The Transcranial Light Therapy Improves Synaptic Plasticity in the Alzheimer’s Disease Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. TLTC Device
- Box: An acrylic box measuring 8 × 4.8 × 4.8 cm, with an opening at the top (2.3 cm2) was designed to reduce the stress factor in mice and to ensure transcranial irradiation and attenuation of light to other regions of the animal’s body.
- Light module: The module is based on the LSMCC-x4X3-LP single-color LED module. The module contains four high-power 5050SMD LEDs. Each LED has a viewing angle of 120 degrees, a wavelength of 630 nm, and 1 W of power. The coverage area of the module is 2.3 cm2. The module is powered by 12 volts and is controlled by a relay in the control unit. The light module is mounted on the top of the box.
- Control unit: The unit contains an electronic board with an ATmega328 microcontroller, a push button, a relay, and additional supporting components (voltage regulators, discrete transistors, connectors, etc.). When the push button is pressed, the microcontroller turns on the relay for 125 s, turning on the light module.
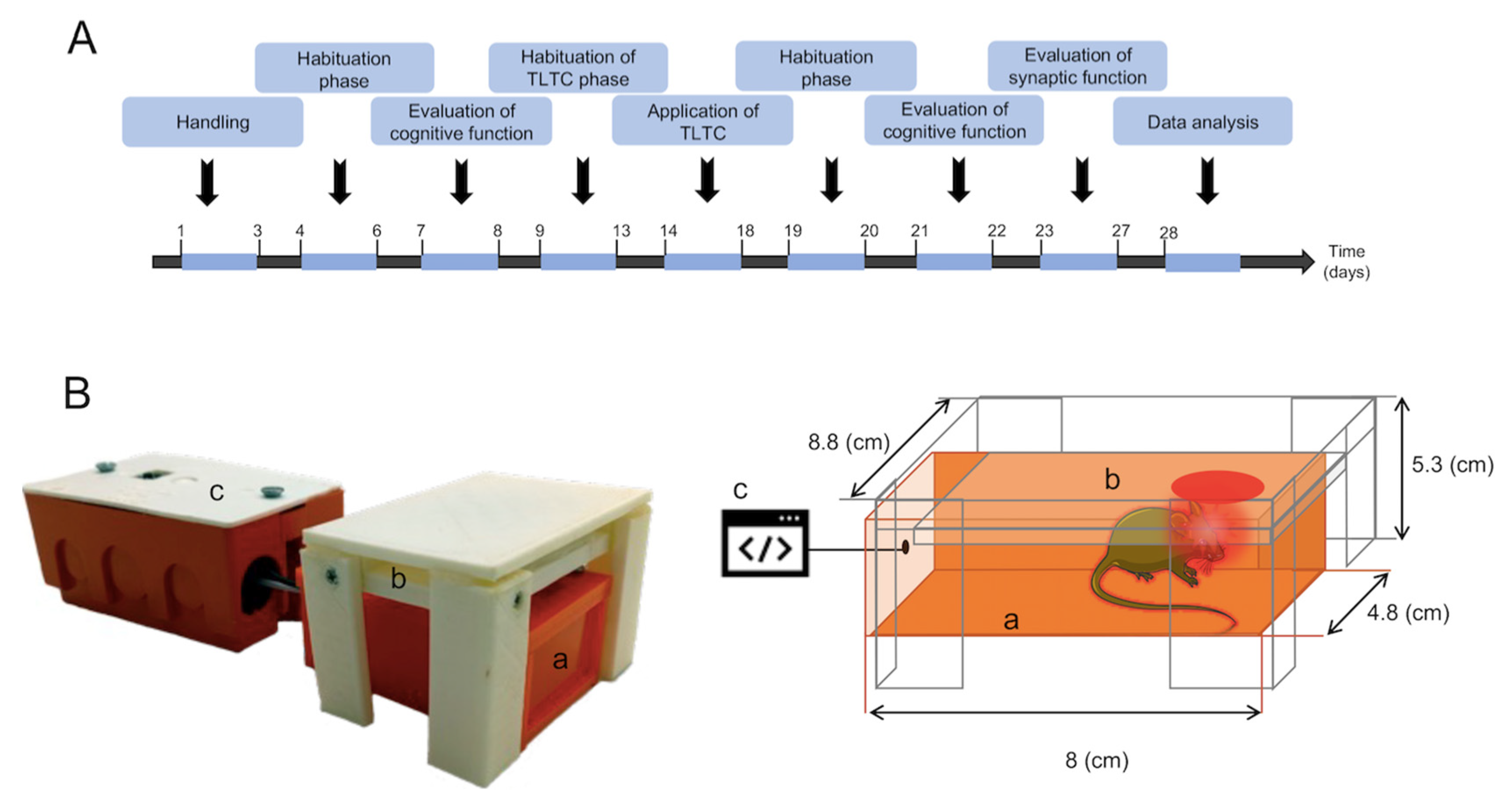
2.3. Experimental Design
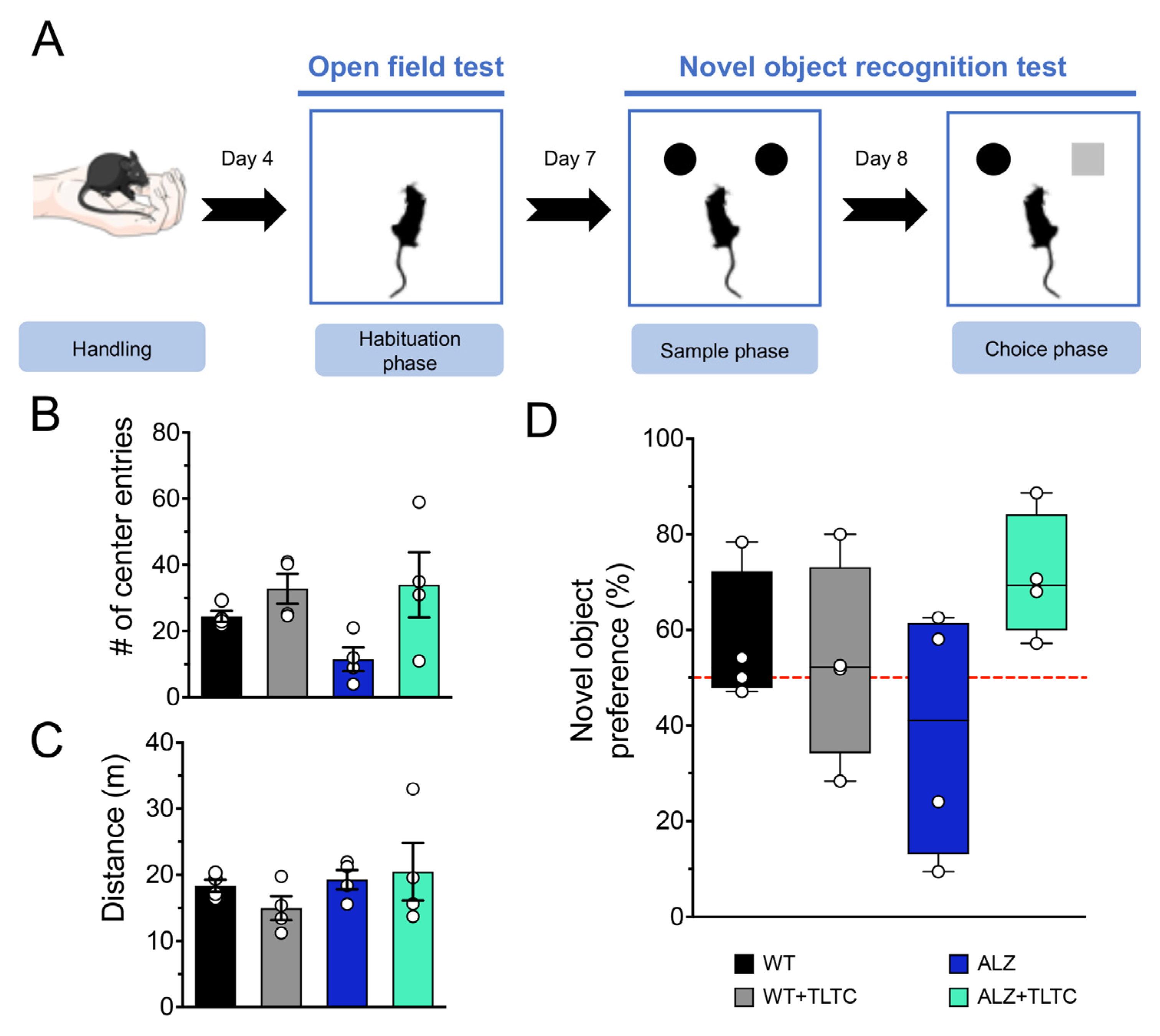
2.3.1. Novel Object Recognition (NOR) Task
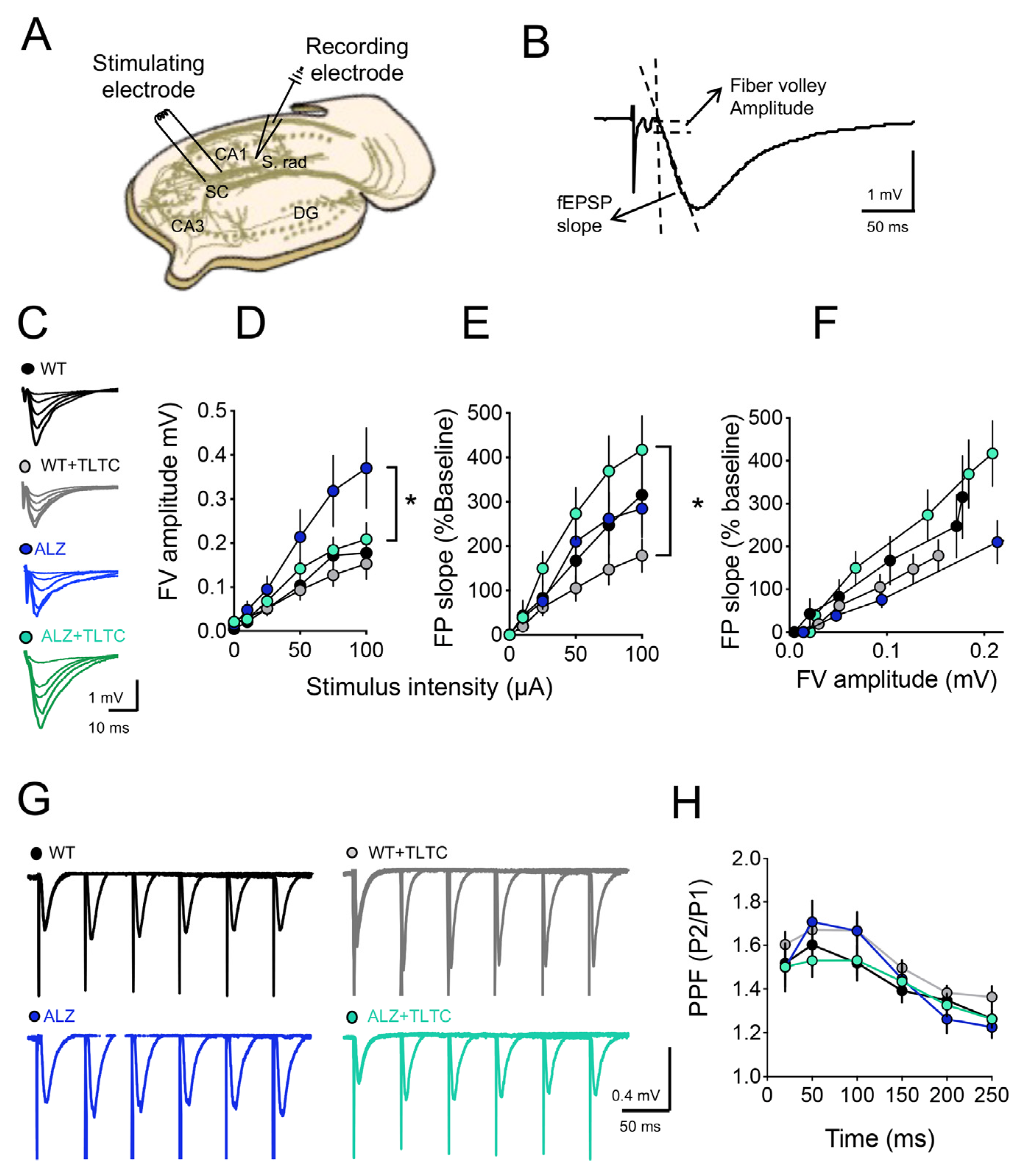
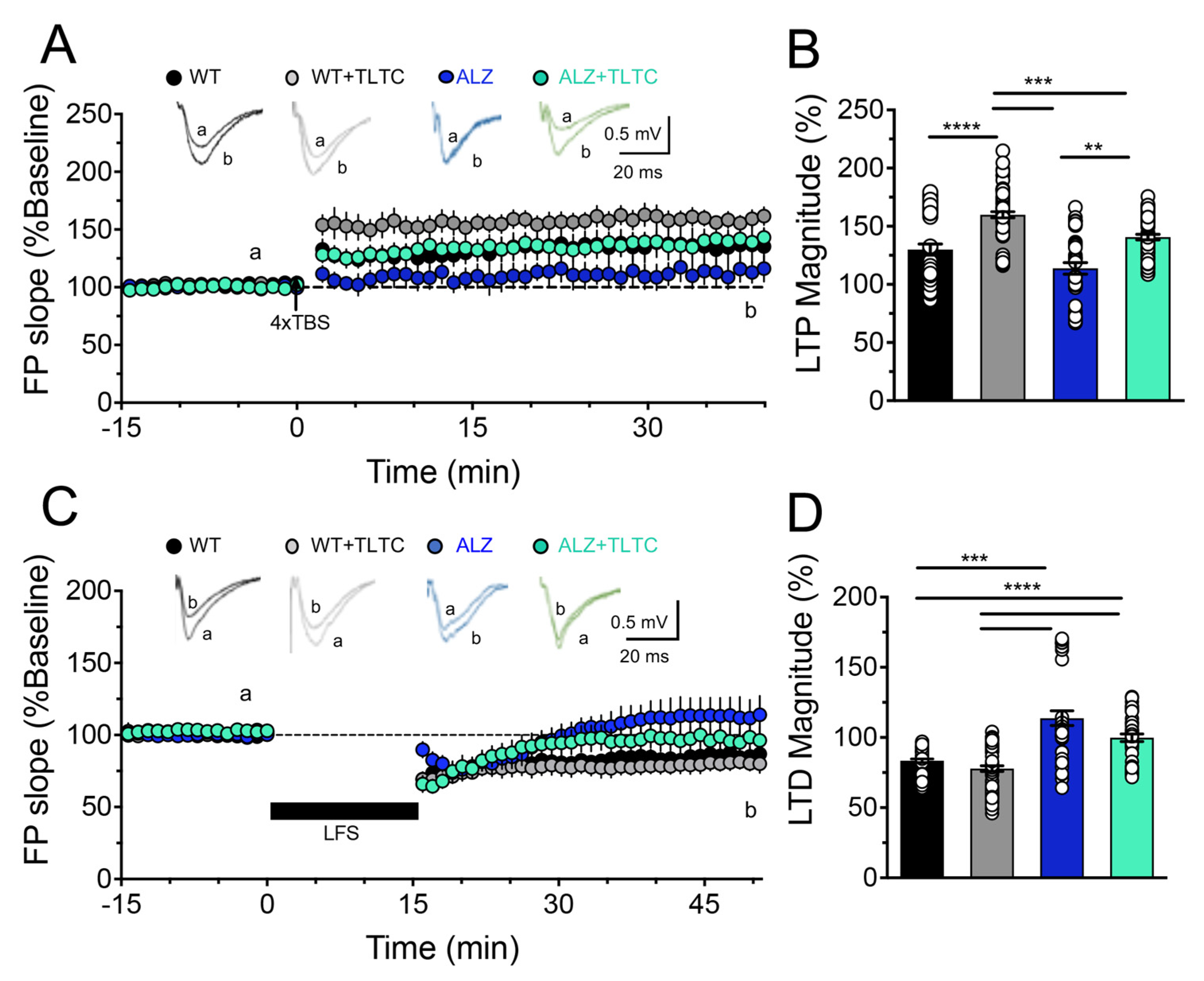
2.3.2. Synaptic Plasticity
2.4. Statistical Analysis
3. Results
3.1. TLTC Shows No Significant Changes in Recognition Memory in Alzheimer’s Disease Model Mice
3.2. TLTC Fully Rescues LTP and Partially Restores LTD in Alzheimer’s Disease Model Mice
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaz, M.; Silvestre, S. Alzheimer’s disease: Recent treatment strategies. Eur. J. Pharmacol. 2020, 887, 173554. [Google Scholar] [CrossRef] [PubMed]
- Haines, J.L. Alzheimer disease: Perspectives from epidemiology and genetics. JLME 2018, 46, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Álvarez, A.; Guillén, F.; Aguinaga, I. Prevalence and incidence of alzheimer’s disease in europe: A meta-analysis. Neurología 2017, 32, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Custodio, N.; Wheelock, A.; Thumala, D.; Slachevsky, A. Dementia in latin america: Epidemiological evidence and implications for public policy. Front. Aging Neurosci. 2017, 9, 221. [Google Scholar] [CrossRef]
