The Facial Skin Blood Flow Change of Stroke Patients with Facial Paralysis after Peripheral Magnetic Stimulation: A Pilot Study
Abstract
:1. Introduction
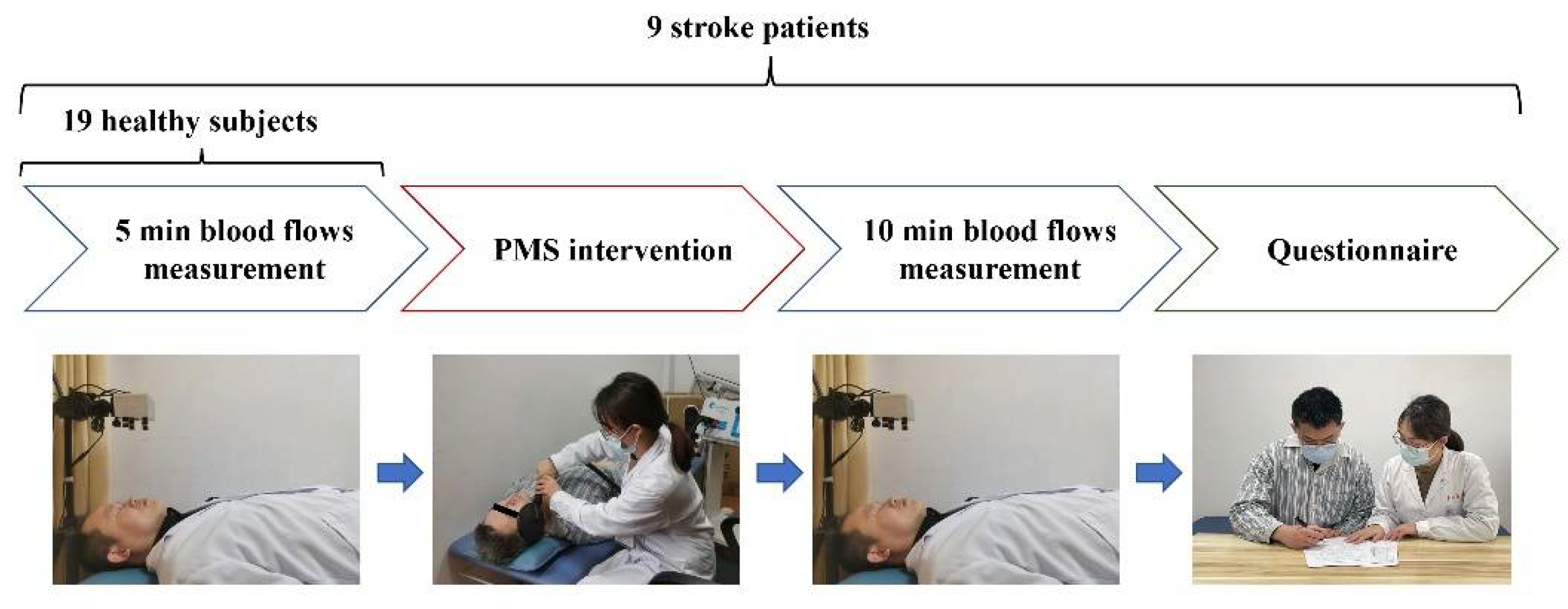
2. Materials and Methods
2.1. Patients Recruitment
2.2. Measurement of Facial Skin Blood Flow
2.3. PMS Intervention
2.4. The Facial Regions of Interest (ROIs)
2.5. Safety Section
2.6. Statistics
3. Results
3.1. Left and Right Facial Skin Blood Flow of Healthy and Stroke with FP
3.2. Left and Right Facial Skin Blood Flow before and after PMS in Stroke with FP
3.3. The Change of Facial Skin Blood Flow before and after PMS in Stroke with FP
3.4. Safety Section
4. Discussion
4.1. Facial Blood Flow on Both Sides of the Participants
4.2. The Effects of PMS on Facial Skin Blood Flow
4.3. The Acceptance of the Facial PMS Intervention
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yew, K.S.; Cheng, E.M. Diagnosis of acute stroke. Am. Fam. Physician 2015, 91, 528–536. [Google Scholar] [PubMed]
- Choi, J.B. Effect of neuromuscular electrical stimulation on facial muscle strength and oral function in stroke patients with facial palsy. J. Phys. Ther. Sci. 2016, 28, 2541–2543. [Google Scholar] [CrossRef]
- Hägg, M.; Tibbling, L. Four-quadrant facial function in dysphagic patients after stroke and in healthy controls. Neurol. Res. Int. 2014, 2014, 672685. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.; Sanches, E.E.; Chaiet, S.R.; de Jongh, F.W.; Beurskens, C.; Monstrey, S.J.; Luijmes, R.E.; Siemann, I.; Ramnarain, D.; Marres, H.; et al. Association between duration of peripheral facial palsy, severity, and age of the patient, and psychological distress. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 3048–3054. [Google Scholar] [CrossRef]
- Coulson, S.E.; O’Dwyer, N.J.; Adams, R.D.; Croxson, G.R. Expression of emotion and quality of life after facial nerve paralysis. Otol. Neurotol. 2004, 25, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.J.; Valbuza, J.S.; Prado, G.F. Physical therapy for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst. Rev. 2011, CD006283. [Google Scholar] [CrossRef]
- Kang, J.; Chun, M.H.; Choi, S.J.; Chang, M.C.; Yi, Y.G. Effects of Mirror Therapy Using a Tablet PC on Central Facial Paresis in Stroke Patients. Ann. Rehabil. Med. 2017, 41, 347. [Google Scholar] [CrossRef]
- Liang, L.; Qiang, F. Observation on the Clinical Effect of Acupuncture and Moxibustion Combined with Repeated Transcranial Magnetic Stimulation on Facial Paralysis. Comput. Math. Methods Med. 2021, 2021, 9642677. [Google Scholar] [CrossRef]
- Yildiz, N.; Yildiz, S.; Ertekin, C.; Aydoğdu, I.; Uludag, B. Changes in the perioral muscle responses to cortical TMS induced by decrease of sensory input and electrical stimulation to lower facial region. Clin. Neurophysiol. 2004, 115, 2343–2349. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Jiao, L.; Wang, H.; Li, J.; Zhong, G.; Zhu, D.; Xu, W.; Jin, M. The efficacy and safety of cupping therapy for treating of intractable peripheral facial paralysis: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e25388. [Google Scholar] [CrossRef]
