Conventional and HD-tDCS May (or May Not) Modulate Overt Attentional Orienting: An Integrated Spatio-Temporal Approach and Methodological Reflections
Abstract
:1. Introduction
- (1)
- Does the modulation of right frontal (FEF) and parietal (PPC) areas induce contralateral, leftward attentional shifts et in a more ecological task? Are these effects different depending on the stimulated area?
- (2)
- Considering the reduced current spread, does HD-tDCS induce more consistent effects than conventional tDCS? Do its aftereffects emerge at different times post-stimulation (e.g., [12])?
2. Experiment 1—Conventional tDCS
2.1. Materials and Methods
2.1.1. Participants and Sample Size Estimation
2.1.2. Free Visual Exploration (FVE) Task
2.1.3. tDCS Protocol and Experimental Procedure
2.2. Data Analyses
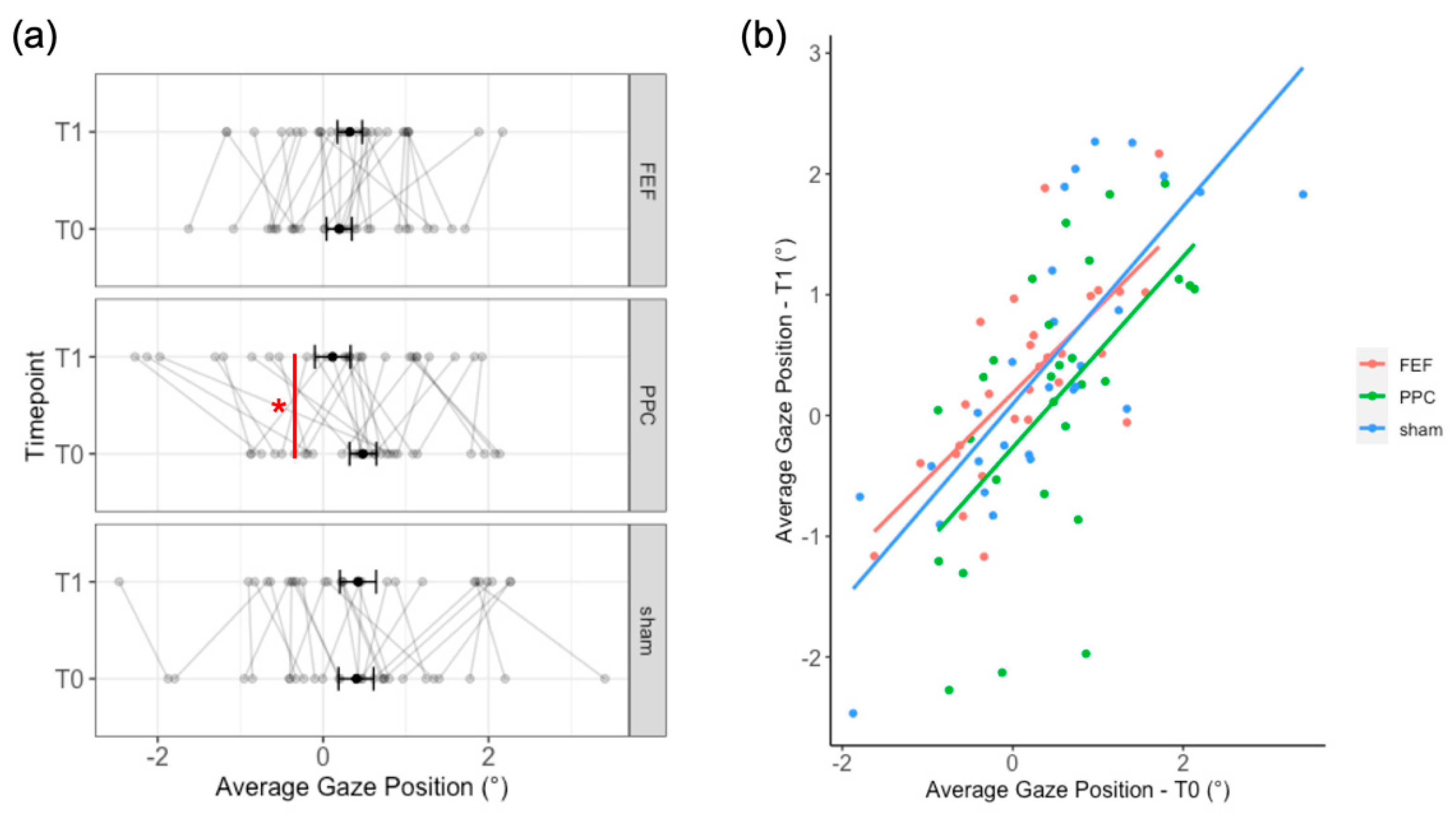
2.2.1. Can tDCS Induce a Contralateral Attentional Shift?
2.2.2. The Effects of tDCS on Visual-Exploration Patterns from a Temporal Perspective
2.3. Results
2.4. Discussion
3. Experiment 2—HD-tDCS
3.1. Materials and Methods
3.1.1. Participants
3.1.2. FVE Task
3.1.3. HD-tDCS Protocol and Experimental Procedure
3.1.4. Data Analyses
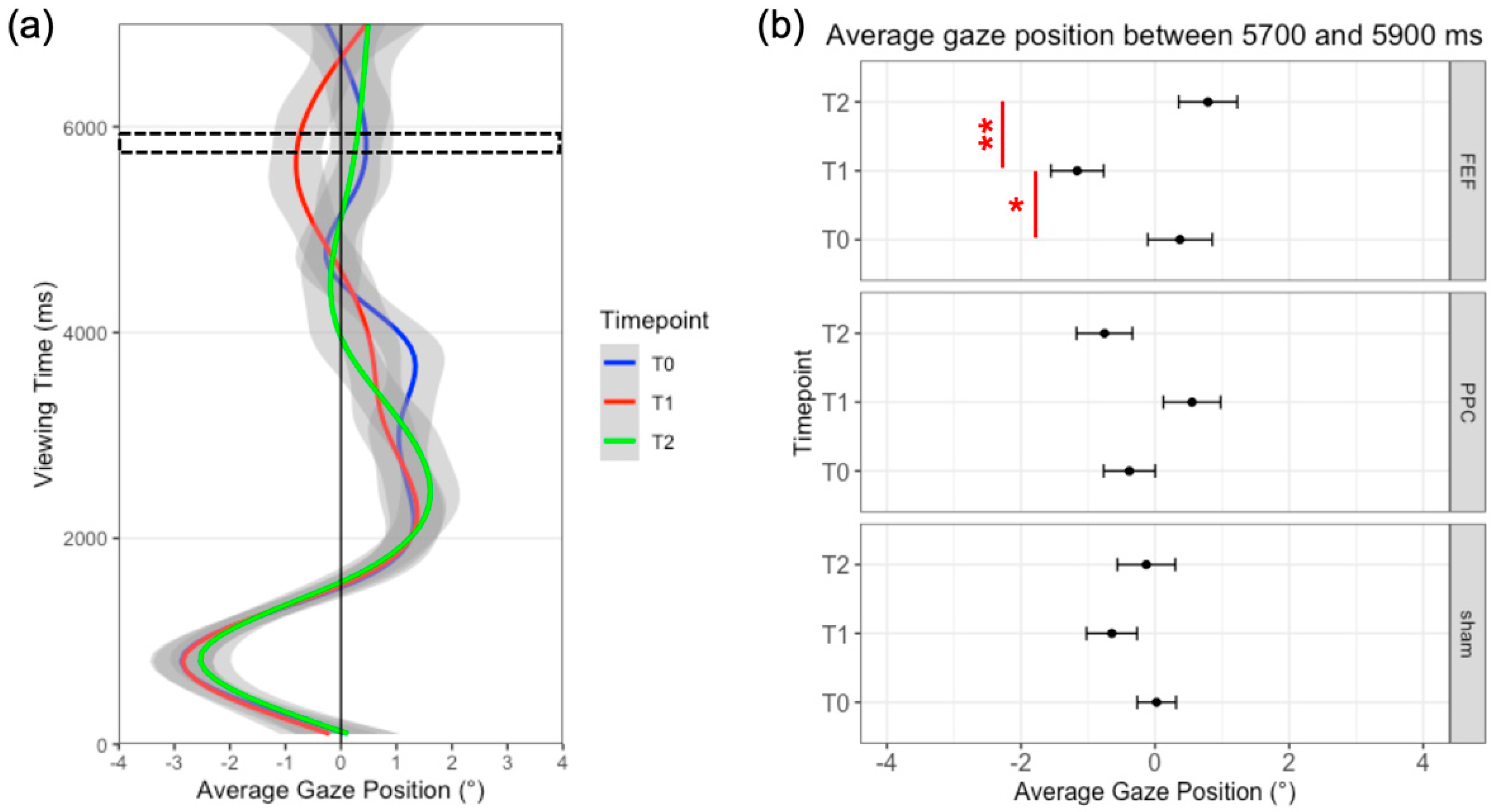
3.2. Results
3.3. Discussion
4. General Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Electric Field Simulation with SimNIBS 3.2.4
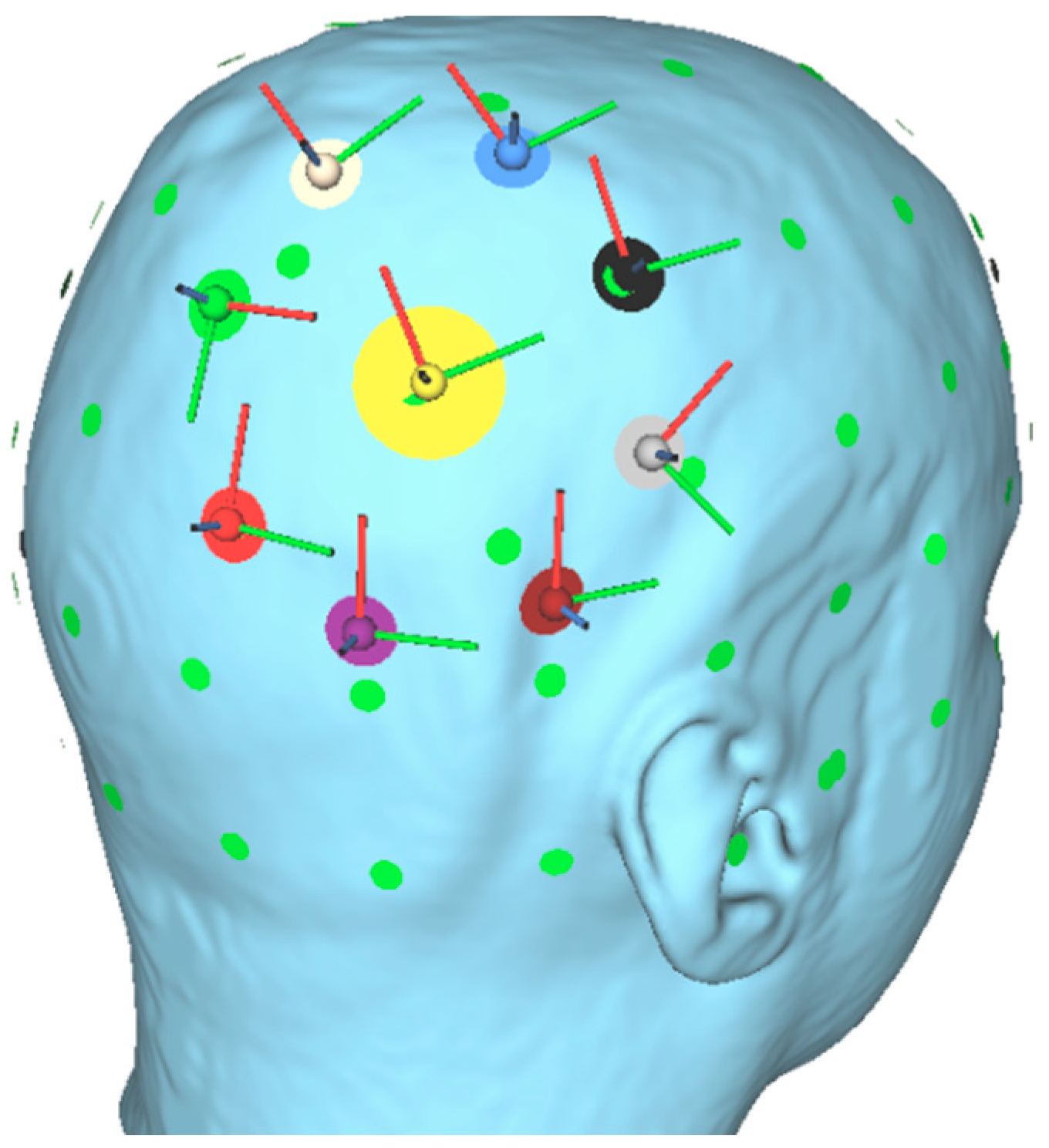
Appendix B. Experiment 1—Conventional tDCS
Appendix B.1. tDCS-Related Sensations and Sham Blinding
| Item | FEF | PPC | Sham | |||
|---|---|---|---|---|---|---|
| N | Most Reported | N | Most Reported | N | Most Reported | |
| Itching | 23 | Mild | 25 | Mild | 20 | Mild |
| Pain | 2 | Mild, Moderate | 2 | Mild | 0 | |
| Burning | 18 | Mild | 17 | Mild | 12 | Mild |
| Heat | 11 | Mild | 11 | Mild | 12 | Mild |
| Pinching | 25 | Mild | 24 | Mild | 18 | Mild |
| Metallic Taste | 1 | Moderate | 0 | 0 | ||
| Fatigue | 8 | Moderate | 5 | Mild | 5 | Mild |
| FEF | PPC | Sham | ||||
|---|---|---|---|---|---|---|
| Count | Adapted Residuals | Count | Adapted Residuals | Count | Adapted Residuals | |
| Correct | 23 | 1.3 | 21 | 1.8 | 11 | −3.1 |
| Wrong | 5 | −1.3 | 7 | −1.8 | 17 | 3.1 |
| Factor | Start | End | Cluster Mass | p (>Mass) |
|---|---|---|---|---|
| Stimulation | 62 | 62 | 4.88 | 0.491 |
| Timepoint | 3 | 3 | 5.70 | 0.73 |
| 19 | 19 | 5.3 | 0.79 | |
| 24 | 24 | 4.13 | 0.95 | |
| 30 | 30 | 5.04 | 0.83 | |
| 44 | 44 | 4.25 | 0.93 | |
| 53 | 53 | 4.41 | 0.92 |
Appendix B.2. The Relationship between Baseline and the Change in Gaze Position