- Mielke, M.; Vemuri, P.; Rocca, W. Clinical epidemiology of alzheimer’s disease: Assessing sex and gender differences. Clin. Epidemiol. 2014, 6, 37–48. [Google Scholar] [CrossRef]
- Abner, E.; Nelson, P.; Kryscio, R.; Schmitt, F.; Fardo, D.; Woltjer, R.; Cairns, N.; Yu, L.; Dodge, H.; Xiong, C.; et al. Diabetes is associated with cerebrovascular but not alzheimer’s disease neuropathology. J. Alzheimer’s Dis. 2017, 60, 1035–1043. [Google Scholar] [CrossRef]
- Frigerio, S.; Wolfs, L.; Fattorelli, N.; Thrupp, N.; Voytyuk, I.; Schmidt, I.; Mancuso, R.; Chen, W.; Woodbury, M.; Srivastava, G.; et al. The major risk factors for alzheimer’s disease: Age, sex, and genes modulate the microglia response to a plaques. Cell Rep. 2019, 27, 1293–1306. [Google Scholar] [CrossRef]
- Gottesman, R.; Schneider, A.; Zhou, Y.; Coresh, J.; Green, E.; Gupta, N.; Knopman, D.; Mintz, A.; Rahmim, A.; Sharrett, A.; et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017, 317, 1443–1450. [Google Scholar] [CrossRef]
- Crous, M.; Minguillon, C.; Gramunt, N.; Molinuevo, J. Alzheimer’s disease prevention: From risk factors to early intervention. Alzheimer’s Res. Ther. 2017, 9, 71. [Google Scholar] [CrossRef]
- Jack, C.; Bennett, D.; Blennow, K.; Carrillo, M.; Dunn, B.; Haeberlein, S.; Holtzman, D.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Jicha, G.; Carr, S. Conceptual evolution in Alzheimer’s disease: Implications for understanding the clinical phenotype of progressive neurodegenerative disease. Alzheimer’s Dis. 2010, 19, 252–272. [Google Scholar] [CrossRef] [PubMed]
- Sarah, E.; Charles, M.; Jason, H.; Catherine, M.; Nigel, J.; John, C.; Walter, K. Neuropsychological changes in asymptomatic persons with Alzheimer disease neuropathology. Neurology 2014, 83, 434–440. [Google Scholar]
