Anxiety and Attentional Processes: The Role of Resting Heart Rate Variability
Abstract
:1. Introduction
Aims
- Given the higher arousal associated with anxiety [32], greater trait anxiety should be related to larger attentional bias for the change detection in a flicker paradigm, regardless of the emotional value of the stimulus. The ability to detect changes was expected to be greater for negative stimuli. In fact, according to previous studies, individuals with high trait anxiety would seem to focus on negative information [4] preferentially and would present greater difficulties, compared to subjects with low trait anxiety, to disengage themselves from threatening information [33].
- HRV should be associated in attentional processes related to the change blindness paradigm and anxiety.
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.2.1. Self-Report Measures
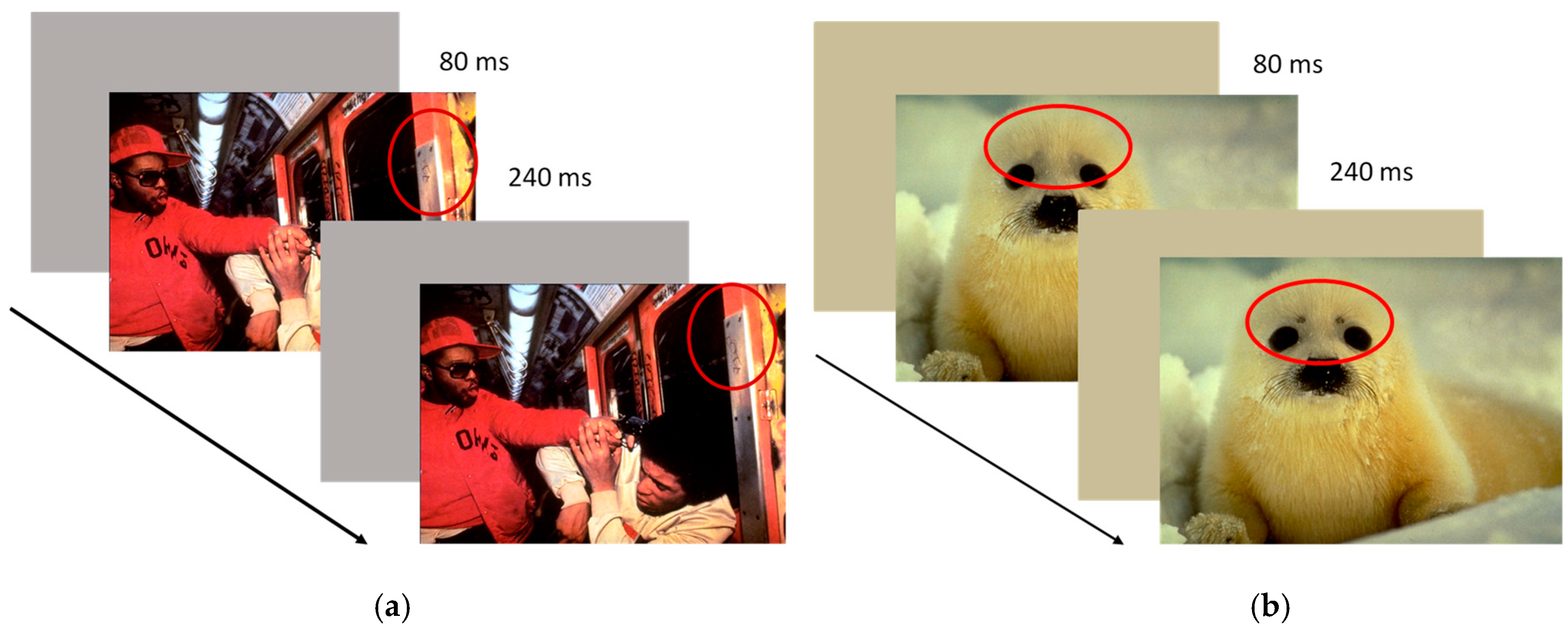
2.2.2. Emotional Flicker Task
Apparatus
Stimuli
Procedure
2.2.3. Physiological Measures
- Blood pressure: Blood pressure was measured using an OMRON digital sphygmomanometer in line with the European guidelines for measuring blood pressure [40]. Systolic and diastolic blood pressure and heart rate were collected.
