Molecular Study of the Protective Effect of a Low-Carbohydrate, High-Fat Diet against Brain Insulin Resistance in an Animal Model of Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Method
2.1. Animals and Experimental Protocol
2.2. Induction of Metabolic Syndrome
2.3. Anthropometric Measurement and Tissue Sampling
2.4. Biochemical Investigations
2.5. Histological Study
2.6. Immunohistochemical (IHC) Study
2.7. Morphometric Study
2.8. Molecular Studies
2.9. Statistical Analysis
3. Results
3.1. Effect of LCHF and HCLF Diet on the Anthropometric Parameters and HOMA-IR
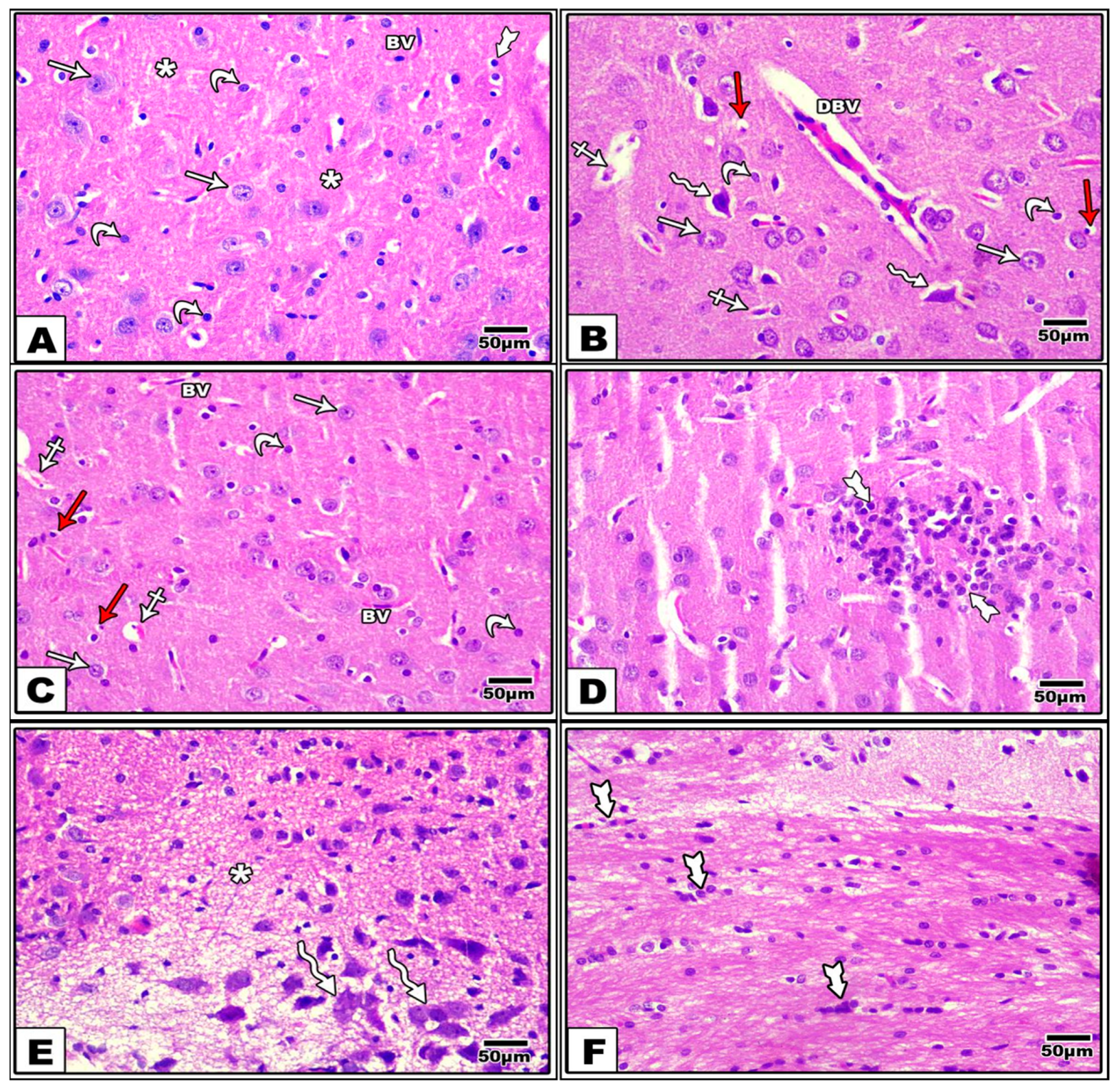
3.2. Effect of LCHF & HCLF Diet on Brain Histology
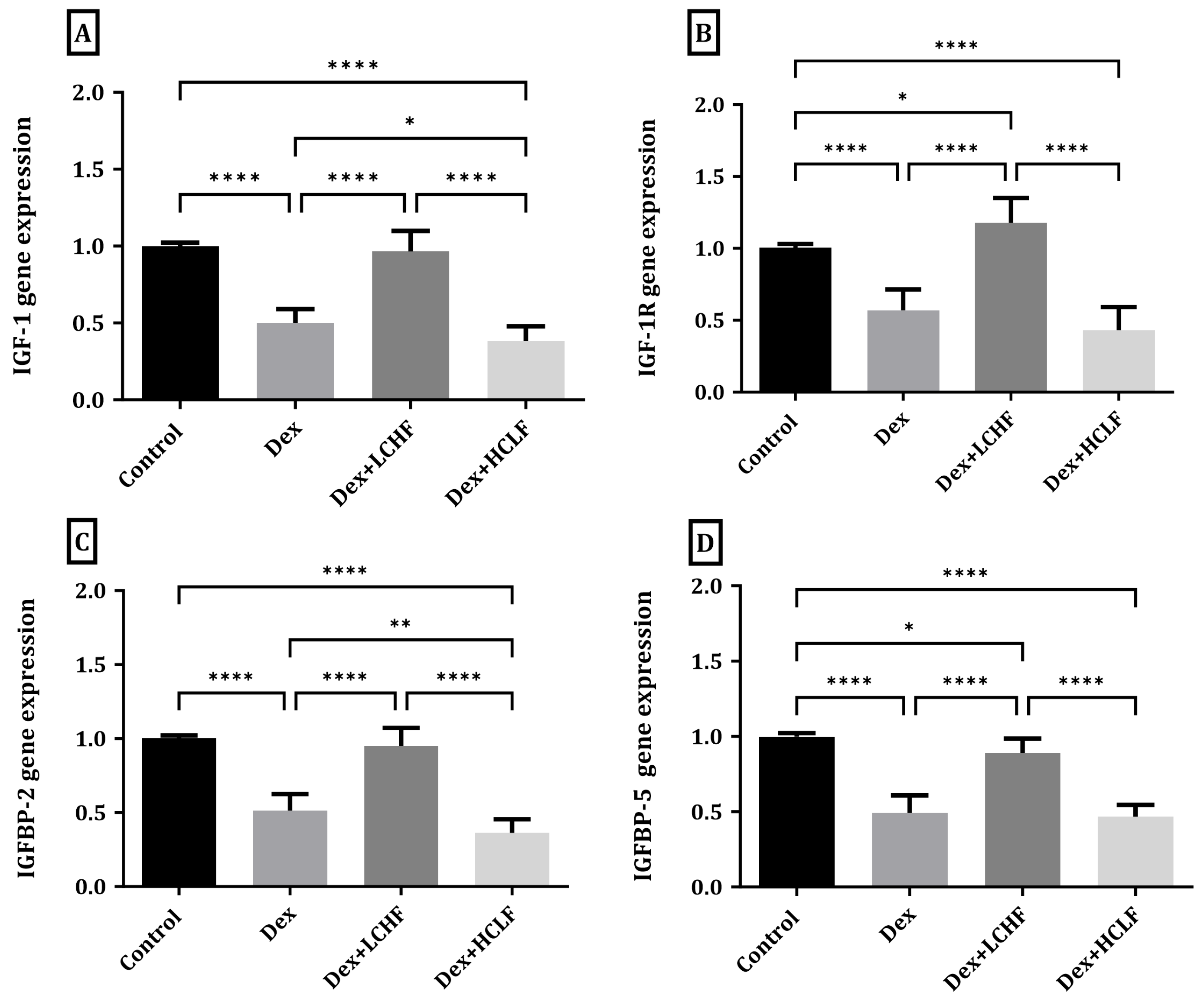
3.3. Effect of LCHF and HCLF Diet on the Expression of IGF-1, IGF-1R, IGFBP-2, and IGFBP-5 Expression in DEX-Treated Rats
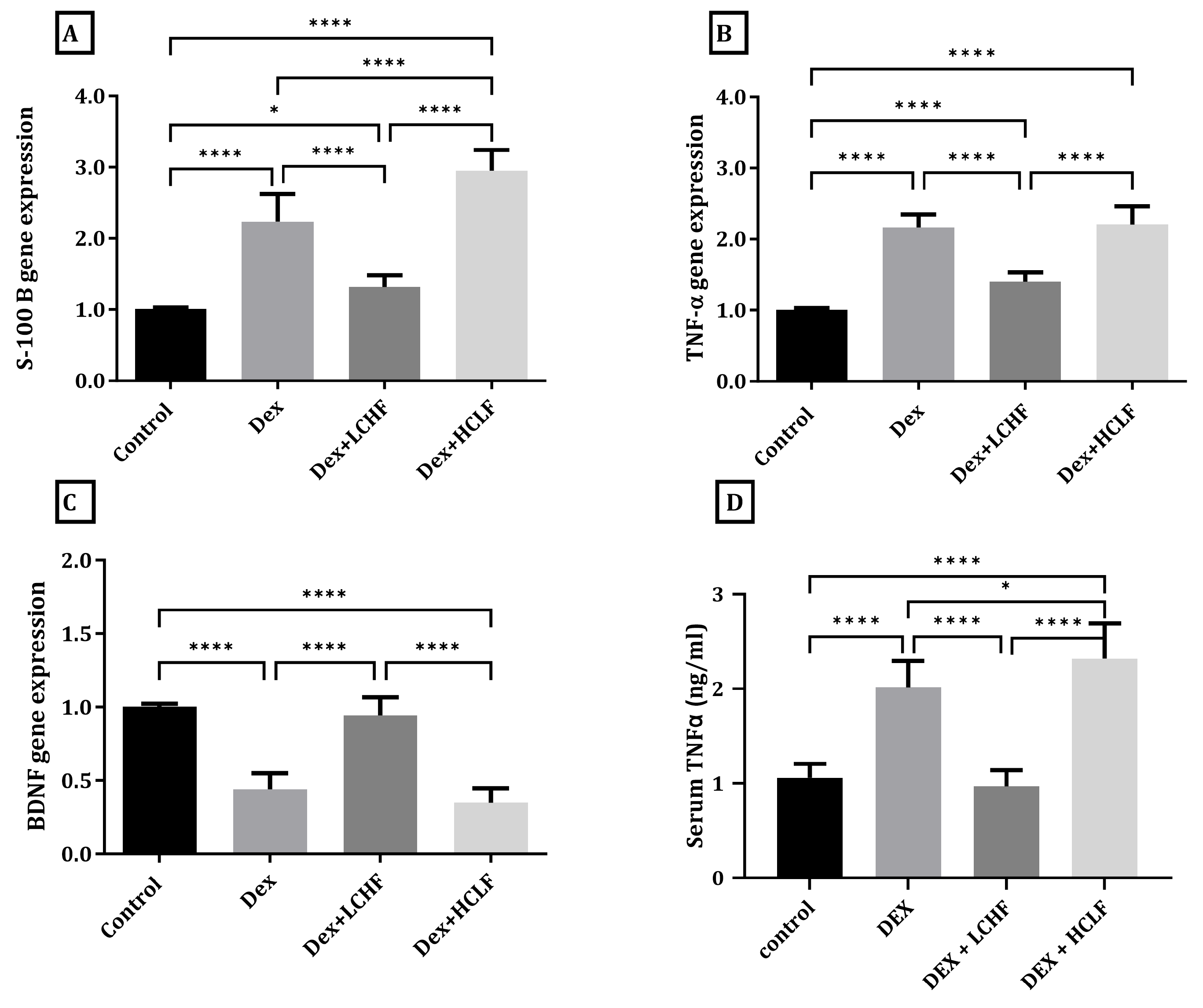
3.4. Effect of LCHF and HCLF Diet on the Expression of S100B, TNF-α, and BDNF in DEX-Treated Rats
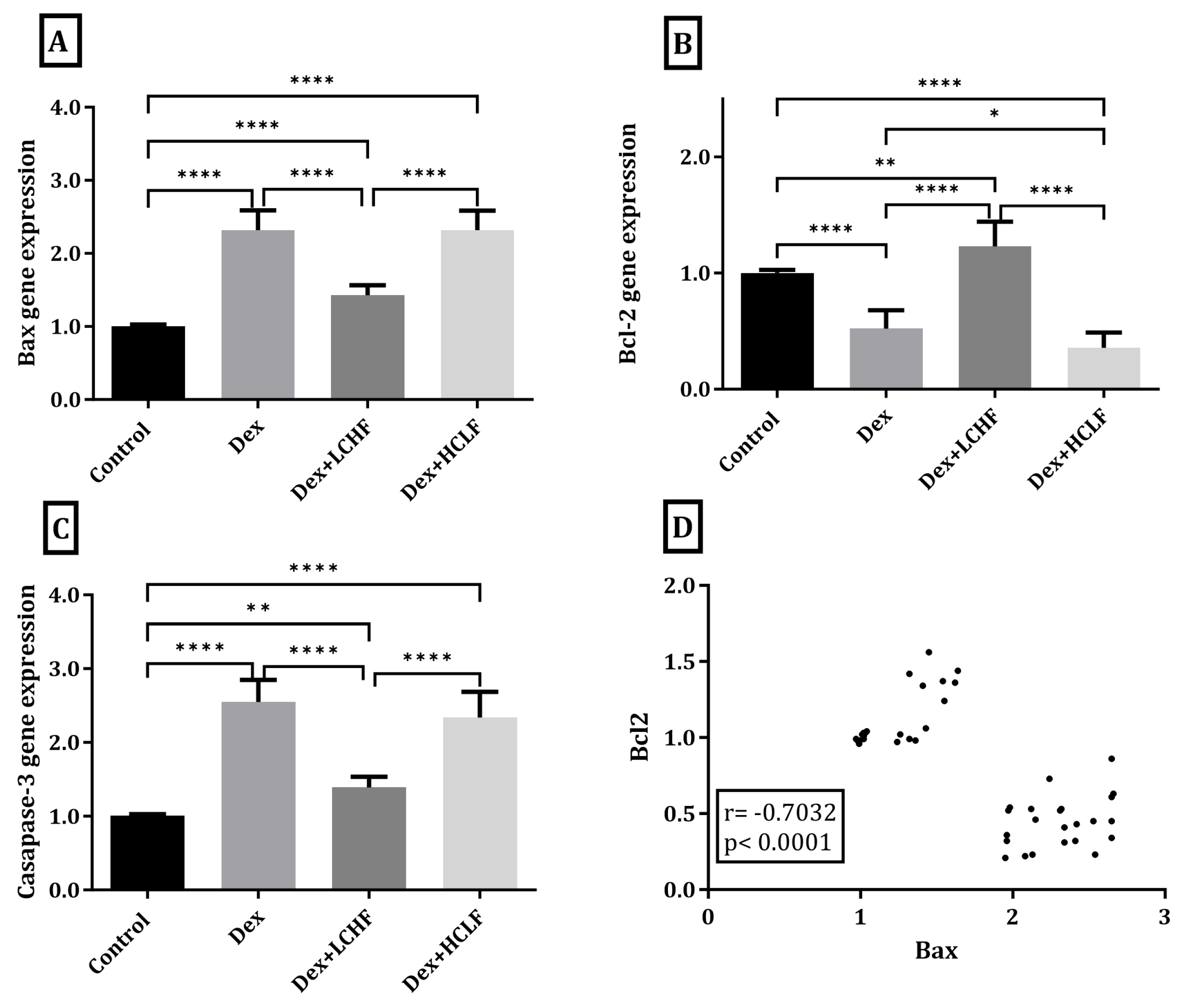
3.5. Effect of LCHF and HCLF Diet on the Expression of Caspase-3, Bax, and Bcl-2 Expression in DEX-Treated Rats
3.6. Effect of LCHF and HCLF Diet on Brain Caspase-3 and S100B Expression
3.7. Correlations of All Measured and Calculated Data in All Studied Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Gene | The Primer Sequence | Reference | |
|---|---|---|---|
| S100B | forward | 5′-TAG TCC TTG GAC ACC GAA GC-3′ | [87] |
| reverse | 5′-CAT CAA TGA GGG CAA CCA T-3′ | ||
| BDNF | forward | 5′-CAG GGG CAT AGA CAA AAG-3′ | [88] |
| reverse | 5′-CTT CCC CTT TTA ATG GTC-3′ | ||
| TNF-α | forward | 5′-AAT GGC CTC CCT CAT CAG TT-3′ | [89] |
| reverse | 5′-CCA CTT GGT GGT TTG CTA CGA-3′ | ||
| IGF-1 | forward | 5′-AAC CTG CAA AAC ATC GGA AC-3′ | [90] |
| reverse | 5′-GCA GCC AAA ATT CAG AGA GG-3′ | ||
| IGF-1 R | forward | 5′-GAC AGT GAA TGA GGC TGC AA-3′ | [90] |
| reverse | 5′-CCA GCC ATC TGG ATC ATC TT-3′ | ||
| IGFBP-2 | forward | 5′-AGA ATG ATG GGG CTC ACG C-3′ | [91] |
| reverse | 5′-TTT CTG CTG GTG TTT GGG GT-3′ | ||
| IGFBP-5 | forward | 5′-GGG CTC TTT CGT GCA TTG TG-3′ | [91] |
| reverse | 5′-ATC TTG GTT TGC TCG CCG TA-3′ | ||
| Bax | forward | 5′-CGG CGA ATT GGA GAT GAA CTG G-3′ | [92] |
| reverse | 5′-CTA GCA AAG TAG AAG AGG GCA ACC-3′ | ||
| Bcl-2 | forward | 5′-TGT GGA TGA CTG ACT ACC TGA ACC-3′ | [93] |
| reverse | 5′-CAG CCA GGA GAA ATC AAA CAG AGG-3′ | ||
| Caspase-3 | forward | 5′-GTG GAA CTG ACG ATG ATA TGG C-3′ | [93] |
| reverse | 5′-CGC AAA GTG ACT GGA TGA ACC-3′ | ||
| GAPDH | forward | 5′-TCC CTC AAG ATT GTC AGC AA-3′ | [89] |
| reverse | 5′-AGA TCC ACA ACG GAT ACA TT-3′ |
References
- Raichle, M.E.; Gusnard, D.A. Appraising the brain’s energy budget. Proc. Natl. Acad. Sci. USA 2002, 99, 10237–10239. [Google Scholar] [CrossRef] [PubMed]
- Milstein, J.L.; Ferris, H.A. The brain as an insulin-sensitive metabolic organ. Mol. Metab. 2021, 52, 101234. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, S.; Arafa, D.; Tropea, D. Insulin-Like Growth Factor 1: At the Crossroads of Brain Development and Aging. Front. Cell Neurosci. 2017, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Csajbók, É.A.; Tamás, G. Cerebral cortex: A target and source of insulin? Diabetologia 2016, 59, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.M.; Barrett, E.J. Insulin transport into the brain. Am. J. Physiol. Cell Physiol. 2018, 315, C125–C136. [Google Scholar] [CrossRef]