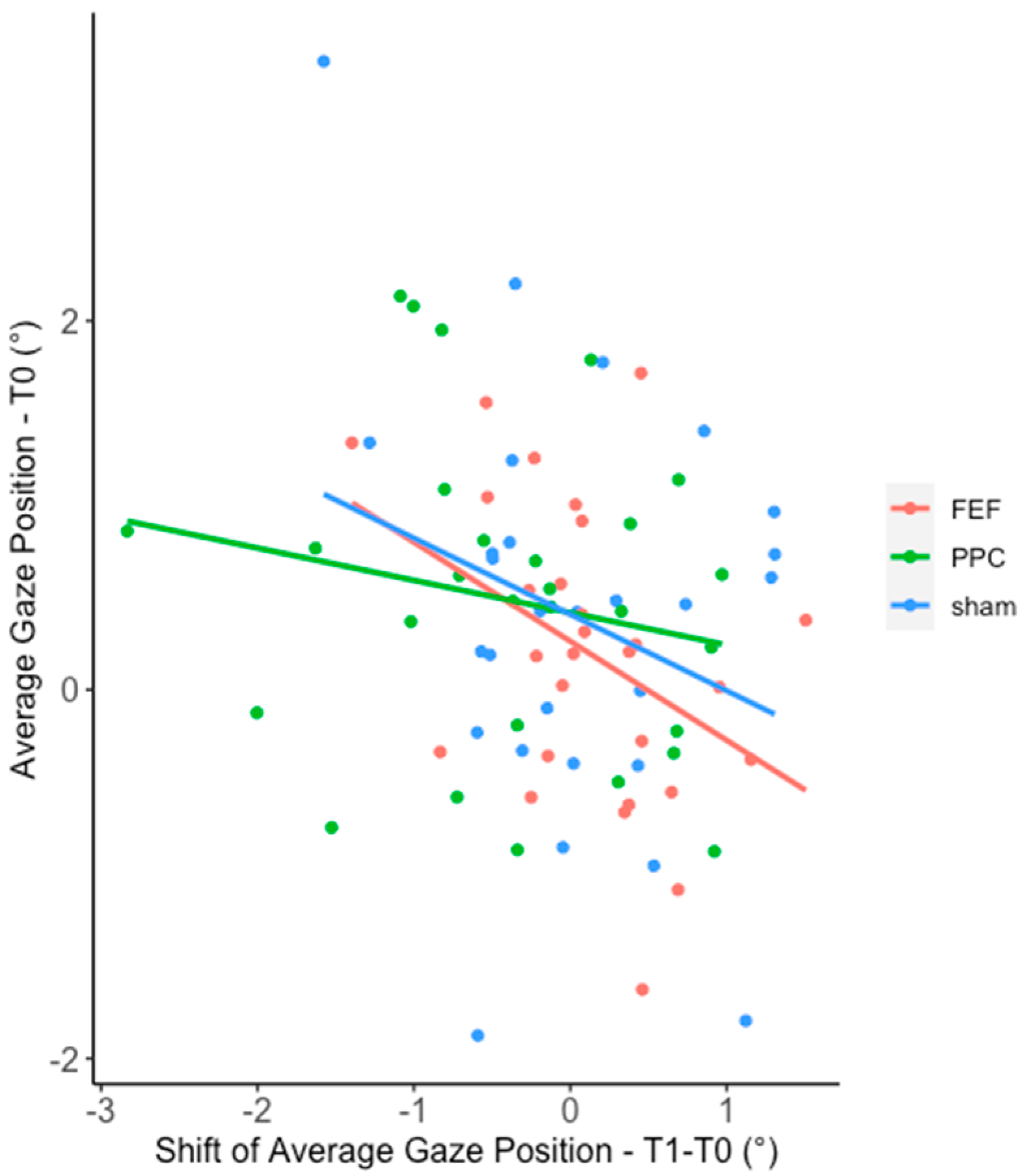
Appendix C. Experiment 2—HD-tDCS
Appendix C.1. HD-tDCS-Related Sensations and Sham-Blinding
| Item | FEF | PPC | Sham | |||
|---|---|---|---|---|---|---|
| N | Most Reported | N | Most Reported | N | Most Reported | |
| Itching | 17 | Mild | 16 | Moderate | 15 | Mild |
| Pain | 9 | Mild | 8 | Mild | 7 | Mild |
| Burning | 15 | Mild | 14 | Mild | 12 | Mild |
| Heat | 8 | Mild | 13 | Mild | 8 | Mild |
| Pinching | 20 | Mild | 18 | Mild | 17 | Mild |
| Metallic Taste | 0 | 1 | Mild | 0 | ||
| Fatigue | 4 | Mild | 5 | Mild | 7 | Mild |
| FEF | PPC | Sham | ||||
|---|---|---|---|---|---|---|
| Count | Adapted Residuals | Count | Adapted Residuals | Count | Adapted Residuals | |
| Correct | 14 | 1.3 | 14 | 1.8 | 7 | −3.1 |
| Wrong | 8 | −1.3 | 8 | −1.8 | 15 | 3.1 |
Appendix C.2. “HD-Baseline” Model–Detailed Statistics
| Factor | Sum Sq | MeanSq | NumDF | DenDF | F Value | p |
|---|---|---|---|---|---|---|
| Stim | 482.22 | 241.11 | 2 | 101.96 | 0.39 | 0.676 |
| Timepoint | 120.33 | 120.33 | 1 | 95.5 | 0.2 | 0.659 |
| Baseline | 808.69 | 808.69 | 1 | 117.91 | 1.32 | 0.254 |
| Stim*Timepoint | 2779.49 | 1389.74 | 2 | 95.5 | 2.27 | 0.11 |
| Stim*Baseline | 507.20 | 253.60 | 2 | 101.96 | 0.41 | 0.663 |
| Timepoint*baseline | 127.11 | 127.11 | 1 | 95.5 | 0.21 | 0.65 |
| 3-ways interaction | 2885.26 | 1442.63 | 2 | 95.5 | 2.35 | 0.101 |
References
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-Based Guidelines on the Therapeutic Use of Transcranial Direct Current Stimulation (TDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained Excitability Elevations Induced by Transcranial DC Motor Cortex Stimulation in Humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Monte-Silva, K.; Kuo, M.F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of Late LTP-Like Plasticity in the Human Motor Cortex by Repeated Non-Invasive Brain Stimulation. Brain Stimul. 2013, 6, 424–432. [Google Scholar] [CrossRef]
- Romero Lauro, L.J.; Rosanova, M.; Mattavelli, G.; Convento, S.; Pisoni, A.; Opitz, A.; Bolognini, N.; Vallar, G. TDCS Increases Cortical Excitability: Direct Evidence from TMS–EEG. Cortex 2014, 58, 99–111. [Google Scholar] [CrossRef]
- Horvath, J.C.; Forte, J.D.; Carter, O. Quantitative Review Finds No Evidence of Cognitive Effects in Healthy Populations From Single-Session Transcranial Direct Current Stimulation (TDCS). Brain Stimul. 2015, 8, 535–550. [Google Scholar] [CrossRef]