- Azuma, T.; Nakamura, K.; Takahashi, M.; Ohyama, S.; Toda, N.; Iwasaki, H.; Kalubi, B.; Takeda, N. Mirror biofeedback rehabilitation after administration of single-dose botulinum toxin for treatment of facial synkinesis. Otolaryngol. Head Neck Surg. 2012, 146, 40–45. [Google Scholar] [CrossRef]
- Vanswearingen, J.M.; Brach, J.S. Changes in facial movement and synkinesis with facial neuromuscular reeducation. Plast Reconstr. Surg. 2003, 111, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, M.; Gaffurini, P.; Vacchi, L.; Lazzarini, S.; Villafane, J.; Orizio, C.; Negrini, S.; Bissolotti, L. Hand Passive Mobilization Performed with Robotic Assistance: Acute Effects on Upper Limb Perfusion and Spasticity in Stroke Survivors. Biomed Res. Int. 2017, 2017, 2796815. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, S.; Takahashi, R. Repetitive peripheral magnetic stimulation improves severe upper limb paresis in early acute phase stroke survivors. Neurorehabilitation 2020, 46, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Grefkes, C.; Fink, G.R. Recovery from stroke: Current concepts and future perspectives. Neurol. Res. Pract. 2020, 2, 17. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, S.B.; Lee, J.H.; Lee, S.J.; Park, J.G.; Jang, K.W. Effects of Neuromuscular Electrical Stimulation for Masseter Muscle on Oral Dysfunction After Stroke. Ann. Rehabil. Med. 2019, 43, 11–18. [Google Scholar] [CrossRef]
- Oh, D.H.; Park, J.S.; Kim, W.J. Effect of neuromuscular electrical stimulation on lip strength and closure function in patients with dysphagia after stroke. J. Phys. Ther. Sci. 2017, 29, 1974–1975. [Google Scholar] [CrossRef]
- Bampouras, T.M.; Reeves, N.D.; Baltzopoulos, V.; Maganaris, C.N. Muscle activation assessment: Effects of method, stimulus number, and joint angle. Muscle Nerve 2006, 34, 740–746. [Google Scholar] [CrossRef]
- Man, W.D.; Moxham, J.; Polkey, M.I. Magnetic stimulation for the measurement of respiratory and skeletal muscle function. Eur. Respir. J. 2004, 24, 846–860. [Google Scholar] [CrossRef]
- Lampropoulou, S.I.; Nowicky, A.V.; Marston, L. Magnetic versus electrical stimulation in the interpolation twitch technique of elbow flexors. J. Sports Sci. Med. 2012, 11, 709–718. [Google Scholar]
- Krewer, C.; Hartl, S.; Müller, F.; Koenig, E. Effects of Repetitive Peripheral Magnetic Stimulation on Upper-Limb Spasticity and Impairment in Patients with Spastic Hemiparesis: A Randomized, Double-Blind, Sham-Controlled Study. Arch. Phys. Med. Rehabil. 2014, 95, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Abe, G.; Oyama, H.; Liao, Z.; Honda, K.; Yashima, K.; Asao, A.; Izumi, S. Difference in Pain and Discomfort of Comparable Wrist Movements Induced by Magnetic or Electrical Stimulation for Peripheral Nerves in the Dorsal Forearm. Med. Devices 2020, 13, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Flamand, V.H.; Schneider, C. Noninvasive and painless magnetic stimulation of nerves improved brain motor function and mobility in a cerebral palsy case. Arch. Phys. Med. Rehabil. 2014, 95, 1984–1990. [Google Scholar] [CrossRef]