- Agrawal, R.; Reno, C.M.; Sharma, S.; Christensen, C.; Huang, Y.; Fisher, S.J. Insulin action in the brain regulates both central and peripheral functions. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E156–E163. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.J. Insulin resistance as the core defect in type 2 diabetes mellitus. Am. J. Cardiol. 2002, 90, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.-Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Knapp, A.; Johnson, A.; Wong, J.M.W.; Greco, K.F.; Ma, C.; Mora, S.; Ludwig, D.S. Effects of a low-carbohydrate diet on insulin-resistant dyslipoproteinemia-a randomized controlled feeding trial. Am. J. Clin. Nutr. 2022, 115, 154–162. [Google Scholar] [CrossRef]
- Lee, S.; Keirsey, K.I.; Kirkland, R.; Grunewald, Z.I.; Fischer, J.G.; de La Serre, C.B. Blueberry Supplementation Influences the Gut Microbiota, Inflammation, and Insulin Resistance in High-Fat-Diet-Fed Rats. J. Nutr. 2018, 148, 209–219. [Google Scholar] [CrossRef]
- Shippy, D.C.; Wilhelm, C.; Viharkumar, P.A.; Raife, T.J.; Ulland, T.K. β-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology. J. Neuroinflamm. 2020, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, C.; Shang, F.-F.; Luo, M.; You, Y.; Zhai, Q.; Xia, Y.; Suxin, L. Ketogenic Diet Ameliorates Cardiac Dysfunction via Balancing Mitochondrial Dynamics and Inhibiting Apoptosis in Type 2 Diabetic Mice. Aging Dis. 2020, 11, 229–240. [Google Scholar] [CrossRef]
- Alnami, A.; Bima, A.; Alamoudi, A.; Eldakhakhny, B.; Sakr, H.; Elsamanoudy, A. Modulation of Dyslipidemia Markers Apo B/Apo A and Triglycerides/HDL-Cholesterol Ratios by Low-Carbohydrate High-Fat Diet in a Rat Model of Metabolic Syndrome. Nutrients 2022, 14, 1903. [Google Scholar] [CrossRef]
- Sivabalan, S.; Renuka, S.; Menon, V.P. Fat feeding potentiates the diabetogenic effect of dexamethasone in Wistar rats. Int. Arch. Med. 2008, 1, 7. [Google Scholar] [CrossRef]
- Ble-Castillo, J.L.; Aparicio-Trapala, M.A.; Juárez-Rojop, I.E.; Torres-Lopez, J.E.; Mendez, J.D.; Aguilar-Mariscal, H.; Olvera-Hernández, V.; Palma-Cordova, L.C.; Diaz-Zagoya, J.C. Differential effects of high-carbohydrate and high-fat diet composition on metabolic control and insulin resistance in normal rats. Int. J. Environ. Res. Public Health 2012, 9, 1663–1676. [Google Scholar] [CrossRef] [PubMed]
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.H.; Cicogna, A.C.; Novelli Filho, J.L.V.B. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: London, UK, 2019. [Google Scholar]
- Jackson, P.; Blythe, D. Immunohistochemical techniques. In Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Elsevier: London, UK, 2019. [Google Scholar]
- Bima, A.I.; Mahdi, A.S.; Al Fayez, F.F.; Khawaja, T.M.; Abo El-Khair, S.M.; Elsamanoudy, A.Z. Cellular Senescence and Vitamin D Deficiency Play a Role in the Pathogenesis of Obesity-Associated Subclinical Atherosclerosis: Study of the Potential Protective Role of Vitamin D Supplementation. Cells 2021, 10, 920. [Google Scholar] [CrossRef]