- Minarik, T.; Berger, B.; Althaus, L.; Bader, V.; Biebl, B.; Brotzeller, F.; Fusban, T.; Hegemann, J.; Jesteadt, L.; Kalweit, L.; et al. The Importance of Sample Size for Reproducibility of TDCS Effects. Front. Hum. Neurosci. 2016, 10, SEP2016. [Google Scholar] [CrossRef] [Green Version]
- Fertonani, A.; Miniussi, C. Transcranial Electrical Stimulation: What We Know and Do Not Know about Mechanisms. Neuroscientist 2017, 23, 109–123. [Google Scholar] [CrossRef]
- Learmonth, G.; Thut, G.; Benwell, C.S.Y.; Harvey, M. The Implications of State-Dependent TDCS Effects in Aging: Behavioural Response Is Determined by Baseline Performance. Neuropsychologia 2015, 74, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Datta, A.; Bansal, V.; Diaz, J.; Patel, J.; Reato, D.; Bikson, M. Gyri-Precise Head Model of Transcranial Direct Current Stimulation: Improved Spatial Focality Using a Ring Electrode versus Conventional Rectangular Pad. Brain Stimul. 2009, 2, 201–207.e1. [Google Scholar] [CrossRef] [Green Version]
- Bortoletto, M.; Rodella, C.; Salvador, R.; Miranda, P.C.; Miniussi, C. Reduced Current Spread by Concentric Electrodes in Transcranial Electrical Stimulation (TES). Brain Stimul. 2016, 9, 525–528. [Google Scholar] [CrossRef]
- Kuo, H.I.; Bikson, M.; Datta, A.; Minhas, P.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Comparing Cortical Plasticity Induced by Conventional and High-Definition 4 × 1 Ring TDCS: A Neurophysiological Study. Brain Stimul. 2013, 6, 644–648. [Google Scholar] [CrossRef]
- Sparing, R.; Thimm, M.; Hesse, M.D.; Küst, J.; Karbe, H.; Fink, G.R. Bidirectional Alterations of Interhemispheric Parietal Balance by Non-Invasive Cortical Stimulation. Brain 2009, 132, 3011–3020. [Google Scholar] [CrossRef]
- Bolognini, N.; Olgiati, E.; Rossetti, A.; Maravita, A. Enhancing Multisensory Spatial Orienting by Brain Polarization of the Parietal Cortex. Eur. J. Neurosci. 2010, 31, 1800–1806. [Google Scholar] [CrossRef]
- Loftus, A.M.; Nicholls, M.E.R. Testing the Activation–Orientation Account of Spatial Attentional Asymmetries Using Transcranial Direct Current Stimulation. Neuropsychologia 2012, 50, 2573–2576. [Google Scholar] [CrossRef]
- Kinsbourne, M. Mechanisms of Unilateral Neglect. Adv. Psychol. 1987, 45, 69–86. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of Goal-Directed and Stimulus-Driven Attention in the Brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Thiebaut de Schotten, M.; Dell’Acqua, F.; Forkel, S.; Simmons, A.; Vergani, F.; Murphy, D.G.M.; Catani, M. A Lateralized Brain Network for Visuo-Spatial Attention. Nat. Preced. 2011, 1, 1. [Google Scholar] [CrossRef]