- Beaulieu, L.D.; Schneider, C. Effects of repetitive peripheral magnetic stimulation on normal or impaired motor control—A review. Neurophysiol. Clin. 2013, 43, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Flamand, V.H.; Beaulieu, L.D.; Nadeau, L.; Schneider, C. Peripheral magnetic stimulation to decrease spasticity in cerebral palsy. Pediatr. Neurol. 2012, 47, 345–348. [Google Scholar] [CrossRef]
- Gallasch, E.; Christova, M.; Kunz, A.; Rafolt, D.; Golaszewski, S. Modulation of sensorimotor cortex by repetitive peripheral magnetic stimulation. Front. Hum. Neurosci. 2015, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Struppler, A.; Binkofski, F.; Angerer, B.; Bernhardt, M.; Spiegel, S.; Drzezga, A.; Bartenstein, P. A fronto-parietal network is mediating improvement of motor function related to repetitive peripheral magnetic stimulation: A PET-H2O15 study. Neuroimage 2007, 36, T174–T186. [Google Scholar] [CrossRef]
- Beaulieu, L.D.; Massé-Alarie, H.; Camiré-Bernier, S.; Ribot-Ciscar, É.; Schneider, C. After-effects of peripheral neurostimulation on brain plasticity and ankle function in chronic stroke: The role of afferents recruited. Neurophysiol. Clin. 2017, 47, 275–291. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, D.; Zhang, J.; Hai, H.; Zhao, Y.; Ma, Y. A Randomized Controlled Trial of Repetitive Peripheral Magnetic Stimulation applied in Early Subacute Stroke: Effects on Severe Upper-limb Impairment. Clin. Rehabil. 2022, 36, 693–702. [Google Scholar] [CrossRef]
- Miller, T.; Qin, L.; Hung, V.W.Y.; Ying, M.T.C.; Tsang, C.S.L.; Ouyang, H.; Chung, R.C.K.; Pang, M.Y.C. Gait speed and spasticity are independently associated with estimated failure load in the distal tibia after stroke: An HR-pQCT study. Osteoporosis. Int. 2022, 33, 713–724. [Google Scholar] [CrossRef]
- Huang, M.; Miller, T.; Ying, M.; Pang, M.Y.C. Whole-body vibration modulates leg muscle reflex and blood perfusion among people with chronic stroke: A randomized controlled crossover trial. Sci. Rep. 2020, 10, 1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durand, M.J.; Murphy, S.A.; Schaefer, K.K.; Hunter, S.K.; Schmit, B.D.; Gutterman, D.D.; Hyngstrom, A.S. Impaired Hyperemic Response to Exercise Post Stroke. PLoS ONE 2015, 10, e0144023. [Google Scholar] [CrossRef]