- Aguirre, G.A.; De Ita, J.R.; de la Garza, R.G.; Castilla-Cortazar, I. Insulin-like growth factor-1 deficiency and metabolic syndrome. J. Transl. Med. 2016, 14, 3. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Tong, M.; Schiano, I.; Didsbury, J. Improved Brain Insulin/IGF Signaling and Reduced Neuroinflammation with T3D-959 in an Experimental Model of Sporadic Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Chesik, D.; De Keyser, J.; Wilczak, N. Insulin-like Growth Factor System Regulates Oligodendroglial Cell Behavior: Therapeutic Potential in CNS. J. Mol. Neurosci. 2008, 35, 81–90. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M.; Tong, M.; Bowling, N.; Moskal, P. si-RNA inhibition of brain insulin or insulin-like growth factor receptors causes developmental cerebellar abnormalities: Relevance to fetal alcohol spectrum disorder. Mol. Brain 2011, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M.; Wands, J.R. Role of central nervous system insulin resistance in fetal alcohol spectrum disorders. J. Popul. Ther. Clin. Pharmacol. = J. Ther. Popul. Pharmacol. Clin. 2010, 17, e390–e404. [Google Scholar]
- Garg, N.; Thakur, S.; McMahan, C.A.; Adamo, M.L. High fat diet induced insulin resistance and glucose intolerance are gender-specific in IGF-1R heterozygous mice. Biochem. Biophys. Res. Commun. 2011, 413, 476–480. [Google Scholar] [CrossRef]
- Bassil, F.; Fernagut, P.-O.; Bezard, E.; Meissner, W.G. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: Targets for disease modification? Progress Neurobiol. 2014, 118, 1–18. [Google Scholar] [CrossRef]
- Bassil, F.; Canron, M.H.; Vital, A.; Bezard, E.; Li, Y.; Greig, N.H.; Gulyani, S.; Kapogiannis, D.; Fernagut, P.O.; Meissner, W.G. Insulin resistance and exendin-4 treatment for multiple system atrophy. Brain 2017, 140, 1420–1436. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef]
- Boughanem, H.; Yubero-Serrano, E.M.; López-Miranda, J.; Tinahones, F.J.; Macias-Gonzalez, M. Potential Role of Insulin Growth-Factor-Binding Protein 2 as Therapeutic Target for Obesity-Related Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 1133. [Google Scholar] [CrossRef]
- Xiao, Z.; Chu, Y.; Qin, W. IGFBP5 modulates lipid metabolism and insulin sensitivity through activating AMPK pathway in non-alcoholic fatty liver disease. Life Sci. 2020, 256, 117997. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kaneda, A.; Kamata, K. Possible involvement of IGF-1 receptor and IGF-binding protein in insulin-induced enhancement of noradrenaline response in diabetic rat aorta. Br. J. Pharmacol. 2003, 140, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Heald, A.; Kaushal, K.; Siddals, K.; Rudenski, A.; Anderson, S.; Gibson, J. Insulin-like Growth Factor Binding Protein-2 (IGFBP-2) is a Marker for the Metabolic Syndrome. Exp. Clin. Endocrinol. Diabetes 2006, 114, 371–376. [Google Scholar] [CrossRef]
- Olszanecka, A.; Dragan, A.; Kawecka-Jaszcz, K.; Fedak, D.; Czarnecka, D. Relationships of insulin-like growth factor-1, its binding proteins, and cardiometabolic risk in hypertensive perimenopausal women. Metabolism 2017, 69, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Rodriguez, R.; Lifshitz, L.M.; Bellve, K.D.; Min, S.Y.; Pires, J.; Leung, K.; Boeras, C.; Sert, A.; Draper, J.T.; Corvera, S.; et al. Human adipose tissue expansion in pregnancy is impaired in gestational diabetes mellitus. Diabetologia 2015, 58, 2106–2114. [Google Scholar] [CrossRef] [PubMed]