- Giglia, G.; Mattaliano, P.; Puma, A.; Rizzo, S.; Fierro, B.; Brighina, F. Neglect-like Effects Induced by TDCS Modulation of Posterior Parietal Cortices in Healthy Subjects. Brain Stimul. 2011, 4, 294–299. [Google Scholar] [CrossRef]
- Ball, K.; Lane, A.R.; Smith, D.T.; Ellison, A. Site-Dependent Effects of TDCS Uncover Dissociations in the Communication Network Underlying the Processing of Visual Search. Brain Stimul. 2013, 6, 959–965. [Google Scholar] [CrossRef] [Green Version]
- Roy, L.B.; Sparing, G.; Fink, G.R.; Hesse, M.D. Modulation of Attention Functions by Anodal TDCS on Right PPC. Neuropsychologia 2015, 74, 96–107. [Google Scholar] [CrossRef]
- Corbetta, M.; Akbudak, E.; Conturo, T.E.; Snyder, A.Z.; Ollinger, J.M.; Drury, H.A.; Linenweber, M.R.; Petersen, S.E.; Raichle, M.E.; Van Essen, D.C.; et al. A Common Network of Functional Areas for Attention and Eye Movements. Neuron 1998, 21, 761–773. [Google Scholar] [CrossRef] [Green Version]
- Grosbras, M.H.; Laird, A.R.; Paus, T. Cortical Regions Involved in Eye Movements, Shifts of Attention, and Gaze Perception. Hum. Brain Mapp. 2005, 25, 140–154. [Google Scholar] [CrossRef]
- De Haan, B.; Morgan, P.S.; Rorden, C. Covert Orienting of Attention and Overt Eye Movements Activate Identical Brain Regions. Brain Res. 2008, 1204, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Li, H.H.; Hanning, N.M.; Carrasco, M. To Look or Not to Look: Dissociating Presaccadic and Covert Spatial Attention. Trends Neurosci. 2021, 44, 669–686. [Google Scholar] [CrossRef]
- Casteau, S.; Smith, D.T. Covert Attention beyond the Range of Eye-Movements: Evidence for a Dissociation between Exogenous and Endogenous Orienting. Cortex 2020, 122, 170–186. [Google Scholar] [CrossRef]
- Kanai, R.; Muggleton, N.; Walsh, V. Transcranial Direct Current Stimulation of the Frontal Eye Fields during Pro- and Antisaccade Tasks. Front. Psychiatry 2012, 3, 45. [Google Scholar] [CrossRef] [Green Version]
- Reteig, L.C.; Knapen, T.; Roelofs, F.J.F.W.; Ridderinkhof, K.R.; Slagter, H.A. No Evidence That Frontal Eye Field TDCS Affects Latency or Accuracy of Prosaccades. Front. Neurosci. 2018, 12, 617. [Google Scholar] [CrossRef] [Green Version]