- Tiftik, T.; Kara, M.; özcan, H.N.; Türkkan, C.; Ural, F.G.; Ekiz, T.; Akkuş, S.; özçakar, L. Doppler ultrasonographic evaluation of the radial and ulnar arteries in hemiparetic patients after stroke. J. Clin. Ultrasound 2014, 42, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Lee, C.; Kang, H.G.; Kim, K.W.; Kim, M.; Jeong, H.; Shin, B. Peripheral vasoreactivity in acute ischemic stroke with hemiplegia. Sci. Rep. 2021, 11, 8531. [Google Scholar] [CrossRef] [PubMed]
- Briers, J.D. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiol. Meas. 2001, 22, R35–R66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ding, L.; Li, M.; Zhang, X.; Su, D.; Jia, J.; Miao, P. Dual-Wavelength Laser Speckle Contrast Imaging (dwLSCI) Improves Chronic Measurement of Superficial Blood Flow in Hands. Sensors 2017, 17, 2811. [Google Scholar] [CrossRef]
- Mahé, G.; Humeau-Heurtier, A.; Durand, S.; Leftheriotis, G.; Abraham, P. Assessment of skin microvascular function and dysfunction with laser speckle contrast imaging. Circ. Cardiovasc. Imaging 2012, 5, 155–163. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Li, J.; Wang, J.; Yu, H. Microcirculation evaluation of facial nerve palsy using laser speckle contrast imaging: A prospective study. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 685–692. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Luan, J. Facial Paralysis Detection in Infrared Thermal Images Using Asymmetry Analysis of Temperature and Texture Features. Diagnostics 2021, 11, 2309. [Google Scholar] [CrossRef]
- Kashima, H.; Ikemura, T.; Hayashi, N. Regional differences in facial skin blood flow responses to the cold pressor and static handgrip tests. Eur. J. Appl. Physiol. 2013, 113, 1035–1041. [Google Scholar] [CrossRef]
- Wang, S.Y.; Qu, X.X.; Song, X.J.; Li, S.Y.; Ma, H.M.; Zhang, D. Blood perfusion in different facial acupoint areas and its changes after acupuncture stimulation of Hegu (LI 4) displayed by laser Doppler imager in healthy volunteers. Zhen Ci Yan Jiu 2012, 37, 482–487. [Google Scholar] [PubMed]
- Billinger, S.A.; Kluding, P.M. Use of Doppler Ultrasound to Assess Femoral Artery Adaptations in the Hemiparetic Limb in People with Stroke. Cerebrovasc. Dis. 2009, 27, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Durand, M.; Negro, F.; Farina, D.; Hunter, S.; Schmit, B.; Gutterman, D.; Hyngstrom, A. The Relationship Between Blood Flow and Motor Unit Firing Rates in Response to Fatiguing Exercise Post-stroke. Front. Physiol. 2019, 10, 545. [Google Scholar] [CrossRef]
- Yonezawa, T.; Nomura, K.; Ichimura, S.; Mizoguchi, H.; Takemura, H. Blood Flow-Promoting Effect of Parallel Link Type Human Ankle Rehabilitation Assistive Device—Influence of Difference in Ankle Exercise on Blood Flow in the Lower Limb—. Trans. Jpn. Soc. Med. Biol. Eng. 2016, 54, 49–57. [Google Scholar]
- Khedr, E.M.; Ahmed, M.A.; Alkady, E.A.M.; Mostafa, M.G.; Said, H.G. Therapeutic effects of peripheral magnetic stimulation on traumatic brachial plexopathy: Clinical and neurophysiological study. Neurophysiol. Clin. 2012, 42, 111–118. [Google Scholar] [CrossRef]