- Villemagne, V.; Pike, K.; Chételat, G.; Ellis, K.; Mulligan, R.; Bourgeat, P.; Rowe, C. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann. Neurol. 2011, 69, 181–192. [Google Scholar] [CrossRef]
- Villemagne, V.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.; Salvado, O.; Masters, C. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013, 12, 357–367. [Google Scholar] [CrossRef]
- Long, J.; Holtzman, D. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Battaglia, S.; Fabius, J.; Moravkova, K.; Fracasso, A.; Borgomaneri, S. The Neurobiological Correlates of Gaze Perception in Healthy Individuals and Neurologic Patients. Biomedicines 2022, 10, 627. [Google Scholar] [CrossRef]
- Nam, U.; Lee, K.; Ko, H.; Lee, J.; Lee, E. Analyzing Facial and Eye Movements to Screen for Alzheimer’s Disease. Sensors 2020, 20, 5349. [Google Scholar] [CrossRef]
- Serrano, A.; Qian, J.; Monsell, S.; Blacker, D.; Gómez, T.; Betensky, R.; Hyman, B. Mild to moderate Alzheimer dementia with insufficient neuropathological changes. Ann. Neurol. 2014, 75, 597–601. [Google Scholar] [CrossRef]
- Török, N.; Tanaka, M.; Vécsei, L. Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. Int. J. Mol. Sci. 2020, 21, 9338. [Google Scholar] [CrossRef]
- Jevtic, S.; Sengar, A.; Salter, M.; McLaurin, J. The role of the immune system in alzheimer disease: Etiology and treatment. Ageing Res. Rev. 2017, 40, 84–94. [Google Scholar] [CrossRef]
- Yiannopoulou, K.; Papageorgiou, S. Current and future treatments in alzheimer disease: An update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Fu, A.; Ip, N. Synaptic dysfunction in alzheimer’s disease: Mechanisms and therapeutic strategies. Pharmacol. Ther. 2019, 195, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wei, Q.; Liu, F.; Hu, F.; Xie, A.; Zhu, L.; Liu, D. Synaptic dysfunction in alzheimer’s disease: Aβ, tau, and epigenetic alterations. Mol. Neurobiol. 2018, 55, 3021–3032. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline: 2019. Alzheimer’s Dement. 2019, 5, 272–293. [Google Scholar] [CrossRef]