- Diana, L.; Pilastro, P.; Aiello, E.N.; Eberhard-Moscicka, A.K.; Müri, R.M.; Bolognini, N. Saccades, Attentional Orienting and Disengagement: The Effects of Anodal TDCS over Right Posterior Parietal Cortex (PPC) and Frontal Eye Field (FEF). Eye Track. Res. Appl. Symp. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Cazzoli, D.; Wurtz, P.; Müri, R.M.; Hess, C.W.; Nyffeler, T. Interhemispheric Balance of Overt Attention: A Theta Burst Stimulation Study. Eur. J. Neurosci. 2009, 29, 1271–1276. [Google Scholar] [CrossRef]
- Chiffi, K.; Diana, L.; Hartmann, M.; Cazzoli, D.; Bassetti, C.L.; Müri, R.M.; Eberhard-Moscicka, A.K. Spatial Asymmetries (“Pseudoneglect”) in Free Visual Exploration—Modulation of Age and Relationship to Line Bisection. Exp. Brain Res. 2021, 239, 2693–2700. [Google Scholar] [CrossRef]
- Paladini, R.E.; Wyss, P.; Kaufmann, B.C.; Urwyler, P.; Nef, T.; Cazzoli, D.; Nyffeler, T.; Müri, R.M. Re-Fixation and Perseveration Patterns in Neglect Patients during Free Visual Exploration. Eur. J. Neurosci. 2019, 49, 1244–1253. [Google Scholar] [CrossRef] [Green Version]
- Delazer, M.; Sojer, M.; Ellmerer, P.; Boehme, C.; Benke, T. Eye-Tracking Provides a Sensitive Measure of Exploration Deficits After Acute Right MCA Stroke. Front. Neurol. 2018, 9, 359. [Google Scholar] [CrossRef]
- Kaufmann, B.C.; Cazzoli, D.; Pflugshaupt, T.; Bohlhalter, S.; Vanbellingen, T.; Müri, R.M.; Nef, T.; Nyffeler, T. Eyetracking during Free Visual Exploration Detects Neglect More Reliably than Paper-Pencil Tests. Cortex 2020, 129, 223–235. [Google Scholar] [CrossRef]
- Nuthmann, A.; Matthias, E. Time Course of Pseudoneglect in Scene Viewing. Cortex 2014, 52, 113–119. [Google Scholar] [CrossRef]
- Hartmann, M.; Sommer, N.R.; Diana, L.; Müri, R.M.; Eberhard-Moscicka, A.K. Further to the Right: Viewing Distance Modulates Attentional Asymmetries (‘Pseudoneglect’) during Visual Exploration. Brain Cogn. 2019, 129, 40–48. [Google Scholar] [CrossRef]
- Cazzoli, D.; Jung, S.; Nyffeler, T.; Nef, T.; Wurtz, P.; Mosimann, U.P.; Müri, R.M. The Role of the Right Frontal Eye Field in Overt Visual Attention Deployment as Assessed by Free Visual Exploration. Neuropsychologia 2015, 74, 37–41. [Google Scholar] [CrossRef]
- Brysbaert, M.; Stevens, M. Power Analysis and Effect Size in Mixed Effects Models: A Tutorial. J. Cogn. 2018, 1. [Google Scholar] [CrossRef] [Green Version]
- Oldfield, R.C. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Bikson, M.; Grossman, P.; Thomas, C.; Louis Zannou, A.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef] [Green Version]
- Thair, H.; Holloway, A.L.; Newport, R.; Smith, A.D. Transcranial Direct Current Stimulation (TDCS): A Beginner’s Guide for Design and Implementation. Front. Neurosci. 2017, 11, 641. [Google Scholar] [CrossRef] [Green Version]