- Weight and height: A digital balance was used to measure the participants’ weight (kg). The height of the subjects was measured with a standardized anthropometer (cm). These data allowed the assessment of the body mass index (BMI: kg/m2).
- Heart rate variability: Electrocardiography (ECG) was recorded using disposable Ag/AgCI electrodes through the Firstbeat bodyguard 2. Two electrodes were positioned on the right side of the body, above the clavicle, and on the left side, at the rib cage level. HRV was analyzed using the Kubios HRV program, and artifacts were manually and automatically removed (ver. 2) [41]. The results in the time domain and the frequency domain were analyzed. In the time domain, in line with previous studies and according to guidelines, given the high correlation among HRV indices, only the root mean square of successive differences (RMSSD) that reflects vagal tone [20,42,43] was considered. In the frequency domain, the low-frequency range (LF; 0.04–0.15 Hz) that reflects a mix of sympathetic and vagal influences [16,42] and high frequencies (HF; 0.15–0.40 Hz), an index of the parasympathetic cardiac tone [17,42,44], were considered.
2.2.4. General Procedure
2.2.5. Data Analysis
3. Results
3.1. Demographic variables
3.1.1. Flicker Task: Attentional Effects
3.1.2. Flicker Task: Group Differences (Neutral vs. Emotional Stimuli)
3.1.3. Flicker Task: Group Differences (Neutral vs. Positive vs. Negative Stimuli)
3.2. Anxiety, Emotional Flicker Task, and Heart Rate Variability (HRV)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graeff, F.G. Anxiety, panic and the hypothalamic-pituitary-adrenal axis. Rev. Bras. Psiquiatr. 2007, 29, s3–s6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grillon, C. Models and mechanisms of anxiety: Evidence from startle studies. Psychopharmacol 2008, 199, 421–437. [Google Scholar] [CrossRef] [Green Version]
- Van Bockstaele, B.; Verschuere, B.; Tibboel, H.; De Houwer, J.; Crombez, G.; Koster, E.H. A review of current evidence for the causal impact of attentional bias on fear and anxiety. Psychol. Bull. 2014, 140, 682. [Google Scholar] [CrossRef]
- Wieser, M.J.; Keil, A. Attentional threat biases and their role in anxiety: A neurophysiological perspective. Int. J. Psychophys 2020, 153, 148–158. [Google Scholar] [CrossRef]
- Eysenck, M.W.; Derakshan, N.; Santos, R.; Calvo, M.G. Anxiety and cognitive performance: Attentional control theory. Emotion 2007, 7, 336. [Google Scholar] [CrossRef] [Green Version]
- Iijima, Y.; Takano, K.; Tanno, Y. Attentional bias and its association with anxious mood dynamics. Emotion 2018, 18, 725. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, A.J.; Gawronski, B.; Dozois, D.J. Cognitive vulnerability to anxiety: A review and an integrative model. Clin. Psychol. Rev. 2009, 29, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Keogh, E.; Dillon, C.; Georgiou, G.; Hunt, C. Selective attentional biases for physical threat in physical anxiety sensitivity. J. Anxiety Disord. 2001, 15, 299–315. [Google Scholar] [CrossRef]