- Minchenko, D.O.; Tsymbal, D.O.; Davydov, V.V.; Minchenko, O.H. Expression of genes encoding IGF1, IGF2, and IGFBPs in blood of obese adolescents with insulin resistance. Endocr. Regul. 2019, 53, 34–45. [Google Scholar] [CrossRef]
- Abdulsada, M.M.; Wilhelm, Z.R.; Opekun, A.R.; Devaraj, S.; Jalal, P.K.; Mindikoglu, A.L. The effect of four-week intermittent fasting from dawn to sunset on circulating brain-derived neurotrophic factor levels in subjects with metabolic syndrome and healthy subjects. Metabol. Open 2020, 9, 100070. [Google Scholar] [CrossRef]
- Lee, I.T.; Wang, J.-S.; Fu, C.-P.; Lin, S.-Y.; Sheu, W.H.-H. Relationship between body weight and the increment in serum brain-derived neurotrophic factor after oral glucose challenge in men with obesity and metabolic syndrome: A prospective study. Medicine 2016, 95, e5260. [Google Scholar] [CrossRef]
- Motamedi, S.; Karimi, I.; Jafari, F. The interrelationship of metabolic syndrome and neurodegenerative diseases with focus on brain-derived neurotrophic factor (BDNF): Kill two birds with one stone. Metab. Brain Dis. 2017, 32, 651–665. [Google Scholar] [CrossRef]
- Jamali, A.; Shahrbanian, S.; Morteza Tayebi, S. The Effects of Exercise Training on the Brain-Derived Neurotrophic Factor (BDNF) in the Patients with Type 2 Diabetes: A Systematic Review of the Randomized Controlled Trials. J. Diabetes Metab. Disord. 2020, 19, 633–643. [Google Scholar] [CrossRef]
- Stålnacke, B.-M.; Sojka, P. Repeatedly Heading a Soccer Ball Does Not Increase Serum Levels of S-100B, a Biochemical Marker of Brain Tissue Damage: An Experimental Study. Biomark. Insights 2008, 3, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.A.; Hamed, E.A.; Zakary, M.M. Oxidative stress and S-100B protein in children with bacterial meningitis. BMC Neurol. 2009, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Bjursten, S.; Pandita, A.; Zhao, Z.; Fröjd, C.; Ny, L.; Jensen, C.; Ullerstam, T.; Jespersen, H.; Borén, J.; Levin, M.; et al. Early rise in brain damage markers and high ICOS expression in CD4+ and CD8+ T cells during checkpoint inhibitor-induced encephalomyelitis. J. Immunother. Cancer 2021, 9, e002732. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Zhang, X.; Tang, J. Oxymatrine Ameliorates Memory Impairment in Diabetic Rats by Regulating Oxidative Stress and Apoptosis: Involvement of NOX2/NOX4. Oxid. Med. Cell Longev. 2020, 2020, 3912173. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Żebrowska, E.; Nesterowicz, M.; Żendzian-Piotrowska, M.; Zalewska, A. α-Lipoic Acid Strengthens the Antioxidant Barrier and Reduces Oxidative, Nitrosative, and Glycative Damage, as well as Inhibits Inflammation and Apoptosis in the Hypothalamus but Not in the Cerebral Cortex of Insulin-Resistant Rats. Oxid. Med. Cell Longev. 2022, 2022, 7450514. [Google Scholar] [CrossRef]