- Itti, L.; Koch, C.; Niebur, E. A Model of Saliency-Based Visual Attention for Rapid Scene Analysis. IEEE Trans. Pattern Anal. Mach. Intell. 1998, 20, 1254–1259. [Google Scholar] [CrossRef] [Green Version]
- Koessler, L.; Maillard, L.; Benhadid, A.; Vignal, J.P.; Felblinger, J.; Vespignani, H.; Braun, M. Automated Cortical Projection of EEG Sensors: Anatomical Correlation via the International 10-10 System. Neuroimage 2009, 46, 64–72. [Google Scholar] [CrossRef]
- Kincade, J.M.; Abrams, R.A.; Astafiev, S.V.; Shulman, G.L.; Corbetta, M. An Event-Related Functional Magnetic Resonance Imaging Study of Voluntary and Stimulus-Driven Orienting of Attention. J. Neurosci. 2005, 25, 4593–4604. [Google Scholar] [CrossRef]
- Thielscher, A.; Antunes, A.; Saturnino, G.B. Field Modeling for Transcranial Magnetic Stimulation: A Useful Tool to Understand the Physiological Effects of TMS? In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2015; Volume 2015, pp. 222–225. [Google Scholar] [CrossRef]
- Fertonani, A.; Ferrari, C.; Miniussi, C. What Do You Feel If I Apply Transcranial Electric Stimulation? Safety, Sensations and Secondary Induced Effects. Clin. Neurophysiol. 2015, 126, 2181–2188. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. 2016. Available online: https://ggplot2.tidyverse.org (accessed on 7 November 2021).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares. R Package Version 1.5.0. Means. 2020. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 7 November 2021). [CrossRef]
- Frossard, J.; Renaud, O. Permutation Tests for Regression, ANOVA, and Comparison of Signals: The Permuco Package. 2019. Available online: https://www.jstatsoft.org/article/view/v099i15 (accessed on 7 November 2021).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Salvaggio, S.; Masson, N.; Andres, M. Eye Position Reflects the Spatial Coding of Numbers During Magnitude Comparison. J. Exp. Psychol. Learn. Mem. Cogn. 2019, 45, 1910–1921. [Google Scholar] [CrossRef]
- Clifton, L.; Clifton, D.A. The Correlation between Baseline Score and Post-Intervention Score, and Its Implications for Statistical Analysis. Trials 2019, 20, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Masina, F.; Arcara, G.; Galletti, E.; Cinque, I.; Gamberini, L.; Mapelli, D. Neurophysiological and Behavioural Effects of Conventional and High Definition TDCS. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Maris, E.; Oostenveld, R. Nonparametric Statistical Testing of EEG- and MEG-Data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef]