- Miziak, B.; Błaszczyk, B.; Czuczwar, S.J. Some Candidate Drugs for Pharmacotherapy of Alzheimer’s Disease. Pharmaceuticals 2021, 14, 458. [Google Scholar] [CrossRef]
- Knopman, D.; Jones, D.; Greicius, M. Failure to demonstrate e cacy of aducanumab: An analysis of the emerge and engage trials as reported by biogen, december 2019. Alzheimer’s Dement. 2021, 17, 696–701. [Google Scholar] [CrossRef]
- Ramezani, F.; Neshasteh, A.; Ghadaksaz, A.; Fazeli, S.M.; Janzadeh, A.; Hamblin, M. Mechanistic aspects of photobiomodulation therapy in the nervous system. Láseres Med. Sci. 2021, 37, 11–18. [Google Scholar] [CrossRef]
- Hamblin, M. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef]
- Hamblin, M. Photobiomodulation for alzheimer’s disease: Has the light dawned? Photonics 2019, 6, 77. [Google Scholar] [CrossRef]
- Hu, X.; Das, B.; Hou, H.; He, W.; Yan, R. Bace 1 deletion in the adult mouse reverses preformed amyloid deposition and improves cognitive functions. Exp. Med. 2018, 215, 927–940. [Google Scholar] [CrossRef]
- Purushothuman, S.; Johnstone, D.; Nandasena, C.; Mitrofanis, J.; Stone, J. Photobiomodulation with near infrared light mitigates alzheimer’s disease-related pathology in cerebral cortex–evidence from two transgenic mouse models. Alzheimer’s Res. Ther. 2014, 6, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Taboada, L.; Hamblin, M. Transcranial photobiomodulation treats alzheimer’s disease in amyloid-protein precursor transgenic mice. In Photobiomodulation in the Brain, 1st ed.; Hamblin, M., Huang, Y., Eds.; Academic Press: London, UK, 2019; pp. 207–212. [Google Scholar]
- Da Luz, E.; Salgado, A.; Zângaro, R.; Da Silva, M.; Kerppers, I.; Da Silva, L.; Parreira, R. Transcranial led therapy on amyloid-toxin 25–35 in the hippocampal region of rats. Lasers Med. Sci. 2017, 32, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.; Barrett, D.; Saucedo, C.; Huang, L.; Abraham, J.; Tanaka, H.; Haley, A.; Gonzalez-Lima, F. Beneficial neurocognitive effects of transcranial laser in older adults. Lasers Med. Sci. 2017, 32, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Saltmarche, E.; Naeser, M.; Ho, K.; Hamblin, M.; Lim, L. Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: Case series report. Photomed. Laser Surg. 2017, 35, 432–441. [Google Scholar] [CrossRef]