- Nuzzo, D.; Galizzi, G.; Amato, A.; Terzo, S.; Picone, P.; Cristaldi, L.; Mulè, F.; Di Carlo, M. Regular Intake of Pistachio Mitigates the Deleterious Effects of a High Fat-Diet in the Brain of Obese Mice. Antioxidants 2020, 9, 317. [Google Scholar] [CrossRef]
- Rahmati, M.; Keshvari, M.; Mirnasouri, R.; Chehelcheraghi, F. Exercise and Urtica dioica extract ameliorate hippocampal insulin signaling, oxidative stress, neuroinflammation, and cognitive function in STZ-induced diabetic rats. Biomed. Pharmacother. 2021, 139, 111577. [Google Scholar] [CrossRef]
- Feng, C.; Jiang, Y.; Li, S.; Ge, Y.; Shi, Y.; Tang, X.; Le, G. Methionine Restriction Improves Cognitive Ability by Alleviating Hippocampal Neuronal Apoptosis through H19 in Middle-Aged Insulin-Resistant Mice. Nutrients 2022, 14, 4503. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, R.; Qu, J. Effect of different glucose supply conditions on neuronal energy metabolism. Cogn. Neurodyn 2016, 10, 563–571. [Google Scholar] [CrossRef]
- Mu, R.; Wu, X.; Yuan, D.; Zhao, J.; Tang, S.; Hong, H.; Long, Y. Activation of TGR5 Ameliorates Streptozotocin-Induced Cognitive Impairment by Modulating Apoptosis, Neurogenesis, and Neuronal Firing. Oxid. Med. Cell Longev. 2022, 2022, 3716609. [Google Scholar] [CrossRef]
- Kim, N.; Chen, D.; Zhou, X.Z.; Lee, T.H. Death-Associated Protein Kinase 1 Phosphorylation in Neuronal Cell Death and Neurodegenerative Disease. Int. J. Mol. Sci. 2019, 20, 3131. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef] [PubMed]
- Brundel, M.; Kappelle, L.J.; Biessels, G.J. Brain imaging in type 2 diabetes. Eur. Neuropsychopharmacol. 2014, 24, 1967–1981. [Google Scholar] [CrossRef] [PubMed]
- Del Bene, A.; Ciolli, L.; Borgheresi, L.; Poggesi, A.; Inzitari, D.; Pantoni, L. Is type 2 diabetes related to leukoaraiosis? an updated review. Acta Neurol. Scand. 2015, 132, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Willette, A.A.; Bendlin, B.B.; Starks, E.J.; Birdsill, A.C.; Johnson, S.C.; Christian, B.T.; Okonkwo, O.C.; La Rue, A.; Hermann, B.P.; Koscik, R.L.; et al. Association of Insulin Resistance With Cerebral Glucose Uptake in Late Middle-Aged Adults at Risk for Alzheimer Disease. JAMA Neurol. 2015, 72, 1013–1020. [Google Scholar] [CrossRef]
- Berhane, F.; Fite, A.; Daboul, N.; Al-Janabi, W.; Msallaty, Z.; Caruso, M.; Lewis, M.K.; Yi, Z.; Diamond, M.P.; Abou-Samra, A.-B.; et al. Plasma Lactate Levels Increase during Hyperinsulinemic Euglycemic Clamp and Oral Glucose Tolerance Test. J. Diabetes Res. 2015, 2015, 102054. [Google Scholar] [CrossRef]
- Pelvig, D.P.; Pakkenberg, H.; Stark, A.K.; Pakkenberg, B. Neocortical glial cell numbers in human brains. Neurobiol. Aging 2008, 29, 1754–1762. [Google Scholar] [CrossRef]
- Ding, X.; Jian, T.; Wu, Y.; Zuo, Y.; Li, J.; Lv, H.; Ma, L.; Ren, B.; Zhao, L.; Li, W.; et al. Ellagic acid ameliorates oxidative stress and insulin resistance in high glucose-treated HepG2 cells via miR-223/keap1-Nrf2 pathway. Biomed. Pharmacother. 2019, 110, 85–94. [Google Scholar] [CrossRef]
- de la Monte, S.M. Insulin Resistance and Neurodegeneration: Progress Towards the Development of New Therapeutics for Alzheimer’s Disease. Drugs 2017, 77, 47–65. [Google Scholar] [CrossRef]