- Splittgerber, M.; Salvador, R.; Brauer, H.; Breitling-Ziegler, C.; Prehn-Kristensen, A.; Krauel, K.; Nowak, R.; Ruffini, G.; Moliadze, V.; Siniatchkin, M. Individual Baseline Performance and Electrode Montage Impact on the Effects of Anodal TDCS Over the Left Dorsolateral Prefrontal Cortex. Front. Hum. Neurosci. 2020, 14. [Google Scholar] [CrossRef]
- Martin, A.K.; Dzafic, I.; Ramdave, S.; Meinzer, M. Causal Evidence for Task-Specific Involvement of the Dorsomedial Prefrontal Cortex in Human Social Cognition. Soc. Cogn. Affect. Neurosci. 2017, 12, 1209–1218. [Google Scholar] [CrossRef] [Green Version]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.-F.; Nitsche, M.A. Partially Non-Linear Stimulation Intensity-Dependent Effects of Direct Current Stimulation on Motor Cortex Excitability in Humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef]
- Chew, T.; Ho, K.A.; Loo, C.K. Inter- and Intra-Individual Variability in Response to Transcranial Direct Current Stimulation (TDCS) at Varying Current Intensities. Brain Stimul. 2015, 8, 1130–1137. [Google Scholar] [CrossRef]
- Esmaeilpour, Z.; Marangolo, P.; Hampstead, B.M.; Bestmann, S.; Galletta, E.; Knotkova, H.; Bikson, M. Incomplete Evidence That Increasing Current Intensity of TDCS Boosts Outcomes. Brain Stimul. 2018, 11, 310–321. [Google Scholar] [CrossRef]
- Fiori, V.; Nitsche, M.A.; Cucuzza, G.; Caltagirone, C.; Marangolo, P. High-Definition Transcranial Direct Current Stimulation Improves Verb Recovery in Aphasic Patients Depending on Current Intensity. Neuroscience 2019, 406, 159–166. [Google Scholar] [CrossRef]
- Papazova, I.; Strube, W.; Becker, B.; Henning, B.; Schwippel, T.; Fallgatter, A.J.; Padberg, F.; Palm, U.; Falkai, P.; Plewnia, C.; et al. Improving Working Memory in Schizophrenia: Effects of 1 mA and 2 mA Transcranial Direct Current Stimulation to the Left DLPFC. Schizophr. Res. 2018, 202, 203–209. [Google Scholar] [CrossRef]
- Ehrhardt, S.E.; Filmer, H.L.; Wards, Y.; Mattingley, J.B.; Dux, P.E. The Influence of TDCS Intensity on Decision-Making Training and Transfer Outcomes. J. Neurophysiol. 2021, 125, 385–397. [Google Scholar] [CrossRef]
