Stimulation of Different Sectors of the Human Dorsal Premotor Cortex Induces a Shift from Reactive to Predictive Action Strategies and Changes in Motor Inhibition: A Dense Transcranial Magnetic Stimulation (TMS) Mapping Study
Abstract
1. Introduction
1.1. The Neural Bases of Immobility Behavior
1.2. A Specific Dorsal Premotor-Primary Motor Circuit Controls Immobility during the SET-Period
1.3. Dense TMS Mapping and Experimental Protocol
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
2.3. Apparatus for Lip Response Collection
2.4. Building of the TMS Dense Grid
2.5. TMS
2.6. Data Analysis
2.7. Post-Hoc Data Analysis on Trials Displaying Predictive Behavior
2.8. Post-Hoc Data Analysis on Anticipation Errors
3. Results
4. Discussion
4.1. A Shift from Reactive to Predictive Behavior
4.2. A Caudal-Rostral Specialization in the dPM
4.3. Limitation of the Present Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Duque, J.; Lew, D.; Mazzocchio, R.; Olivier, E.; Ivry, R.B. Evidence for Two Concurrent Inhibitory Mechanisms during Response Preparation. J. Neurosci. 2010, 30, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Duque, J.; Greenhouse, I.; Labruna, L.; Ivry, R.B. Physiological Markers of Motor Inhibition during Human Behavior. Trends Neurosci. 2017, 40, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Boucher, L.; Palmeri, T.J.; Logan, G.D.; Schall, J.D. Inhibitory Control in Mind and Brain: An Interactive Race Model of Countermanding Saccades. Psychol. Rev. 2007, 114, 376–397. [Google Scholar] [CrossRef]
- Verbruggen, F.; Logan, G.D. Response Inhibition in the Stop-Signal Paradigm. Trends Cogn. Sci. 2008, 12, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Noorani, I. Towards a Unifying Mechanism for Cancelling Movements. Philos. Trans. R. Soc. B 2017, 372, 20160191. [Google Scholar] [CrossRef]
- Aron, A.R. From Reactive to Proactive and Selective Control: Developing a Richer Model for Stopping Inappropriate Responses. Biol. Psychiatry 2011, 69, e55–e68. [Google Scholar] [CrossRef]
- Hoshi, E. Functional Specialization within the Dorsolateral Prefrontal Cortex: A Review of Anatomical and Physiological Studies of Non-Human Primates. Neurosci. Res. 2006, 54, 73–84. [Google Scholar] [CrossRef]
- Monaco, S.; Malfatti, G.; Culham, J.C.; Cattaneo, L.; Turella, L. Decoding Motor Imagery and Action Planning in the Early Visual Cortex: Overlapping but Distinct Neural Mechanisms. Neuroimage 2020, 218, 116981. [Google Scholar] [CrossRef] [PubMed]
- Hasbroucq, T.; Kaneko, H.; Akamatsu, M.; Possamaı, C.-A. The Time-Course of Preparatory Spinal and Cortico-Spinal Inhibition: An H-Reflex and Transcranial Magnetic Stimulation Study in Man. Exp. Brain Res. 1999, 124, 33–41. [Google Scholar] [CrossRef]
- Davranche, K.; Tandonnet, C.; Burle, B.; Meynier, C.; Vidal, F.; Hasbroucq, T. The Dual Nature of Time Preparation: Neural Activation and Suppression Revealed by Transcranial Magnetic Stimulation of the Motor Cortex. Eur. J. Neurosci. 2007, 25, 3766–3774. [Google Scholar] [CrossRef] [PubMed]
