiologies of Dysfunction. Chem. Senses 2016, 41, 763–770. [Google Scholar] [CrossRef]
- Dibattista, M.; Pifferi, S.; Menini, A.; Reisert, J. Alzheimer’s Disease: What Can We Learn from the Peripheral Olfactory System? Front. Neurosci. 2020, 14, 440. [Google Scholar] [CrossRef]
- Zhou, G.; Lane, G.; Cooper, S.L.; Kahnt, T.; Zelano, C. Characterizing functional pathways of the human olfactory system. eLife 2019, 8, e47177. [Google Scholar] [CrossRef]
- Milardi, D.; Cacciola, A.; Calamuneri, A.; Ghilardi, M.F.; Caminiti, F.; Cascio, F.; Andronaco, V.; Anastasi, G.; Mormina, E.; Arrigo, A.; et al. The Olfactory System Revealed: Non-Invasive Mapping by using Constrained Spherical Deconvolution Tractography in Healthy Humans. Front. Neuroanat. 2017, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Fjældstad, A. Testing olfactory function and mapping the structural olfactory networks in the brain. Dan. Med. J. 2018, 65, B5428. [Google Scholar]
- Fjaeldstad, A.; Fernandes, H.M.; Van Hartevelt, T.J.; Gleesborg, C.; Møller, A.; Ovesen, T.; Kringelbach, M.L. Brain fingerprints of olfaction: A novel structural method for assessing olfactory cortical networks in health and disease. Sci. Rep. 2017, 7, 42534. [Google Scholar] [CrossRef] [Green Version]
- Vasavada, M.M.; Wang, J.; Eslinger, P.J.; Gill, D.J.; Sun, X.; Karunanayaka, P.; Yang, Q.X. Olfactory cortex degeneration in Alzheimer’s disease and mild cognitive impairment. J. Alzheimers Dis. 2015, 45, 947–958. [Google Scholar] [CrossRef]
- Bitter, T.; Gudziol, H.; Burmeister, H.P.; Mentzel, H.J.; Guntinas-Lichius, O.; Gaser, C. Anosmia leads to a loss of gray matter in cortical brain areas. Chem. Senses 2010, 35, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Pinto, J.M.; Yi, X.; Li, L.; Peng, P.; Wei, Y. Gray matter volume reduction of olfactory cortices in patients with idiopathic olfactory loss. Chem. Senses 2014, 39, 755–760. [Google Scholar] [CrossRef] [Green Version]
- Growdon, M.E.; Schultz, A.P.; Dagley, A.S.; Amariglio, R.E.; Hedden, T.; Rentz, D.M.; Johnson, K.A.; Sperling, R.A.; Albers, M.W.; Marshall, G.A. Odor identification and Alzheimer disease biomarkers in clinically normal elderly. Neurology 2015, 84, 2153–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.; Jernigan, T.L.; Fennema-Notestine, C. Left hippocampal volume loss in Alzheimer’s disease is reflected in performance on odor identification: A structural MRI study. J. Int. Neuropsychol. Soc. 2003, 9, 459–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, H.S.; Jeon, S.; Chung, S.J.; Yun, M.; Lee, P.H.; Sohn, Y.H.; Evans, A.C.; Ye, B.S. Olfactory dysfunction in Alzheimer’s disease- and Lewy body-related cognitive impairment. Alzheimers Dement. 2018, 14, 1243–1252. [Google Scholar] [CrossRef]
- Wu, X.; Geng, Z.; Zhou, S.; Bai, T.; Wei, L.; Ji, G.J.; Zhu, W.; Yu, Y.; Tian, Y.; Wang, K. Brain Structural Correlates of Odor Identification in Mild Cognitive Impairment and Alzheimer’s Disease Revealed by Magnetic Resonance Imaging and a Chinese Olfactory Identification Test. Front. Neurosci. 2019, 13, 842. [Google Scholar] [CrossRef] [Green Version]