- Roberts, R.O.; Knopman, D.S.; Cha, R.H.; Mielke, M.M.; Pankratz, V.S.; Boeve, B.F.; Kantarci, K.; Geda, Y.E.; Jack, C.R., Jr.; Petersen, R.C.; et al. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J. Nucl. Med. 2014, 55, 759–764. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Bagheri, R.; Bavi, H.; Baker, J.S.; Moro, T.; Mancin, L.; Paoli, A. Ketogenic diets, physical activity and body composition: A review. Br. J. Nutr. 2022, 127, 1898–1920. [Google Scholar] [CrossRef] [PubMed]
- Nuwaylati, D.; Eldakhakhny, B.; Bima, A.; Sakr, H.; Elsamanoudy, A. Low-Carbohydrate High-Fat Diet: A SWOC Analysis. Metabolites 2022, 12, 1126. [Google Scholar] [CrossRef] [PubMed]
- Montella, L.; Sarno, F.; Altucci, L.; Cioffi, V.; Sigona, L.; Di Colandrea, S.; De Simone, S.; Marinelli, A.; Facchini, B.A.; De Vita, F.; et al. A Root in Synapsis and the Other One in the Gut Microbiome-Brain Axis: Are the Two Poles of Ketogenic Diet Enough to Challenge Glioblastoma? Front. Nutr. 2021, 8, 703392. [Google Scholar] [CrossRef] [PubMed]
- Lilamand, M.; Mouton-Liger, F.; Di Valentin, E.; Sànchez Ortiz, M.; Paquet, C. Efficacy and Safety of Ketone Supplementation or Ketogenic Diets for Alzheimer’s Disease: A Mini Review. Front. Nutr. 2022, 8, 807970. [Google Scholar] [CrossRef]
- Walsh, J.J.; Myette-Côté, É.; Little, J.P. The Effect of Exogenous Ketone Monoester Ingestion on Plasma BDNF During an Oral Glucose Tolerance Test. Front. Physiol. 2020, 11, 1094. [Google Scholar] [CrossRef]
- Walsh, J.J.; Neudorf, H.; Little, J.P. 14-Day Ketone Supplementation Lowers Glucose and Improves Vascular Function in Obesity: A Randomized Crossover Trial. J. Clin. Endocrinol. Metab. 2021, 106, e1738–e1754. [Google Scholar] [CrossRef]
- Kackley, M.L.; Buga, A.; Crabtree, C.D.; Sapper, T.N.; McElroy, C.A.; Focht, B.C.; Kraemer, W.J.; Volek, J.S. Influence of Nutritional Ketosis Achieved through Various Methods on Plasma Concentrations of Brain Derived Neurotropic Factor. Brain Sci. 2022, 12, 1143. [Google Scholar] [CrossRef]
- Lan, Y.; Huang, Z.; Jiang, Y.; Zhou, X.; Zhang, J.; Zhang, D.; Wang, B.; Hou, G. Strength exercise weakens aerobic exercise-induced cognitive improvements in rats. PLoS ONE 2018, 13, e0205562. [Google Scholar] [CrossRef]
- Park, C.H.; Kwak, Y.-S. Analysis of energy restriction and physical activity on brain function: The role of ketone body and brain-derived neurotrophic factor. J. Exerc. Rehabil. 2017, 13, 378–380. [Google Scholar] [CrossRef]
- Vizuete, A.F.; de Souza, D.F.; Guerra, M.C.; Batassini, C.; Dutra, M.F.; Bernardi, C.; Costa, A.P.; Goncalves, C.A. Brain changes in BDNF and S100B induced by ketogenic diets in Wistar rats. Life Sci. 2013, 92, 923–928. [Google Scholar] [CrossRef]
- Murugan, M.; Boison, D. Ketogenic diet, neuroprotection, and antiepileptogenesis. Epilepsy Res. 2020, 167, 106444. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.R.; Oliveira, D.L.; Pires, C.; Ribeiro, L.; Leite, M.; Mendez, A.; Goncalves, D.; Tramontina, F.; Portela, L.V.; Wofchuk, S.T.; et al. Ketogenic diet fed rats have low levels of S100B in cerebrospinal fluid. Neurosci. Res. 2004, 50, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yin, X.; Wang, M.; Chen, T.; Wang, Y.; Gao, Z.; Wang, Z. Effects of Ketogenic Diet on Neuroinflammation in Neurodegenerative Diseases. Aging Dis. 2022, 13, 1146–1165. [Google Scholar] [CrossRef]