- Touge, T.; Taylor, J.L.; Rothwell, J.C. Reduced Excitability of the Cortico-Spinal System during the Warning Period of a Reaction Time Task. Electroencephalogr. Clin. Neurophysiol./Electromyogr. Mot. Control 1998, 109, 489–495. [Google Scholar] [CrossRef]
- Duque, J.; Ivry, R.B. Role of Corticospinal Suppression during Motor Preparation. Cereb. Cortex 2009, 19, 2013–2024. [Google Scholar] [CrossRef]
- Parmigiani, S.; Zattera, B.; Barchiesi, G.; Cattaneo, L. Spatial and Temporal Characteristics of Set-Related Inhibitory and Excitatory Inputs from the Dorsal Premotor Cortex to the Ipsilateral Motor Cortex Assessed by Dual-Coil Transcranial Magnetic Stimulation. Brain Topogr. 2018, 31, 795–810. [Google Scholar] [CrossRef]
- Ibáñez, J.; Hannah, R.; Rocchi, L.; Rothwell, J.C. Premovement Suppression of Corticospinal Excitability May Be a Necessary Part of Movement Preparation. Cereb. Cortex 2020, 30, 2910–2923. [Google Scholar] [CrossRef]
- Weinrich, M.; Wise, S.P.; Mauritz, K.-H. A Neurophysiological study of the premotor cortex in the rhesus monkey. Brain 1984, 107, 385–414. [Google Scholar] [CrossRef]
- Godschalk, M.; Lemon, R.N.; Kuypers, H.; Van der Steen, J. The Involvement of Monkey Premotor Cortex Neurones in Preparation of Visually Cued Arm Movements. Behav. Brain Res. 1985, 18, 143–157. [Google Scholar] [CrossRef]
- Wise, S.P. The Primate Premotor Cortex: Past, Present, and Preparatory. Annu. Rev. Neurosci. 1985, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kurata, K.; Wise, S.P. Premotor and Supplementary Motor Cortex in Rhesus Monkeys: Neuronal Activity during Externally-and Internally-Instructed Motor Tasks. Exp. Brain Res. 1988, 72, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Filevich, E. Negative Motor Phenomena in Cortical Stimulation: Implications for Inhibitory Control of Human Action. Cortex 2012, 48, 1251–1261. [Google Scholar] [CrossRef]
- Mirabella, G.; Pani, P.; Ferraina, S. Neural Correlates of Cognitive Control of Reaching Movements in the Dorsal Premotor Cortex of Rhesus Monkeys. J. Neurophysiol. 2011, 106, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- di Pellegrino, G.; Wise, S. Visuospatial versus Visuomotor Activity in the Premotor and Prefrontal Cortex of a Primate. J. Neurosci. 1993, 13, 1227–1243. [Google Scholar] [CrossRef]
- Koch, G.; Franca, M.; Del Olmo, M.F.; Cheeran, B.; Milton, R.; Sauco, M.A.; Rothwell, J.C. Time Course of Functional Connectivity between Dorsal Premotor and Contralateral Motor Cortex during Movement Selection. J. Neurosci. 2006, 26, 7452–7459. [Google Scholar] [CrossRef]
- Burle, B.; Vidal, F.; Tandonnet, C.; Hasbroucq, T. Physiological Evidence for Response Inhibition in Choice Reaction Time Tasks. Brain Cogn. 2004, 56, 153–164. [Google Scholar] [CrossRef]
- Nachev, P.; Kennard, C.; Husain, M. Functional Role of the Supplementary and Pre-Supplementary Motor Areas. Nat. Rev. Neurosci. 2008, 9, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Meletti, S.; Tinuper, P.; Bisulli, F.; Santucci, M. Epileptic Negative Myoclonus and Brief Asymmetric Tonic Seizures. A Supplementary Sensorimotor Area Involvement for Both Negative and Positive Motor Phenomena. Epileptic Disord. Int. Epilepsy J. Videotape 2000, 2, 163–168. [Google Scholar]