- Pisoni, A.; Mattavelli, G.; Papagno, C.; Rosanova, M.; Casali, A.G.; Romero Lauro, L.J. Cognitive Enhancement Induced by Anodal TDCS Drives Circuit-Specific Cortical Plasticity. Cereb. Cortex 2018, 28, 1132–1140. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.T.; Rogasch, N.C.; Fitzgerald, P.B.; Hoy, K.E. Effects of Single versus Dual-Site High-Definition Transcranial Direct Current Stimulation (HD-TDCS) on Cortical Reactivity and Working Memory Performance in Healthy Subjects. Brain Stimul. 2018, 11, 1033–1043. [Google Scholar] [CrossRef]
- Hill, A.T.; Rogasch, N.C.; Fitzgerald, P.B.; Hoy, K.E. Impact of Concurrent Task Performance on Transcranial Direct Current Stimulation (TDCS)-Induced Changes in Cortical Physiology and Working Memory. Cortex 2019, 113, 37–57. [Google Scholar] [CrossRef]
- Li, L.M.; Violante, I.R.; Leech, R.; Ross, E.; Hampshire, A.; Opitz, A.; Rothwell, J.C.; Carmichael, D.W.; Sharp, D.J. Brain State and Polarity Dependent Modulation of Brain Networks by Transcranial Direct Current Stimulation. Hum. Brain Mapp. 2019, 40, 904–915. [Google Scholar] [CrossRef] [Green Version]
- Salazar, A.P.S.; Vaz, P.G.; Marchese, R.R.; Stein, C.; Pinto, C.; Pagnussat, A.S. Noninvasive Brain Stimulation Improves Hemispatial Neglect After Stroke: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2018, 99, 355–366.e1. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diana, L.; Scotti, G.; Aiello, E.N.; Pilastro, P.; Eberhard-Moscicka, A.K.; Müri, R.M.; Bolognini, N. Conventional and HD-tDCS May (or May Not) Modulate Overt Attentional Orienting: An Integrated Spatio-Temporal Approach and Methodological Reflections. Brain Sci. 2022, 12, 71. https://doi.org/10.3390/brainsci12010071
Diana L, Scotti G, Aiello EN, Pilastro P, Eberhard-Moscicka AK, Müri RM, Bolognini N. Conventional and HD-tDCS May (or May Not) Modulate Overt Attentional Orienting: An Integrated Spatio-Temporal Approach and Methodological Reflections. Brain Sciences. 2022; 12(1):71. https://doi.org/10.3390/brainsci12010071
Chicago/Turabian StyleDiana, Lorenzo, Giulia Scotti, Edoardo N. Aiello, Patrick Pilastro, Aleksandra K. Eberhard-Moscicka, René M. Müri, and Nadia Bolognini. 2022. "Conventional and HD-tDCS May (or May Not) Modulate Overt Attentional Orienting: An Integrated Spatio-Temporal Approach and Methodological Reflections" Brain Sciences 12, no. 1: 71. https://doi.org/10.3390/brainsci12010071
APA StyleDiana, L., Scotti, G., Aiello, E. N., Pilastro, P., Eberhard-Moscicka, A. K., Müri, R. M., & Bolognini, N. (2022). Conventional and HD-tDCS May (or May Not) Modulate Overt Attentional Orienting: An Integrated Spatio-Temporal Approach and Methodological Reflections. Brain Sciences, 12(1), 71. https://doi.org/10.3390/brainsci12010071