- Harun-Or-Rashid, M.; Inman, D.M. Reduced AMPK activation and increased HCAR activation drive anti-inflammatory response and neuroprotection in glaucoma. J. Neuroinflamm. 2018, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wang, X.; Zhao, Y.; Yang, Q.; Ding, H.; Dong, Q.; Chen, X.; Cui, M. Ketogenic Diet Improves Brain Ischemic Tolerance and Inhibits NLRP3 Inflammasome Activation by Preventing Drp1-Mediated Mitochondrial Fission and Endoplasmic Reticulum Stress. Front. Mol. Neurosci. 2018, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qi, W.; Zou, C.; Xie, Z.; Zhang, M.; Naito, M.G.; Mifflin, L.; Liu, Z.; Najafov, A.; Pan, H.; et al. NEK1-mediated retromer trafficking promotes blood-brain barrier integrity by regulating glucose metabolism and RIPK1 activation. Nat. Commun. 2021, 12, 4826. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, X.; Cheng, Z.; Dong, Q.; Ruan, G. The Anti-Inflammatory Effect of Preventive Intervention with Ketogenic Diet Mediated by the Histone Acetylation of mGluR5 Promotor Region in Rat Parkinson’s Disease Model: A Dual-Tracer PET Study. Park. Dis. 2022, 2022, 3506213. [Google Scholar] [CrossRef]
- Sourbron, J.; Klinkenberg, S.; van Kuijk, S.M.J.; Lagae, L.; Lambrechts, D.; Braakman, H.M.H.; Majoie, M. Ketogenic diet for the treatment of pediatric epilepsy: Review and meta-analysis. Child’s Nerv. Syst. 2020, 36, 1099–1109. [Google Scholar] [CrossRef]
- Tao, Y.; Leng, S.X.; Zhang, H. Ketogenic Diet: An Effective Treatment Approach for Neurodegenerative Diseases. Curr. Neuropharmacol. 2022, 20, 2303–2319. [Google Scholar] [CrossRef]
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef]
- Davis, J.J.; Fournakis, N.; Ellison, J. Ketogenic Diet for the Treatment and Prevention of Dementia: A Review. J. Geriatr. Psychiatry Neurol. 2020, 34, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Mayengbam, S.; Ellegood, J.; Kesler, M.; Reimer, R.A.; Shearer, J.; Murari, K.; Rho, J.M.; Lerch, J.P.; Cheng, N. A ketogenic diet affects brain volume and metabolome in juvenile mice. NeuroImage 2021, 244, 118542. [Google Scholar] [CrossRef] [PubMed]
- Altayyar, M.; Nasser, J.A.; Thomopoulos, D.; Bruneau, M., Jr. The Implication of Physiological Ketosis on The Cognitive Brain: A Narrative Review. Nutrients 2022, 14, 513. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Parvathy, S.; Varma, K.K. Hyperinsulinemia Induced Altered Insulin Signaling Pathway in Muscle of High Fat- and Carbohydrate-Fed Rats: Effect of Exercise. J. Diabetes Res. 2021, 2021, 5123241. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, F.; Ge, W.; Mi, C.; Wang, R.; Sun, R. Protective effects of naloxone in two-hit seizure model. Epilepsia 2010, 51, 344–353. [Google Scholar] [CrossRef]
- Ghoneim, F.M.; Khalaf, H.A.; Elsamanoudy, A.Z.; Abo El-Khair, S.M.; Helaly, A.M.; Mahmoud, E.H.M.; Elshafey, S.H. Protective effect of chronic caffeine intake on gene expression of brain derived neurotrophic factor signaling and the immunoreactivity of glial fibrillary acidic protein and Ki-67 in Alzheimer’s disease. Int. J. Clin. Exp. Pathol. 2015, 8, 7710–7728. [Google Scholar]