- Beaulieu, L.D.; Schneider, C. Repetitive peripheral magnetic stimulation to reduce pain or improve sensorimotor impairments: A literature review on parameters of application and afferents recruitment. Neurophysiol. Clin. 2015, 45, 223–237. [Google Scholar] [CrossRef]
- Chipchase, L.S.; Schabrun, S.M.; Hodges, P.W. Peripheral electrical stimulation to induce cortical plasticity: A systematic review of stimulus parameters. Clin. Neurophysiol. 2011, 122, 456–463. [Google Scholar] [CrossRef]
- Quandt, F.; Hummel, F.C. The influence of functional electrical stimulation on hand motor recovery in stroke patients: A review. Exp. Transl. Stroke Med. 2014, 6, 9. [Google Scholar] [CrossRef]
- Kashima, H.; Hamada, Y.; Hayashi, N. Palatability of Tastes Is Associated with Facial Circulatory Responses. Chem. Senses 2014, 39, 243–248. [Google Scholar] [CrossRef]
- Miyaji, A.; Hayashi, S.; Hayashi, N. Regional differences in facial skin blood flow responses to thermal stimulation. Eur. J. Appl. Physiol. 2019, 119, 1195–1201. [Google Scholar] [CrossRef]



| Patients | Sex | Age (y) | Type of Injury | Affected Face | Time Post-Stroke (Month) | House-Brackmann |
|---|---|---|---|---|---|---|
| S1 | Male | 46–50 | hemorrhage | Right | 11 | IV |
| S2 | Male | 66–70 | ischemia | Right | 4 | III |
| S3 | Male | 51–55 | ischemia | Right | 4 | II |
| S4 | Male | 46–50 | hemorrhage | Left | 52 | IV |
| S5 | Female | 66–70 | ischemia | Left | 6 | III |
| S6 | Female | 71–75 | hemorrhage | Right | 2 | II |
| S7 | Female | 66–70 | ischemia | Right | 1 | II |
| S8 | Female | 51–55 | hemorrhage | Right | 105 | II |
| S9 | Male | 71–75 | ischemia | Left | 5 | IV |
| Healthy Subjects (n = 19) | Stroke Patients (n = 9) Pre-Intervention | |||||
|---|---|---|---|---|---|---|
| Region | Left | Right | p Value | Affected | Unaffected | p Value |
| 1 | 151.00 | 151.36 | 0.947 | 145.89 | 156.43 | 0.139 |
| 2 | 203.18 | 201.86 | 0.659 | 200.52 | 206.09 | 0.378 |
| 3 | 209.23 | 215.95 | 0.142 | 181.84 | 199.50 | 0.286 |
| 4 | 233.10 | 230.75 | 0.616 | 235.45 | 240.20 | 0.192 |
| 5 | 191.90 | 191.16 | 0.931 | 134.45 | 166.05 | 0.181 |
| 6 | 237.20 | 236.17 | 0.639 | 225.00 | 237.11 | 0.130 |
| 7 | 232.16 | 238.53 | 0.099 | 219.32 | 231.56 | 0.046 |
| half face | 197.44 | 198.14 | 0.695 | 193.60 | 201.30 | 0.289 |
| Pre_0–5 min (P1) | Post_0–5 min (P2) | Post_6–10 min (P3) | Post_0–10 min (P4) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Affected | Unaffected | p Value | Affected | Unaffected | p Value | Affected | Unaffected | p Value | Affected | Unaffected | p Value |
| 1 | 145.89 | 156.43 | 0.139 | 151.87 | 153.96 | 0.665 | 155.63 | 160.35 | 0.268 | 154.01 | 156.81 | 0.501 |
| 2 | 200.52 | 206.10 | 0.378 | 209.56 | 205.15 | 0.079 | 208.71 | 208.44 | 0.959 | 209.21 | 206.54 | 0.435 |
| 3 | 181.84 | 199.50 | 0.286 | 203.11 | 198.85 | 0.581 | 200.96 | 205.54 | 0.783 | 202.48 | 201.86 | 0.958 |