- Berman, M.; Halper, J.; Nichols, T.; Jarrett, H.; Lundy, A.; Huang, J. Photobiomodulation with near infrared light helmet in a pilot, placebo controlled clinical trial in dementia patients testing memory and cognition. J. Neurol. Neurosci. 2017, 8, 176. [Google Scholar] [CrossRef]
- Lim, L. The Growing Evidence for Photobiomodulation as a Promising Treatment for Alzheimer’s Disease. Int. J. Biosci. Med. 2018, 6, 100–110. [Google Scholar] [CrossRef]
- Kuhn, M.; Popovic, A.; Pezawas, L. Neuroplasticity and memory formation in major depressive disorder: An imaging genetics perspective on serotonin and BDNF. Restor. Neurol. Neurosci. 2014, 32, 25–49. [Google Scholar] [CrossRef]
- Felling, R.; Song, H. Epigenetic mechanisms of neuroplasticity and the implications for stroke recovery. Exp. Neurol. 2015, 268, 37–45. [Google Scholar] [CrossRef]
- Tomaszczyk, J.; Green, N.; Frasca, D.; Colella, B.; Turner, G.; Christensen, B.; Green, R. Negative neuroplasticity in chronic traumatic brain injury and implications for neurorehabilitation. Neuropsychol. Rev. 2014, 24, 409–427. [Google Scholar] [CrossRef]
- Desmet, K.; Paz, D.; Corry, J.; Eells, J.; Wong, M.; Henry, M.; Buchmann, E.; Connelly, M.; Dovi, J.; Ling, H.; et al. Clinical and Experimental Applications of NIR-LED Photobiomodulation. Photomed. Laser Surg. 2006, 24, 121–128. [Google Scholar] [CrossRef]
- Hamblin, M. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Denninger, J.; Smith, B.; Kirby, E. Novel Object Recognition and Object Location Behavioral Testing in Mice on a Budget. J. Vis. Exp. 2018, 141, e58593. [Google Scholar] [CrossRef]