- Battagliese, G.; Lombardo, C. L’attentional bias nella psicopatologia. Psicoter. Cogn. Comport. 2011, 17, 75–98. [Google Scholar]
- Notebaert, L.; Tilbrook, M.; Clarke, P.J.; MacLeod, C. When a bad bias can be good: Anxiety-linked attentional bias to threat in contexts where dangers can be avoided. Clin. Psychol. Sci. 2017, 5, 485–496. [Google Scholar] [CrossRef]
- Eysenck, M.W.; Calvo, M.G. Anxiety and performance: The processing efficiency theory. Cogn. Emot. 1992, 6, 409–434. [Google Scholar] [CrossRef]
- MacLeod, C.; Grafton, B.; Notebaert, L. Anxiety-linked attentional bias: Is it reliable? Annu. Rev. Clin. Psychol. 2019, 15, 529–554. [Google Scholar] [CrossRef]
- Bar-Haim, Y.; Lamy, D.; Pergamin, L.; Bakermans-Kranenburg, M.J.; Van Ijzendoorn, M.H. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol. Bull. 2007, 133, 1. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.P.; Ehlers, A.; Clark, D.M.; Mansell, W. Patients with generalized social phobia direct their attention away from faces. Behav. Res. Ther. 2002, 40, 677–687. [Google Scholar] [CrossRef]
- Cox, J.A.; Christensen, B.K.; Goodhew, S.C. Temporal dynamics of anxiety-related attentional bias: Is affective context a missing piece of the puzzle? Cogn. Emot. 2018, 32, 1329–1338. [Google Scholar] [CrossRef]
- Berntson, G.G.; Thomas, B.J., Jr.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef]
- Reyes del Paso, G.A.; Langewitz, W.; Mulder, L.J.; Van Roon, A.; Duschek, S. The Utility of Low Frequency Heart Rate Variability as an Index of Sympathetic Cardiac Tone: A Review with Emphasis on a Reanalysis of Previous Studies. Psychophysiology 2013, 50, 477–487. [Google Scholar] [CrossRef]
- Porges, S.W. Orienting in a Defensive World: Mammalian Modifications of Our Evolutionary Heritage. A Polyvagal Theory. Psychophysiology 1995, 32, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the Heart—Brain Connection: Further Elaboration of a Model of Neurovisceral Integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Lane, R.D. A Model of Neurovisceral Integration in Emotion Regulation and Dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Ellis, R.J.; Thayer, J.F. Music and autonomic nervous system (dys) function. Music. Percept. 2010, 27, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Favieri, F.; Casagrande, M. Heart Rate Variability and Cognitive Function: A Systematic Review. Front. Neurosci. 2019, 13, 710. [Google Scholar] [CrossRef] [PubMed]
- Bellato, A.; Arora, I.; Hollis, C.; Groom, M.J. Is autonomic nervous system function atypical in attention deficit hyperactivity disorder (ADHD)? A systematic review of the evidence. Neurosci. Biobehav. Rev. 2020, 108, 182–206. [Google Scholar] [CrossRef]
- Chalmers, J.A.; Quintana, D.S.; Abbott, M.J.; Kemp, A.H. Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Front. Psychiatry 2014, 5, 80. [Google Scholar] [CrossRef] [Green Version]