- Yoshii, F.; Onaka, H.; Kohara, S.; Ryo, M.; Takahashi, W. Association of Smell Identification Deficit with Alzheimer’s Disease Assessment Scale-Cognitive Subscale, Japanese Version Scores and Brain Atrophy in Patients with Dementia. Eur. Neurol. 2019, 81, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Eslinger, P.J.; Du, G.; Kong, L.; Lewis, M.M.; Huang, X. Olfactory-related cortical atrophy is associated with olfactory dysfunction in Parkinson’s disease. Mov. Disord. 2014, 29, 1205–1208. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Yu, C.; Fan, F.; Zhang, K.; Zhu, C.; Wu, T.; Li, K.; Chan, P. Correlation between progressive changes in piriform cortex and olfactory performance in early Parkinson’s disease. Eur. Neurol. 2011, 66, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Wattendorf, E.; Welge-Lüssen, A.; Fiedler, K.; Bilecen, D.; Wolfensberger, M.; Fuhr, P.; Hummel, T.; Westermann, B. Olfactory impairment predicts brain atrophy in Parkinson’s disease. J. Neurosci. 2009, 29, 15410–15413. [Google Scholar] [CrossRef] [Green Version]
- Campabadal, A.; Uribe, C.; Segura, B.; Baggio, H.C.; Abos, A.; Garcia-Diaz, A.I.; Marti, M.J.; Valldeoriola, F.; Compta, Y.; Bargallo, N.; et al. Brain correlates of progressive olfactory loss in Parkinson’s disease. Parkinsonism Relat. Disord. 2017, 41, 44–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; You, H.; Liu, J.F.; Ni, D.F.; Zhang, Z.X.; Guan, J. Association of olfactory bulb volume and olfactory sulcus depth with olfactory function in patients with Parkinson disease. AJNR Am. J. Neuroradiol. 2011, 32, 677–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.E.; Cho, K.H.; Ham, J.H.; Song, S.K.; Sohn, Y.H.; Lee, P.H. Olfactory performance acts as a cognitive reserve in non-demented patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2014, 20, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Bartrés-Faz, D.; Arenaza-Urquijo, E.M. Structural and functional imaging correlates of cognitive and brain reserve hypotheses in healthy and pathological aging. Brain Topogr. 2011, 24, 340–357. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [Green Version]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [Green Version]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Čičelienė, J.; Vaičys, Ž.; Rastenytė, D. Development of the Lithuanian Version of Sniffin’ Sticks 12 Odor Identification Test. Medicina 2018, 54, 13. [Google Scholar] [CrossRef] [Green Version]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischl, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef] [Green Version]
- Fischl, B.; Liu, A.; Dale, A.M. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging 2001, 20, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Fischl, B.; Salat, D.H.; van der Kouwe, A.J.; Makris, N.; Ségonne, F.; Quinn, B.T.; Dale, A.M. Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004, 23, S69–S84. [Google Scholar] [CrossRef] [Green Version]