| 4 | 235.45 | 240.20 | 0.192 | 238.36 | 239.37 | 0.496 | 241.08 | 239.47 | 0.284 | 239.71 | 239.41 | 0.820 |
| 5 | 134.45 | 166.05 | 0.181 | 181.21 | 169.77 | 0.457 | 182.27 | 174.71 | 0.633 | 182.72 | 172.68 | 0.509 |
| 6 | 225.00 | 237.11 | 0.130 | 238.16 | 228.50 | 0.054 | 242.69 | 236.06 | 0.124 | 240.61 | 232.49 | 0.064 |
| 7 | 219.32 | 231.55 | 0.046 | 230.06 | 221.67 | 0.116 | 234.33 | 226.93 | 0.170 | 232.53 | 224.50 | 0.107 |
| half face | 193.60 | 201.30 | 0.289 | 203.62 | 200.51 | 0.306 | 205.58 | 205.13 | 0.919 | 204.81 | 202.66 | 0.510 |
| Affected Side | Unaffected Side | |||||
|---|---|---|---|---|---|---|
| Region\Time | P1-P2 | P1-P3 | P1-P4 | P1-P2 | P1-P3 | P1-P4 |
| 1 | 0.483 | 0.289 | 0.362 | 0.579 | 0.357 | 0.929 |
| 2 | 0.150 | 0.195 | 0.152 | 0.823 | 0.599 | 0.921 |
| 3 | 0.088 | 0.080 | 0.072 | 0.937 | 0.538 | 0.792 |
| 4 | 0.273 | 0.067 | 0.115 | 0.773 | 0.833 | 0.800 |
| 5 | 0.014 | 0.009 | 0.010 | 0.778 | 0.533 | 0.617 |
| 6 | 0.069 | 0.021 | 0.035 | 0.173 | 0.832 | 0.388 |
| 7 | 0.046 | 0.023 | 0.023 | 0.107 | 0.403 | 0.197 |
| half face | 0.126 | 0.051 | 0.077 | 0.838 | 0.395 | 0.744 |
| Subcomponent (Description) | Scores (Mean ± SD) |
|---|---|
| Tolerance (I accepted the treatment and stick to finish it until the end easily for me) | 6.33 ± 0.71 |
| Comfort (The treatment process is comfortable) | 5.11 ± 1.36 |
| Preference (If the treatment effects are the same, I would prefer peripheral magnetic stimulation to other facial treatments) | 6.56 ± 0.73 |
| Painful (During the treatment, I felt pain in the treated area) | 1.44 ± 1.33 |
| Numbness (During the treatment, I felt numbness) | 4.11 ± 2.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, S.; Ruan, Y.; Lin, J.; Li, C.; Li, C.; Xu, S.; Yan, Z.; Liu, X.; Miao, P.; et al. The Facial Skin Blood Flow Change of Stroke Patients with Facial Paralysis after Peripheral Magnetic Stimulation: A Pilot Study. Brain Sci. 2022, 12, 1271. https://doi.org/10.3390/brainsci12101271
Zhang Y, Chen S, Ruan Y, Lin J, Li C, Li C, Xu S, Yan Z, Liu X, Miao P, et al. The Facial Skin Blood Flow Change of Stroke Patients with Facial Paralysis after Peripheral Magnetic Stimulation: A Pilot Study. Brain Sciences. 2022; 12(10):1271. https://doi.org/10.3390/brainsci12101271
Chicago/Turabian StyleZhang, Yongli, Shugeng Chen, Yinglu Ruan, Jiaying Lin, Chengdong Li, Chong Li, Shuo Xu, Zhijie Yan, Xiangyun Liu, Peng Miao, and et al. 2022. "The Facial Skin Blood Flow Change of Stroke Patients with Facial Paralysis after Peripheral Magnetic Stimulation: A Pilot Study" Brain Sciences 12, no. 10: 1271. https://doi.org/10.3390/brainsci12101271
APA StyleZhang, Y., Chen, S., Ruan, Y., Lin, J., Li, C., Li, C., Xu, S., Yan, Z., Liu, X., Miao, P., & Jia, J. (2022). The Facial Skin Blood Flow Change of Stroke Patients with Facial Paralysis after Peripheral Magnetic Stimulation: A Pilot Study. Brain Sciences, 12(10), 1271. https://doi.org/10.3390/brainsci12101271