- Chalmers, J.A.; Heathers, J.A.; Abbott, M.J.; Kemp, A.H.; Quintana, D.S. Worry is associated with robust reductions in heart rate variability: A transdiagnostic study of anxiety psychopathology. BMC Psychol. 2016, 4, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rensink, R.A. How much of a scene is seen? The role of attention in scene perception. Investig. Ophthalmol. Vis. Sci. 1997, 38, S707. [Google Scholar]
- Simons, D.J.; Rensink, R.A. Change blindness: Past, present, and future. Trends Cogn. Sci. 2005, 9, 16–20. [Google Scholar] [CrossRef]
- Favieri, F.; Forte, G.; Marotta, A.; Casagrande, M. Food-Related Attentional Bias in Individuals with Normal Weight and Overweight: A Study with a Flicker Task. Nutrients 2020, 12, 492. [Google Scholar] [CrossRef] [Green Version]
- Maccari, L.; Casagrande, M.; Martella, D.; Anolfo, M.; Rosa, C.; Fuentes, L.J.; Pasini, A. Change blindness in children with ADHD: A selective impairment in visual search? JAD 2013, 17, 620–627. [Google Scholar] [CrossRef]
- Turatto, M.; Galfano, G. Color, form and luminance capture attention in visual search. Vis. Res. 2000, 40, 1639–1643. [Google Scholar] [CrossRef] [Green Version]
- McGlynn, F.D.; Wheeler, S.A.; Wilamowska, Z.A.; Katz, J.S. Detection of change in threat-related and innocuous scenes among snake-fearful and snake-tolerant participants: Data from the flicker task. J. Anxiety Disord. 2008, 22, 515–523. [Google Scholar] [CrossRef]
- Roos, A.L.; Goetz, T.; Voracek, M.; Krannich, M.; Bieg, M.; Jarrell, A.; Pekrun, R. Test Anxiety and Physiological Arousal: A Systematic Review and Meta-Analysis. Educ. Psychol. Rev. 2020, 1–40. [Google Scholar] [CrossRef]
- Georgiou, G.; Bleakley, C.; Hayward, J.; Russo, R.; Dutton, K.; Eltiti, S.; Fox, E. Focusing on fear: Attentional disengagement from emotional faces. Visual Cogn. 2005, 12, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Paniccia, M.; Paniccia, D.; Thomas, S.; Taha, T.; Reed, N. Clinical and non-clinical depression and anxiety in young people: A scoping review on heart rate variability. Auton. Neurosci. 2017, 208, 1–14. [Google Scholar] [CrossRef]
- Ramírez, E.; Ortega, A.R.; Del Paso, G.A.R. Anxiety, attention, and decision making: The moderating role of heart rate variability. Int. J. Psychophys. 2015, 98, 490–496. [Google Scholar] [CrossRef]
- Spielberger, C.D. State-Trait Anxiety Inventory for Adults; American Psychological Association: Washington, DC, USA, 1983. [Google Scholar]
- Pedrabissi, L.; Santinello, M. Verifica della validità dello STAI forma Y di Spielberger. Boll. Psicol. Appl. 1989, 191–192, 11–14. [Google Scholar]
- Lang, P.; Bradley, M.M. The International Affective Picture System (IAPS) in the study of emotion and attention. Handb. Emot. Elicitation Assess. 2007, 29, 70–73. [Google Scholar]
- Simons, D.J.; Levin, D.T. Change blindness. Trends Cogn. Sci. 1997, 1, 261–267. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018, 27, 314–340. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, M.P.; Niskanen, J.P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV—Heart Rate Variability Analysis Software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef]