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999, 9, 179–194. [Google Scholar] [CrossRef]
- Dale, A.M.; Sereno, M.I. Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. J. Cogn. Neurosci. 1993, 5, 162–176. [Google Scholar] [CrossRef]
- Reuter, M.; Rosas, H.D.; Fischl, B. Highly accurate inverse consistent registration: A robust approach. Neuroimage 2010, 53, 1181–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, C.G.; Gunter, J.L.; Wiste, H.J.; Przybelski, S.A.; Weigand, S.D.; Ward, C.P.; Senjem, M.L.; Vemuri, P.; Murray, M.E.; Dickson, D.W.; et al. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. Neuroimage Clin. 2016, 11, 802–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Wisse, L.E.M.; Chételat, G.; Daugherty, A.M.; de Flores, R.; la Joie, R.; Mueller, S.G.; Stark, C.E.L.; Wang, L.; Yushkevich, P.A.; Berron, D.; et al. Hippocampal subfield volumetry from structural isotropic 1 mm3 MRI scans: A note of caution. Hum. Brain Mapp. 2021, 42, 539–550. [Google Scholar] [CrossRef]
- Benjamini, Y.; Krieger, A.; Yekutieli, D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 2006, 93, 491–507. [Google Scholar] [CrossRef]
- Pike, N. Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol. Evol. 2011, 2, 278–282. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J. Educ. Behav. Stat. 2000, 25, 60–83. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Tan, H.Y.; Wu, Z.H.; Sun, C.P.; He, J.X.; Li, X.C.; Shao, M. Imaging of olfactory bulb and gray matter volumes in brain areas associated with olfactory function in patients with Parkinson’s disease and multiple system atrophy. Eur. J. Radiol. 2014, 83, 564–570. [Google Scholar] [CrossRef]
- Sengoku, R.; Matsushima, S.; Bono, K.; Sakuta, K.; Yamazaki, M.; Miyagawa, S.; Komatsu, T.; Mitsumura, H.; Kono, Y.; Kamiyama, T.; et al. Olfactory function combined with morphology distinguishes Parkinson’s disease. Parkinsonism Relat. Disord. 2015, 21, 771–777. [Google Scholar] [CrossRef]
- Sunwoo, M.K.; Cha, J.; Ham, J.H.; Song, S.K.; Hong, J.Y.; Lee, J.M.; Sohn, Y.H.; Lee, P.H. Olfactory performance and resting state functional connectivity in non-demented drug naïve patients with Parkinson’s disease. Hum. Brain Mapp. 2015, 36, 1716–1727. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Kassir, M.A.; Wu, J.; Zhang, Q.; Zhou, S.; Xuan, S.Y.; Li, Q.; Ye, Y.; Hu, J. MR volumetric study of piriform-cortical amygdala and orbitofrontal cortices: The aging effect. PLoS ONE 2013, 8, e74526. [Google Scholar] [CrossRef] [PubMed]

| AD (n = 20) | PD (n = 20) | Controls (n = 19) | ANOVA F2,57; p | Group Differences | |
|---|---|---|---|---|---|
| Age in years, mean (SD), range | 72.30 (8.74), 28 | 64.05 (10.13), 35 | 68.11 (6.78), 23 | F = 4.5; p = 0.015 | AD > PD, p = 0.012 a |
| Gender, (Male:Female) | 11:9 | 11:9 | 11:8 | χ2 = 2.3 p = 0.893 b | |
| Education in years, mean (SD) | 12.95 (3.65) | 14.65 (2.94) | 14.95 (3.19) | F = 2.14; p = 0.127 | |
| SS12 score, mean (SD) | 4.50 (2.91) | 6.20(2.98) | 9.60 (1.50) | F = 19.58; p < 0.001 | AD < C, p < 0.001 a PD < C, p < 0.001 a |