- Krainik, A.; Duffau, H.; Capelle, L.; Cornu, P.; Boch, A.-L.; Mangin, J.-F.; Le Bihan, D.; Marsault, C.; Chiras, J.; Lehéricy, S. Role of the Healthy Hemisphere in Recovery after Resection of the Supplementary Motor Area. Neurology 2004, 62, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Potgieser, A.R.E.; De Jong, B.M.; Wagemakers, M.; Hoving, E.W.; Groen, R.J.M. Insights from the Supplementary Motor Area Syndrome in Balancing Movement Initiation and Inhibition. Front. Hum. Neurosci. 2014, 8, 960. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Josephs, K.A. Alien Hand Syndrome. Curr. Neurol. Neurosci. Rep. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Simmonds, D.J.; Pekar, J.J.; Mostofsky, S.H. Meta-Analysis of Go/No-Go Tasks Demonstrating That FMRI Activation Associated with Response Inhibition Is Task-Dependent. Neuropsychologia 2008, 46, 224–232. [Google Scholar] [CrossRef]
- Sebastian, A.; Pohl, M.F.; Klöppel, S.; Feige, B.; Lange, T.; Stahl, C.; Voss, A.; Klauer, K.C.; Lieb, K.; Tüscher, O. Disentangling Common and Specific Neural Subprocesses of Response Inhibition. Neuroimage 2013, 64, 601–615. [Google Scholar] [CrossRef]
- Ariani, G.; Wurm, M.F.; Lingnau, A. Decoding Internally and Externally Driven Movement Plans. J. Neurosci. 2015, 35, 14160–14171. [Google Scholar] [CrossRef]
- Aron, A.R. The Neural Basis of Inhibition in Cognitive Control. Neuroscientist 2007, 13, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Coxon, J.P.; Stinear, C.M.; Byblow, W.D. Intracortical Inhibition during Volitional Inhibition of Prepared Action. J. Neurophysiol. 2006, 95, 3371–3383. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.F.; Wiecki, T.V.; Cohen, M.X.; Figueroa, C.M.; Samanta, J.; Sherman, S.J.; Frank, M.J. Subthalamic Nucleus Stimulation Reverses Mediofrontal Influence over Decision Threshold. Nat. Neurosci. 2011, 14, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.J. Hold Your Horses: A Dynamic Computational Role for the Subthalamic Nucleus in Decision Making. Neural Netw. 2006, 19, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, G.; Iaconelli, S.; Romanelli, P.; Modugno, N.; Lena, F.; Manfredi, M.; Cantore, G. Deep Brain Stimulation of Subthalamic Nuclei Affects Arm Response Inhibition in Parkinson’s Patients. Cereb. Cortex 2012, 22, 1124–1132. [Google Scholar] [CrossRef]
- Van Wouwe, N.C.; Pallavaram, S.; Phibbs, F.T.; Martinez-Ramirez, D.; Neimat, J.S.; Dawant, B.M.; D’Haese, P.F.; Kanoff, K.E.; van den Wildenberg, W.P.M.; Okun, M.S. Focused Stimulation of Dorsal Subthalamic Nucleus Improves Reactive Inhibitory Control of Action Impulses. Neuropsychologia 2017, 99, 37–47. [Google Scholar] [CrossRef]
- van den Wildenberg, W.P.; van Boxtel, G.J.; van der Molen, M.W.; Bosch, D.A.; Speelman, J.D.; Brunia, C.H. Stimulation of the Subthalamic Region Facilitates the Selection and Inhibition of Motor Responses in Parkinson’s Disease. J. Cogn. Neurosci. 2006, 18, 626–636. [Google Scholar] [CrossRef]
- Swann, N.; Poizner, H.; Houser, M.; Gould, S.; Greenhouse, I.; Cai, W.; Strunk, J.; George, J.; Aron, A.R. Deep Brain Stimulation of the Subthalamic Nucleus Alters the Cortical Profile of Response Inhibition in the Beta Frequency Band: A Scalp EEG Study in Parkinson’s Disease. J. Neurosci. 2011, 31, 5721–5729. [Google Scholar] [CrossRef]