- Malik, M. Heart Rate Variability: Standards of Measurement, Physiological Interpretation, and Clinical Use: Task Force of the European Society of Cardiology and the North American Society for Pacing and Electrophysiology. Ann. Noninvasive Electrocardiol. 1996, 1, 151–181. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T., Jr. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Mertgen, A. Vagal Tank Theory: The Three Rs of Cardiac Vagal Control Functioning—Resting, Reactivity, and Recovery. Front. Neurosci. 2018, 12, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillie, B.L.; Thayer, J.F. Individual differences in resting heart rate variability and cognitive control in posttraumatic stress disorder. Front. Psychol. 2014, 5, 758. [Google Scholar] [CrossRef] [Green Version]
- Park, G.; Vasey, M.; VanBavel, J.J.; Thayer, J.F. Cardiac Vagal Tone Is Cor-Related with Selective Attention to Neutral Distractors under Load. Psychophysiology 2013, 50, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Bornas, X.; Llabrés, J.; Noguera, M.; López, A.M.; Barceló, F.; Tortella-Feliu, M.; Fullana, M. Looking at the heart of low and high heart rate variability fearful flyers: Self-reported anxiety when confronting feared stimuli. Biol. Psychol. 2005, 70, 182–187. [Google Scholar] [CrossRef]
- Cocia, I.R.; Uscătescu, L.C.; Rusu, A.S. Attention bias to threat in anxiety-prone individuals: Evidence from disengagement, but not engagement bias using cardiac vagal tone. J. Psychophysiol. 2012, 26, 74. [Google Scholar] [CrossRef]
- Rensink, R.A. Change blindness: Implications for the nature of visual attention. In Vision and Attention; Springer: New York, NY, USA, 2001; pp. 169–188. [Google Scholar]
- Dijksterhuis, A.; Aarts, H. On wildebeests and humans: The preferential detection of negative stimuli. Psychol. Sci. 2003, 14, 14–18. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2020, 23, 155–184. [Google Scholar] [CrossRef]
- Luo, Q.; Holroyd, T.; Majestic, C.; Cheng, X.; Schechter, J.; Blair, R.J. Emotional automaticity is a matter of timing. J. Neurosci. 2010, 30, 5825–5829. [Google Scholar] [CrossRef] [Green Version]
- MacLeod, C.; Rutherford, E.; Campbell, L.; Ebsworthy, G.; Holker, L. Selective attention and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. J. Abnorm. Psychol. 2002, 111, 107. [Google Scholar] [CrossRef]
- Taylor, C.T.; Bomyea, J.; Amir, N. Malleability of attentional bias for positive emotional information and anxiety vulnerability. Emotion 2011, 11, 127. [Google Scholar] [CrossRef] [Green Version]