- Abo El-Khair, S.M.; Ghoneim, F.M.; Shabaan, D.A.; Elsamanoudy, A.Z. Molecular and ultrastructure study of endoplasmic reticulum stress in hepatic steatosis: Role of hepatocyte nuclear factor 4alpha and inflammatory mediators. Histochem. Cell Biol. 2020, 153, 49–62. [Google Scholar] [CrossRef]
- Juárez-Vázquez, C.I.; Gurrola-Díaz, C.M.; Vargas-Guerrero, B.; Domínguez-Rosales, J.A.; Rodriguez-Ortiz, J.F.; Barros-Núñez, P.; Flores-Martínez, S.E.; Sánchez-Corona, J.; Rosales-Reynoso, M.A. Insulin glargine affects the expression of Igf-1r, Insr, and Igf-1 genes in colon and liver of diabetic rats. Iran. J. Basic. Med. Sci. 2018, 21, 489–494. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, W.; Yang, S.; Qi, F.; Zhao, R. Transgenerational Inheritance of Betaine-Induced Epigenetic Alterations in Estrogen-Responsive IGF-2/IGFBP2 Genes in Rat Hippocampus. Mol. Nutr. Food Res. 2020, 64, 1900823. [Google Scholar] [CrossRef]
- He, X.; Sun, J.; Huang, X. Expression of caspase-3, Bax and Bcl-2 in hippocampus of rats with diabetes and subarachnoid hemorrhage. Exp. Ther. Med. 2018, 15, 873–877. [Google Scholar] [CrossRef]
- Ghoneim, F.M.; Abo-Elkhair, S.M.; Elsamanoudy, A.Z.; Shabaan, D.A. Evaluation of Endothelial Dysfunction and Autophagy in Fibromyalgia-Related Vascular and Cerebral Cortical Changes and the Ameliorative Effect of Fisetin. Cells 2021, 11, 48. [Google Scholar] [CrossRef] [PubMed]




| Control | DEX | DEX + LCHF | DEX + HCLF | p-Value (ANOVA) | |
|---|---|---|---|---|---|
| BMI (baseline) [14] | 0.51 ± 0.031 | 0.52 ± 0.05 | 0.53 ± 0.05 | 0.51 ± 0.044 | 0.87 |
| BMI (Dex induction) [14] | 0.65 ± 0.07 | 0.76 ± 0.08 a | 0.51 ± 0.034 a,b | 0.93 ± 0.041 a,b,c | <0.001 |
| BMI (post-stopping Dex) [14] | 0.72 ± 0.05 | 0.88 ± 0.05 a | 0.53 ± 0.06 a,b | 0.97 ± 0.07 a,c | <0.001 |
| Brain weight (g) (post stopping Dex) | 3.89 ± 0.21 | 2.83 ± 0.19 a | 3.76 ± 0.43 b | 2.56 ± 0.17 a,c | <0.001 |
| Brain volume (mm3) (post-stopping Dex) | 1.81 ± 0.11 | 1.52 ± 0.09 a | 1.76 ± 0.06 b | 1.44 ± 0.13 a,c | <0.001 |
| HOMA-IR [14] | 2.43 ± 0.19 | 4.89 ± 0.39 a | 2.67 ± 0.29 b | 5.76 ± 0.94 a,b,c | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bima, A.; Eldakhakhny, B.; Alamoudi, A.A.; Awan, Z.; Alnami, A.; Abo-Elkhair, S.M.; Sakr, H.; Ghoneim, F.M.; Elsamanoudy, A. Molecular Study of the Protective Effect of a Low-Carbohydrate, High-Fat Diet against Brain Insulin Resistance in an Animal Model of Metabolic Syndrome. Brain Sci. 2023, 13, 1383. https://doi.org/10.3390/brainsci13101383
Bima A, Eldakhakhny B, Alamoudi AA, Awan Z, Alnami A, Abo-Elkhair SM, Sakr H, Ghoneim FM, Elsamanoudy A. Molecular Study of the Protective Effect of a Low-Carbohydrate, High-Fat Diet against Brain Insulin Resistance in an Animal Model of Metabolic Syndrome. Brain Sciences. 2023; 13(10):1383. https://doi.org/10.3390/brainsci13101383
Chicago/Turabian StyleBima, Abdulhadi, Basmah Eldakhakhny, Aliaa A. Alamoudi, Zuhier Awan, Abrar Alnami, Salwa Mohamed Abo-Elkhair, Hussein Sakr, Fatma Mohamed Ghoneim, and Ayman Elsamanoudy. 2023. "Molecular Study of the Protective Effect of a Low-Carbohydrate, High-Fat Diet against Brain Insulin Resistance in an Animal Model of Metabolic Syndrome" Brain Sciences 13, no. 10: 1383. https://doi.org/10.3390/brainsci13101383