- Mirabella, G.; Iaconelli, S.; Modugno, N.; Giannini, G.; Lena, F.; Cantore, G. Stimulation of Subthalamic Nuclei Restores a near Normal Planning Strategy in Parkinson’s Patients. PLoS ONE 2013, 8, e62793. [Google Scholar] [CrossRef]
- Parmigiani, S.; Barchiesi, G.; Cattaneo, L. The Dorsal Premotor Cortex Exerts a Powerful and Specific Inhibitory Effect on the Ipsilateral Corticofacial System: A Dual-Coil Transcranial Magnetic Stimulation Study. Exp. Brain Res. 2015, 233, 3253–3260. [Google Scholar] [CrossRef]
- Parmigiani, S.; Cattaneo, L. Stimulation of the Dorsal Premotor Cortex, But Not of the Supplementary Motor Area Proper, Impairs the Stop Function in a STOP Signal Task. Neuroscience 2018, 394, 14–22. [Google Scholar] [CrossRef]
- Kenemans, J.L. Specific Proactive and Generic Reactive Inhibition. Neurosci. Biobehav. Rev. 2015, 56, 115–126. [Google Scholar] [CrossRef]
- Bianco, V.; Di Russo, F.; Perri, R.L.; Berchicci, M. Different Proactive and Reactive Action Control in Fencers’ and Boxers’ Brain. Neuroscience 2017, 343, 260–268. [Google Scholar] [CrossRef]
- Cattaneo, L. Fancies and Fallacies of Spatial Sampling with Transcranial Magnetic Stimulation (TMS). Front. Psychol. 2018, 9, 5. [Google Scholar] [CrossRef]
- Lega, C.; Pirruccio, M.; Bicego, M.; Parmigiani, L.; Chelazzi, L.; Cattaneo, L. The Topography of Visually Guided Grasping in the Premotor Cortex: A Dense-Transcranial Magnetic Stimulation (TMS) Mapping Study. J. Neurosci. 2020, 40, 6790–6800. [Google Scholar] [CrossRef]
- Lega, C.; Chelazzi, L.; Cattaneo, L. Two Distinct Systems Represent Contralateral and Ipsilateral Sensorimotor Processes in the Human Premotor Cortex: A Dense TMS Mapping Study. Cereb. Cortex 2020, 30, 2250–2266. [Google Scholar] [CrossRef] [PubMed]
- Maule, F.; Barchiesi, G.; Brochier, T.; Cattaneo, L. Haptic Working Memory for Grasping: The Role of the Parietal Operculum. Cereb. Cortex 2015, 25, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.-A.; Duque, J.; Labruna, L.; Ivry, R.B. Comparison of the Two Cerebral Hemispheres in Inhibitory Processes Operative during Movement Preparation. NeuroImage 2016, 125, 220–232. [Google Scholar] [CrossRef]
- Mirabella, G.; Fragola, M.; Giannini, G.; Modugno, N.; Lakens, D. Inhibitory Control Is Not Lateralized in Parkinson’s Patients. Neuropsychologia 2017, 102, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Di Caprio, V.; Modugno, N.; Mancini, C.; Olivola, E.; Mirabella, G. Early-stage Parkinson’s Patients Show Selective Impairment in Reactive but Not Proactive Inhibition. Mov. Disord. 2020, 35, 409–418. [Google Scholar] [CrossRef]
- Cattaneo, L.; Pavesi, G. The Facial Motor System. Neurosci. Biobehav. Rev. 2014, 38, 135–159. [Google Scholar] [CrossRef]
- Leocani, L.; Cohen, L.G.; Wassermann, E.M.; Ikoma, K.; Hallett, M. Human Corticospinal Excitability Evaluated with Transcranial Magnetic Stimulation during Different Reaction Time Paradigms. Brain 2000, 123, 1161–1173. [Google Scholar] [CrossRef]