- Mathews, A.; MacLeod, C. Cognitive vulnerability to emotional disorders. Annu. Rev. Clin. Psychol. 2005, 1, 167–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joormann, J.; Gotlib, I.H. Selective attention to emotional faces following recovery from depression. J Abnorm. Psychol. 2007, 116, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sussman, T.J.; Jin, J.; Mohanty, A. Top-down and bottom-up factors in threat-related perception and attention in anxiety. Biol. Psychol. 2016, 121, 160–172. [Google Scholar] [CrossRef]
- Vuilleumier, P.; Pourtois, G. Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia 2007, 45, 174–194. [Google Scholar] [CrossRef] [PubMed]
- Grupe, D.W.; Nitschke, J.B. Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nat. Rev. Neurosci. 2013, 14, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Siennicka, A.; Quintana, D.S.; Fedurek, P.; Wijata, A.; Paleczny, B.; Ponikowska, B.; Danel, D.P. Resting heart rate variability, attention and attention maintenance in young adults. Int. J. Psychophysiol. 2019, 143, 126–131. [Google Scholar] [CrossRef]
- Forte, G.; Morelli, M.; Casagrande, M. Heart Rate Variability and Decision-Making: Autonomic Responses in Making Decisions. Brain Sci. 2021, 11, 243. [Google Scholar] [CrossRef]
- Pittig, A.; Arch, J.J.; Lam, C.W.; Craske, M.G. Heart rate and heart rate variability in panic, social anxiety, obsessive–compulsive, and generalized anxiety disorders at baseline and in response to relaxation and hyperventilation. Int. J. Psychophysiol. 2013, 87, 19–27. [Google Scholar] [CrossRef]
- Guarino, A.; Forte, G.; Giovannoli, J.; Casagrande, M. Executive Functions in the Elderly with Mild Cognitive Impairment: A Systematic Review on Motor and Cognitive Inhibition, Conflict Control and Cognitive Flexibility. Aging Ment. Health 2019, 24, 1028–1045. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; de Pascalis, V.; Favieri, F.; Casagrande, M. Effects of Blood Pressure on Cognitive Performance: A Systematic Review. J. Clin. Med. 2020, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Forte, G.; Casagrande, M. Effects of Blood Pressure on Cognitive Performance in Aging: A Systematic Review. Brain Sci. 2020, 10, 919. [Google Scholar] [CrossRef] [PubMed]
- Favieri, F.; Forte, G.; Casagrande, M. The executive functions in overweight and obesity: A systematic review of neuropsychological cross-sectional and longitudinal studies. Front. Psychol. 2019, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Burgess, M.M.; Cabeleira, C.M.; Cabrera, I.; Bucks, R.S.; MacLeod, C. Examining attentional biases underlying trait anxiety in younger and older adults. Cogn. Emot. 2014, 28, 84–97. [Google Scholar] [CrossRef]


| Low Trait Anxiety (n = 36) | High Trait Anxiety (n = 39) | F | P | |
|---|---|---|---|---|
| Female (%) | 20 (55.6) | 20 (51.3) | ||
| Male (%) | 16 (44.4) | 19 (48.7) | ||
| Age | 24.5 (2.7) | 23.5 (2.2) | 3.15 | 0.08 |
| Trait Anxiety score | 28.2 (2.9) | 55.5 (5.0) | 821.0 | 0.0001 * |
| BMI | 23.1 (3.7) | 22.9 (2.2) | <1 | 0.90 |
| SBP | 116.5 (12.5) | 121.5 (10.2) | 2.16 | 0.14 |
| DBP | 72.9 (7.8) | 71.9 (6.9) | <1 | 0.62 |
| HR | 73.9 (11.1) | 76.10 (11.7) | <1 | 0.51 |
| HRV indices in Resting State | ||||