| MMSE score, mean (SD) | 17.60 (5.35) | 28.50(1.57) | 29.26 (1.05) | F = 77.17; p < 0.001 | AD < C, p < 0.001 a AD < PD, p < 0.001 a |
| . | Group | Mean (SD) | ANOVA F2,56; p | Group Differences |
|---|---|---|---|---|
| Cortical thickness, mm | AD | 2.16 (0.20) | F2,56 = 13.34; p < 0.001 | AD < PD; p < 0.001 a |
| PD | 2.38 (0.16) | AD < C; p < 0.001 a | ||
| C | 2.38 (0.11) | |||
| Subcortical gray matter volume, cm3 | AD | 49.61 (5.11) | F2,56 = 4.57; p = 0.014 | AD < PD; p = 0.013 a |
| PD | 55.05 (7.42) | |||
| C | 53.18 (4.27) |
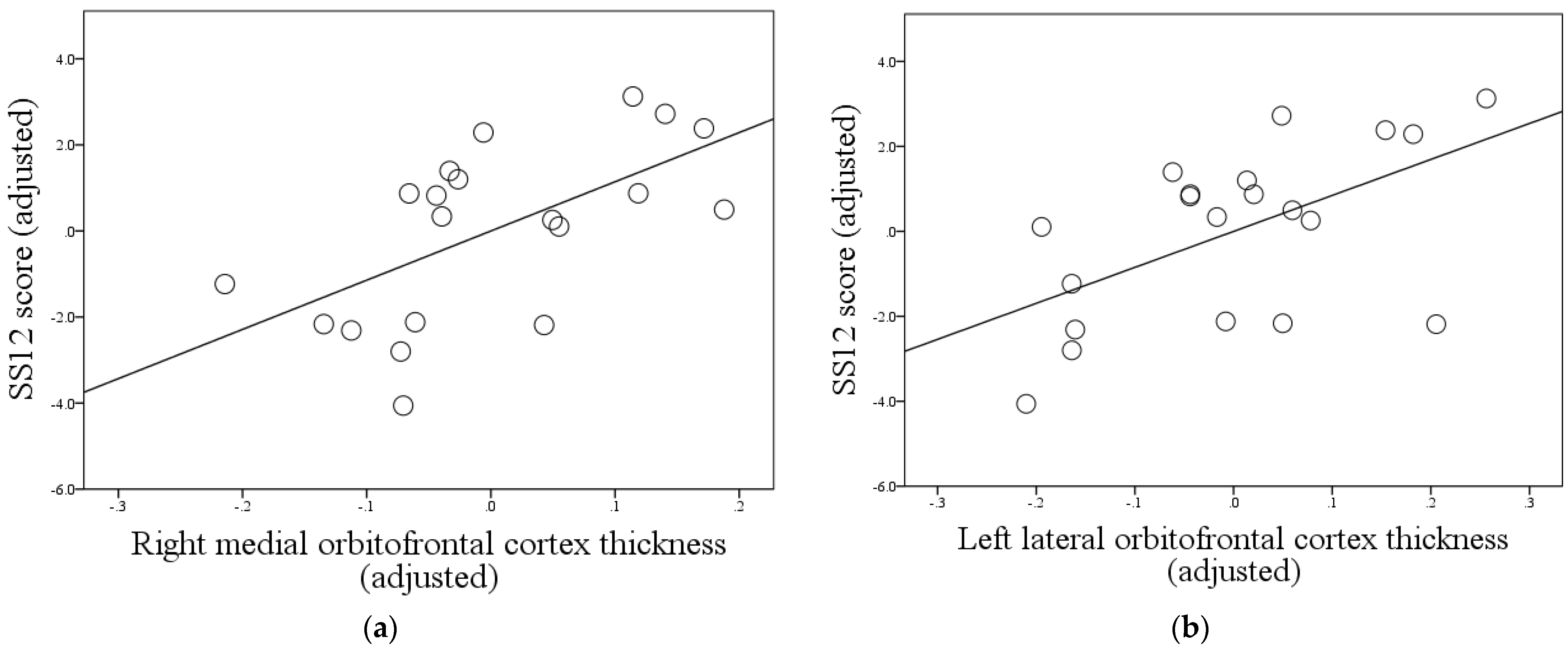
| Gray Matter Region | Correlation with SS12 Score | Uncorrected p Value | FDR-Adjusted p Value a |
|---|---|---|---|
| Right medial orbitofrontal cortex thickness | 0.673 | 0.00115 | 0.034 |
| Left lateral orbitofrontal cortex thickness | 0.649 | 0.00196 | 0.034 |
| Right pars orbitalis cortex thickness | 0.623 | 0.00336 | 0.034 |
| Right frontal pole cortex thickness | 0.620 | 0.00353 | 0.034 |
| Right thalamus proper volume | 0.604 | 0.00480 | 0.034 |
| Right lateral orbitofrontal cortex thickness | 0.599 | 0.00525 | 0.034 |
| Left medial orbitofrontal cortex thickness | 0.595 | 0.00561 | 0.034 |
| Left temporal pole cortex thickness | 0.594 | 0.00575 | 0.034 |
| Right insular cortex thickness | 0.592 | 0.00592 | 0.034 |
| Left accumbens area volume | 0.503 | 0.00611 | 0.034 |
| Right temporal pole cortex thickness | 0.587 | 0.00675 | 0.034 |
| Left rostral anterior cingulate cortex thickness | 0.579 | 0.00750 | 0.034 |
| Left pars orbitalis cortex thickness | 0.573 | 0.00827 | 0.036 |
| Left insular cortex thickness | 0.569 | 0.00877 | 0.036 |
| Right isthmus cingulate cortex thickness | 0.553 | 0.01100 | 0.043 |
| Left entorhinal cortex thickness | 0.537 | 0.01470 | 0.048 |
| Right rostral anterior cingulate cortex thickness | 0.536 | 0.01481 | 0.048 |
| Left frontal pole cortex thickness | 0.533 | 0.01554 | 0.048 |
| Left superior frontal cortex thickness | 0.531 | 0.01592 | 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesmanas, S.; Gleiznienė, R.; Baranauskas, M.; Matijošaitis, V.; Rastenytė, D. Odor Identification and Regional Gray Matter Atrophy in Patients with Alzheimer’s Disease, Parkinson’s Disease, and the Healthy Elderly: A Cross-Sectional Structural MRI Study. Brain Sci. 2021, 11, 1296. https://doi.org/10.3390/brainsci11101296
Jesmanas S, Gleiznienė R, Baranauskas M, Matijošaitis V, Rastenytė D. Odor Identification and Regional Gray Matter Atrophy in Patients with Alzheimer’s Disease, Parkinson’s Disease, and the Healthy Elderly: A Cross-Sectional Structural MRI Study. Brain Sciences. 2021; 11(10):1296. https://doi.org/10.3390/brainsci11101296
Chicago/Turabian StyleJesmanas, Simonas, Rymantė Gleiznienė, Mindaugas Baranauskas, Vaidas Matijošaitis, and Daiva Rastenytė. 2021. "Odor Identification and Regional Gray Matter Atrophy in Patients with Alzheimer’s Disease, Parkinson’s Disease, and the Healthy Elderly: A Cross-Sectional Structural MRI Study" Brain Sciences 11, no. 10: 1296. https://doi.org/10.3390/brainsci11101296