- Chen, R.; Yaseen, Z.; Cohen, L.G.; Hallett, M. Time Course of Corticospinal Excitability in Reaction Time and Self-Paced Movements. Ann. Neurol. 1998, 44, 317–325. [Google Scholar] [CrossRef]
- Jahanshahi, M. Reaction time as an index of motor preparation/programming and speed of response initiation. In Handbook of Clinical Neurophysiology; Elsevier: Amsterdam, The Netherlands, 2003; Volume 1, pp. 203–229. [Google Scholar]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Safety of TMS Consensus Group. Safety, Ethical Considerations, and Application Guidelines for the Use of Transcranial Magnetic Stimulation in Clinical Practice and Research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Niemi, P.; Näätänen, R. Foreperiod and Simple Reaction Time. Psychol. Bull. 1981, 89, 133–162. [Google Scholar] [CrossRef]
- Merchant, H.; Bartolo, R.; Pérez, O.; Méndez, J.C.; Mendoza, G.; Gámez, J.; Yc, K.; Prado, L. Neurophysiology of Timing in the Hundreds of Milliseconds: Multiple Layers of Neuronal Clocks in the Medial Premotor Areas. Neurobiol. Interval Timing 2014, 143–154. [Google Scholar]
- Hoshi, E.; Tanji, J. Distinctions between Dorsal and Ventral Premotor Areas: Anatomical Connectivity and Functional Properties. Curr. Opin. Neurobiol. 2007, 17, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Cisek, P.; Kalaska, J.F. Neural Mechanisms for Interacting with a World Full of Action Choices. Annu. Rev. Neurosci. 2010, 33, 269–298. [Google Scholar] [CrossRef]
- Barchiesi, G.; Cattaneo, L. Early and Late Motor Responses to Action Observation. Soc. Cogn. Affect. Neurosci. 2013, 8, 711–719. [Google Scholar] [CrossRef]
- Michelet, T.; Duncan, G.H.; Cisek, P. Response Competition in the Primary Motor Cortex: Corticospinal Excitability Reflects Response Replacement during Simple Decisions. J. Neurophysiol. 2010, 104, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Duque, J.; Labruna, L.; Verset, S.; Olivier, E.; Ivry, R.B. Dissociating the Role of Prefrontal and Premotor Cortices in Controlling Inhibitory Mechanisms during Motor Preparation. J. Neurosci. 2012, 32, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.D.; Bellgrove, M.A.; Gould, I.C.; English, T.; Garavan, H.; McNaught, E.; Kamke, M.; Mattingley, J.B. Dissociable Mechanisms of Cognitive Control in Prefrontal and Premotor Cortex. J. Neurophysiol. 2007, 98, 10. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Cattaneo, L.; Fabbri-Destro, M.; Rozzi, S. Cortical Mechanisms Underlying the Organization of Goal-Directed Actions and Mirror Neuron-Based Action Understanding. Physiol. Rev. 2014, 94, 655–706. [Google Scholar] [CrossRef]
- Abe, M.; Hanakawa, T. Functional Coupling Underlying Motor and Cognitive Functions of the Dorsal Premotor Cortex. Behav. Brain Res. 2009, 198, 13–23. [Google Scholar] [CrossRef]
- Nakayama, Y.; Yamagata, T.; Hoshi, E. Rostrocaudal Functional Gradient among the Pre-Dorsal Premotor Cortex, Dorsal Premotor Cortex and Primary Motor Cortex in Goal-Directed Motor Behaviour. Eur. J. Neurosci. 2016, 43, 1569–1589. [Google Scholar] [CrossRef]