- Arias, A.; More, J.; Vicente, J.; Adasme, T.; Hidalgo, J.; Valdés, J.; Humeres, A.; Valdés-Undurraga, I.; Sánchez, G.; Hidalgo, C.; et al. Triclosan Impairs Hippocampal Synaptic Plasticity and Spatial Memory in Male Rats. Front. Mol. Neurosci. 2018, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.; Bachstetter, A.; Van, J. Comprehensive Behavioral Characterization of an APP/PS-1 Double Knock-in Mouse Model of Alzheimer’s Disease. Alzheimer’s Res. Ther. 2013, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.; Dao, D.; Kovacsics, C. “The Open Field Test”. In Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests. Neuromethods 2019, 23, 444–456. [Google Scholar]
- Arias, A.; Adasme, T.; Sanchez, G.; Muñoz, P.; Hidalgo, C. Aging impairs hippocampal-dependent recognition memory and LTP and prevents the associated RyR up-regulation. Front. Aging Neurosci. 2017, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xue, G.; Wang, S.; Zhang, L.; Sh, C.I.; Xie, X. Novel object recognition as a facile behavior test for evaluating drug effects in APP/PS1 Alzheimer’s Disease mouse model. JAD 2012, 31, 801–812. [Google Scholar] [CrossRef]
- Jardanhazi, D.; Markus, P.; Terwel, D.; Vogel, K.; Dyrks, T.; Thiele, A.; Michael, T. Induced LC degeneration in APP/PS1 transgenic mice accelerates early cerebral amyloidosis and cognitive deficits. Neurochem. Int. 2010, 57, 375–382. [Google Scholar] [CrossRef]
- Comerota, M.; Krishnan, B.; Taglialatela, G. Near infrared light decreases synaptic vulnerability to amyloid beta oligomers. Sci. Rep. 2017, 7, 15012. [Google Scholar] [CrossRef]
- Im, J.; Jeong, H.; Bikson, M.; Woods, A.; Unal, G.; Oh, J.; Na, S.; Park, J.; Knotkova, H.; Song, I.; et al. Effects of 6-month at-home transcranial direct current stimulation on cognition and cerebral glucose metabolism in Alzheimer’s disease. Brain Stimul. 2019, 12, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Cotelli, M.; Manenti, R.; Brambilla, M.; Petesi, M.; Rosini, S.; Ferrari, C.; Zanetti, O.; Miniussi, C. Anodal tDCS during face-name associations memory training in Alzheimer’s patients. Front. Aging Neurosci. 2014, 6, 38. [Google Scholar]