| RMSSD (ms) | 36.15 (13.9) | 34.8 (15.6) | <1 | 0.63 |
| LF (ln) | 6.9 (0.6) | 6.6 (0.7) | 1.96 | 0.16 |
| HF (ln) | 6.2 (0.9) | 5.9 (0.9) | 1.20 | 0.27 |
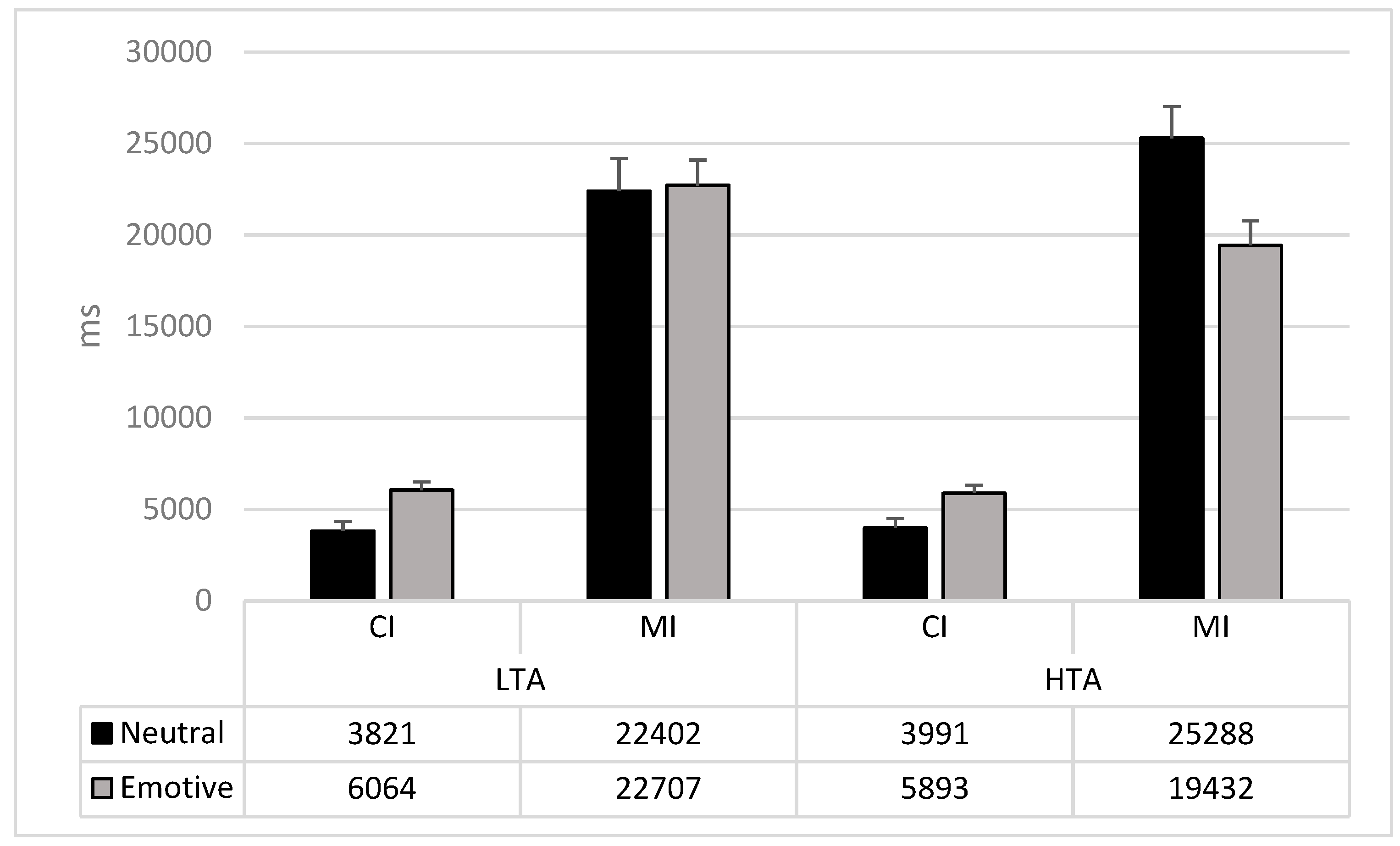
| Change Type | Stimuli Valence | Low Trait Anxiety | High Trait Anxiety |
|---|---|---|---|
| CI | Emotional | 6064 (2513) | 5893 (2696) |
| Neutral | 3821 (3128) | 3992 (3135) | |
| MI | Emotional | 22,707 (8837) | 19,432 (7716) |
| Neutral | 22,402 (8608) | 25,288 (12271) | |
| CI change | 5319 (2132) | 5346 (2270) | |
| MI change | 22,627 (6884) | 21,356 (1103) |
| Change Type | Valence | Low Trait Anxiety | High Trait Anxiety |
|---|---|---|---|
| CI | Negative | 6952 (4326) | 6451 (4389) |
| Neutral | 3820 (3173) | 3992 (3135) | |
| Positive | 5338 (2092) | 5335 (2929) | |
| MI | Negative | 16,713 (8340) | 16,486 (7182) |
| Neutral | 22,335 (8724) | 25,288 (12,271) | |
| Positive | 28,845 (15,409) | 22,378 (10,366) |
| High Anxiety Trait | Low Anxiety Trait | |||||||
|---|---|---|---|---|---|---|---|---|
| RMSSD (ln) | LF (ln) | HF (ln) | RMSSD (ln) | LF (ln) | HF (ln) | |||
| CI | r | −0.37 | −0.30 | −0.40 | 0.09 | −0.20 | −0.18 | |
| p | 0.03 | 0.083 | 0.02 | 0.61 | 0.27 | 0.32 | ||
| MI | r | 0.044 | 0.062 | 0.13 | 0.13 | 0.25 | 0.067 | |
| p | 0.80 | 0.72 | 0.47 | 0.45 | 0.15 | 0.71 | ||
| CI | Negative | r | −0.20 | −0.28 | −0.23 | 0.01 | −0.14 | −0.11 |
| p | 0.24 | 0.10 | 0.19 | 0.95 | 0.43 | 0.52 | ||
| CI | Neutral | r | −0.32 | −0.14 | −0.35 | 0.06 | −0.26 | −0.17 |
| p | 0.06 | 0.42 | 0.04 | 0.73 | 0.13 | 0.33 | ||
| CI | Positive | r | −0.19 | −0.09 | −0.20 | 0.16 | 0.10 | −0.04 |
| p | 0.27 | 0.61 | 0.25 | 0.37 | 0.58 | 0.82 | ||
| MI | Negative | r | −0.16 | −0.04 | −0.04 | 0.05 | 0.02 | 0.004 |
| p | 0.36 | 0.82 | 0.81 | 0.77 | 0.90 | 0.98 | ||
| MI | Neutral | r | 0.021 | 0.06 | 0.10 | −0.16 | 0.12 | −0.09 |
| p | 0.91 | 0.71 | 0.56 | 0.36 | 0.50 | 0.61 | ||
| MI | Positive | r | 0.18 | 0.08 | 0.17 | 0.24 | 0.26 | 0.14 |
| p | 0.31 | 0.66 | 0.345 | 0.18 | 0.16 | 0.45 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forte, G.; Favieri, F.; Oliha, E.O.; Marotta, A.; Casagrande, M. Anxiety and Attentional Processes: The Role of Resting Heart Rate Variability. Brain Sci. 2021, 11, 480. https://doi.org/10.3390/brainsci11040480
Forte G, Favieri F, Oliha EO, Marotta A, Casagrande M. Anxiety and Attentional Processes: The Role of Resting Heart Rate Variability. Brain Sciences. 2021; 11(4):480. https://doi.org/10.3390/brainsci11040480
Chicago/Turabian StyleForte, Giuseppe, Francesca Favieri, Esther Osariemen Oliha, Andrea Marotta, and Maria Casagrande. 2021. "Anxiety and Attentional Processes: The Role of Resting Heart Rate Variability" Brain Sciences 11, no. 4: 480. https://doi.org/10.3390/brainsci11040480
APA StyleForte, G., Favieri, F., Oliha, E. O., Marotta, A., & Casagrande, M. (2021). Anxiety and Attentional Processes: The Role of Resting Heart Rate Variability. Brain Sciences, 11(4), 480. https://doi.org/10.3390/brainsci11040480