- Geyer, S. The Microstructural Border between the Motor and the Cognitive Domain in the Human Cerebral Cortex; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Mattia, M.; Spadacenta, S.; Pavone, L.; Quarato, P.; Esposito, V.; Sparano, A.; Sebastiano, F.; Di Gennaro, G.; Morace, R.; Cantore, G. Stop-Event-Related Potentials from Intracranial Electrodes Reveal a Key Role of Premotor and Motor Cortices in Stopping Ongoing Movements. Front. Neuroeng. 2012, 5, 12. [Google Scholar] [CrossRef]
- Mirabella, G.; Pani, P.; Ferraina, S. Context Influences on the Preparation and Execution of Reaching Movements. Cogn. Neuropsychol. 2008, 25, 996–1010. [Google Scholar] [CrossRef] [PubMed]


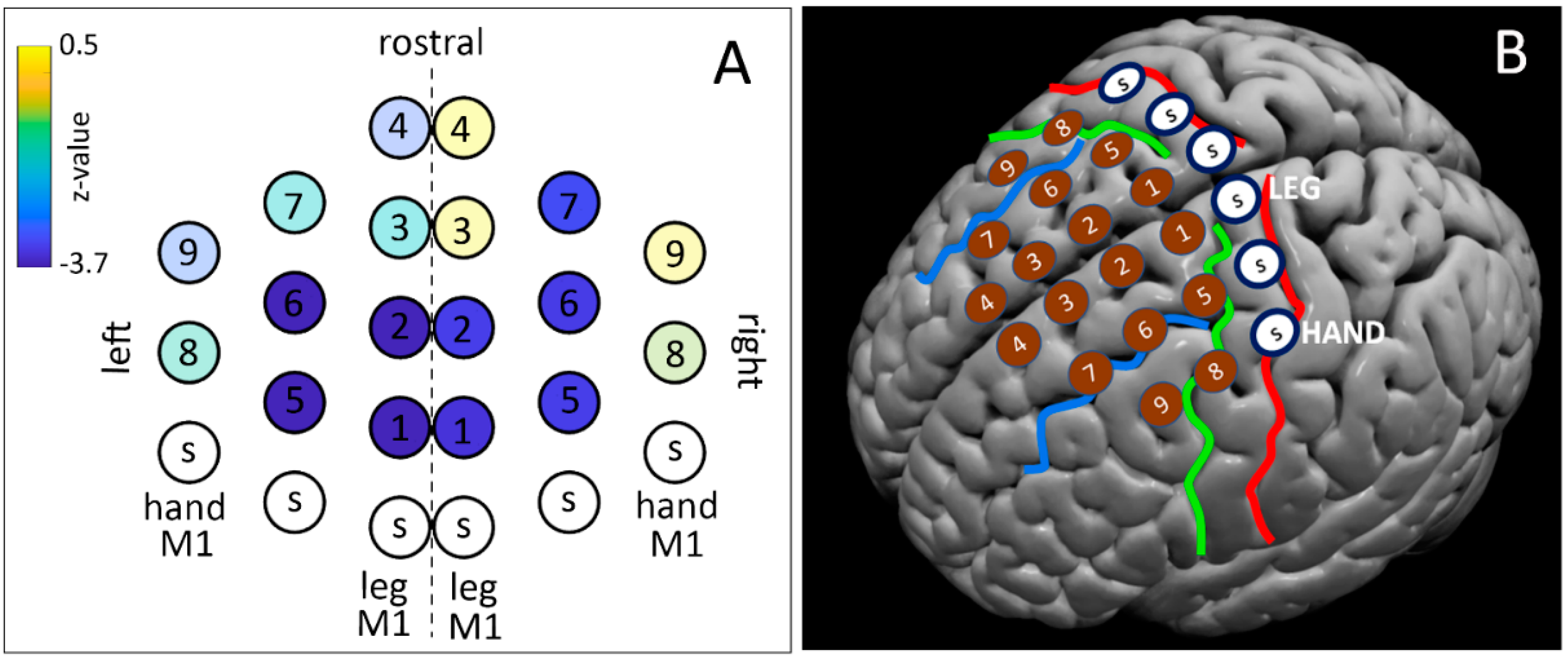



| Grid Spot | Right Hemisphere | Left Hemisphere |
|---|---|---|
| 1 | −3.43 (0.0006) | −3.62 (0.0003) |
| 2 | −3.29 (0.001) | −3.62 (0.0003) |
| 3 | 0.4 (0.69) | −1.63 (0.1) |
| 4 | 0.5 (0.62) | −2.82 (0.005) |
| 5 | −3.29 (0.001) | −3.62 (0.0003) |
| 6 | −3.34 (0.001) | −3.62 (0.0003) |
| 7 | −3.15 (0.002) | −1.59 (0.11) |
| 8 | −0.78 (0.43) | −1.4 (0.16) |
| 9 | 0.36 (0.72) | −2.72 (0.01) |
| Grid Spot | Right Hemisphere | Left Hemisphere |
|---|---|---|
| 1 | −2.26 (0.0238) | −2.66 (0.0079) |
| 2 | −3.01 (0.0026) | −4.35 (0.0001) |
| 3 | −1.49 (0.14) | −1.67 (0.09) |
| 4 | −1.92 (0.05) | −1.42 (0.157) |
| 5 | −2.69 (0.0071) | −1.36 (0.1724) |
| 6 | −3.39 (0.001) | −3.46 (0.0005) |
| 7 | −1.81 (0.07) | −1.56 (0.12) |
| 8 | −0.99 (0.32) | 1.13 (0.26) |
| 9 | −0.28 (0.78) | −2.68 (0.01) |
| Grid Spot | Right Hemisphere | Left Hemisphere |
|---|---|---|
| 1 | 1.1 (0.271) | 1.1 (0.271) |
| 2 | 1.1 (0.271) | −2.79 (0.0052) |
| 3 | −0.56 (0.58) | 1.1 (0.27) |
| 4 | 0 (1) | −1.67 (0.095) |
| 5 | 0.53 (0.5966) | 0 (1) |
| 6 | −0.56 (0.576) | 1.68 (0.0927) |
| 7 | −2.24 (0.025) | 2.27 (0.02) |
| 8 | 0 (1) | 1.68 (0.09) |
| 9 | 0 (1) | 1.1 (0.27) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattaneo, L.; Parmigiani, S. Stimulation of Different Sectors of the Human Dorsal Premotor Cortex Induces a Shift from Reactive to Predictive Action Strategies and Changes in Motor Inhibition: A Dense Transcranial Magnetic Stimulation (TMS) Mapping Study. Brain Sci. 2021, 11, 534. https://doi.org/10.3390/brainsci11050534
Cattaneo L, Parmigiani S. Stimulation of Different Sectors of the Human Dorsal Premotor Cortex Induces a Shift from Reactive to Predictive Action Strategies and Changes in Motor Inhibition: A Dense Transcranial Magnetic Stimulation (TMS) Mapping Study. Brain Sciences. 2021; 11(5):534. https://doi.org/10.3390/brainsci11050534
Chicago/Turabian StyleCattaneo, Luigi, and Sara Parmigiani. 2021. "Stimulation of Different Sectors of the Human Dorsal Premotor Cortex Induces a Shift from Reactive to Predictive Action Strategies and Changes in Motor Inhibition: A Dense Transcranial Magnetic Stimulation (TMS) Mapping Study" Brain Sciences 11, no. 5: 534. https://doi.org/10.3390/brainsci11050534
APA StyleCattaneo, L., & Parmigiani, S. (2021). Stimulation of Different Sectors of the Human Dorsal Premotor Cortex Induces a Shift from Reactive to Predictive Action Strategies and Changes in Motor Inhibition: A Dense Transcranial Magnetic Stimulation (TMS) Mapping Study. Brain Sciences, 11(5), 534. https://doi.org/10.3390/brainsci11050534