- Borgomaneri, S.; Battaglia, S.; Garofalo, S.; Tortora, F.; Avenanti, A.; Di Pellegrino, G. State-Dependent TMS over Prefrontal Cortex Disrupts Fear-Memory Reconsolidation and Prevents the Return of Fear. Curr. Biol. 2020, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.; Hamblin, M. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shen, Q.; Wu, X.; Zhang, D.; Xing, D. Activation of PKA/SIRT1 signaling pathway by photobiomodulation therapy reduces aβ levels in alzheimer’s disease models. Aging Cell 2020, 19, e13054. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.; Lee, S.; Park, J.; Kim, M.; Park, K.; Choi, B.; Shin, Y. Photobiomodulation using a low-level light-emitting diode improves cognitive dysfunction in the 5xfad mouse model of alzheimer’s disease. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 631–639. [Google Scholar] [CrossRef]
- Shen, L.; Liu, X.; Gu, D.; Xing, D. Photobiomodulation suppresses jnk3 by activation of erk/mkp7 to attenuate ampa receptor endocytosis in alzheimer’s disease. Aging Cell 2021, 20, e13289. [Google Scholar] [CrossRef]
- Thomas, G.; Lin, D.; Nuriya, M.; Huganir, R. Rapid and bidirectional regulation of ampa receptor phosphorylation and trafficking by jnk. EMBO Rep. 2021, 27, 361–372. [Google Scholar] [CrossRef]
- Zhao, W.; Santini, F.; Breese, R.; Ross, D.; Zhang, X.; Stone, D.; Ferrer, M.; Townsend, M.; Wolfe, A.; Seager, M.; et al. Inhibition of calcineurin-mediated endocytosis and amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid β oligomer-induced synaptic disruption. J. Biol. Chem. 2010, 285, 7619–7632. [Google Scholar] [CrossRef]
- Hennessy, M.; Hamblin, M. Photobiomodulation and the brain: A new paradigm. J. Opt. 2016, 19, 013003. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Z.; Zhi, Z.; Tian, J.; Zhao, Y.; Sun, J. Targeting the TLR4/NF-κB pathway in β-amyloid-stimulated microglial cells: A possible mechanism that oxysophoridine exerts anti-oxidative and anti-inflammatory effects in an in vitro model of Alzheimer’s disease. Brain Res. Bull. 2021, 175, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, C.; Wang, R. Light modulation of brain and development of relevant equipment. J. Alzheimer’s Dis. 2020, 74, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Chao, L. Effects of home photobiomodulation treatments on cognitive and behavioral function, cerebral perfusion, and resting-state functional connectivity in patients with dementia: A pilot trial. Photomed. Laser Surg. 2019, 37, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, W.; Feen, E.; Grossberg, G. The use of cholinesterase inhibitors across all stages of alzheimer’s disease. Drugs Aging 2015, 32, 537–547. [Google Scholar] [CrossRef]
- Wilcock, G.; Howe, I.; Coles, H.; Lilienfeld, S.; Truyen, L.; Zhu, Y.; Bul-lock, R.; Kershaw, P. A long-term comparison of galan-tamine and donepezil in the treatment of alzheimer’s disease. Drugs Aging 2003, 20, 777–789. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buendía, D.; Guncay, T.; Oyanedel, M.; Lemus, M.; Weinstein, A.; Ardiles, Á.O.; Marcos, J.; Fernandes, A.; Zângaro, R.; Muñoz, P. The Transcranial Light Therapy Improves Synaptic Plasticity in the Alzheimer’s Disease Mouse Model. Brain Sci. 2022, 12, 1272. https://doi.org/10.3390/brainsci12101272
Buendía D, Guncay T, Oyanedel M, Lemus M, Weinstein A, Ardiles ÁO, Marcos J, Fernandes A, Zângaro R, Muñoz P. The Transcranial Light Therapy Improves Synaptic Plasticity in the Alzheimer’s Disease Mouse Model. Brain Sciences. 2022; 12(10):1272. https://doi.org/10.3390/brainsci12101272
Chicago/Turabian StyleBuendía, Débora, Tatiana Guncay, Macarena Oyanedel, Makarena Lemus, Alejandro Weinstein, Álvaro O. Ardiles, José Marcos, Adriana Fernandes, Renato Zângaro, and Pablo Muñoz. 2022. "The Transcranial Light Therapy Improves Synaptic Plasticity in the Alzheimer’s Disease Mouse Model" Brain Sciences 12, no. 10: 1272. https://doi.org/10.3390/brainsci12101272
APA StyleBuendía, D., Guncay, T., Oyanedel, M., Lemus, M., Weinstein, A., Ardiles, Á. O., Marcos, J., Fernandes, A., Zângaro, R., & Muñoz, P. (2022). The Transcranial Light Therapy Improves Synaptic Plasticity in the Alzheimer’s Disease Mouse Model. Brain Sciences, 12(10), 1272. https://doi.org/10.3390/brainsci12101272







