Motivations for Cannabis Use in Individuals with Social Anxiety Disorder (SAD)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Procedures
2.3. Outcome Measures
2.4. In-Depth Interviews
2.5. Data Analysis
3. Results
3.1. Demographic and Clinical Characteristics
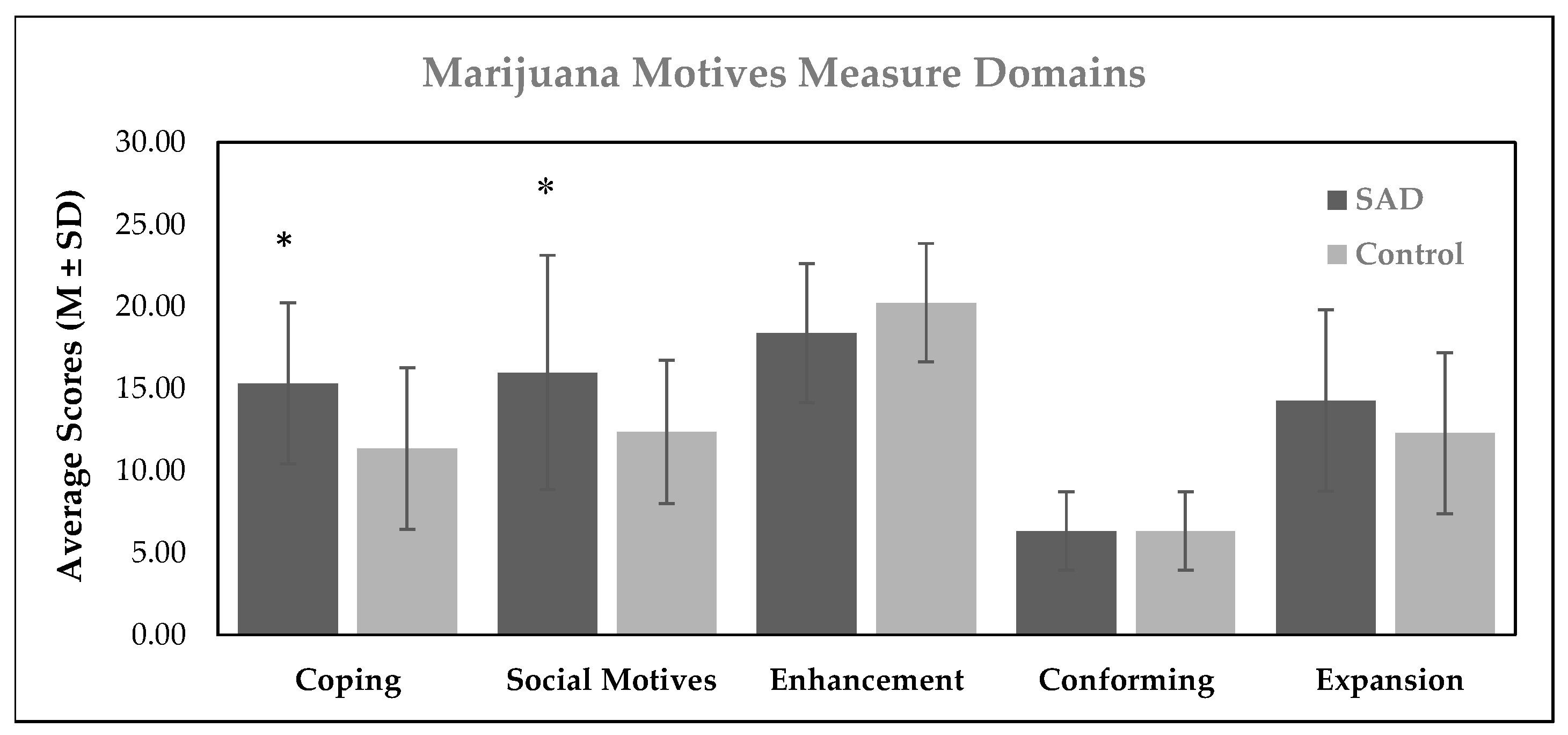
3.2. Results from Outcome Measures at the Maintenance Stage
3.3. Motivation for Cannabis Use across Different Time Trajectories
3.3.1. Stage I: Motivations for Initiating Cannabis Use
“Yeah, I was more just curious about how it [cannabis] would make me feel and anything I did, like if it had any effects.”
“My baseline was to be very anxious, and I was very in my own head, and I thought weed would help with that.”
“…so especially when you’re young, we all want to fit in, we want to part of a clique, and especially in high school you want to be accepted. So, of course, my anxiety was surrounded by that as a teenager. When I was smoking weed I was part of, I guess you would say the popular people. So, when you’re part of that, I was feeling more inclusive. I was always with people that I wanted to be a part of and I felt accepted.”
3.3.2. Stage II: Motivations for Continuing Cannabis Use
3.3.3. Stage III: Motivations for Maintaining Cannabis Use
“Today, I smoke anytime before going into a store, a busy store, or even just going out, going for a walk because it makes me feel more relaxed and more comfortable with myself. Maybe so I can more focus on myself, and I’m not as focused on other people around me, and I’m not as focused on if they’re thinking about me or I don’t feel as embarrassed to be out……Today I think it helps with panic attacks, and I haven’t had a panic attack in a while because I feel like it helps you focus on your breathing and it helps calm you down…”
“Yeah. Okay. It just takes my black-and-white world and throws a little bit of color into it. That’s the best way I can describe [cannabis use]”
3.3.4. Cannabis Use Setting
4. Discussion
4.1. Motivations for Cannabis Use
4.2. Scientific Relevance
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leichsenring, F.; Leweke, F. Social Anxiety Disorder. N. Engl. J. Med. 2017, 376, 2255–2264. [Google Scholar] [CrossRef]
- MacKenzie, M.B.; Fowler, K.F. Social anxiety disorder in the Canadian population: Exploring gender differences in sociodemographic profile. J. Anxiety Disord. 2013, 27, 427–434. [Google Scholar] [CrossRef]
- Shields, M. Social anxiety disorder—beyond shyness. Health Rep. 2004, 15, 45–61. [Google Scholar]
- Stein, D.J.; Lim, C.C.; Roest, A.M.; De Jonge, P.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Benjet, C.; Bromet, E.J.; Bruffaerts, R. The cross-national epidemiology of social anxiety disorder: Data from the World Mental Health Survey Initiative. BMC Med. 2017, 15, 143. [Google Scholar] [CrossRef]
- Bruffaerts, R.; Harris, M.G.; Kazdin, A.E.; Vigo, D.V.; Sampson, N.A.; Chiu, W.T.; Al-Hamzawi, A.; Alonso, J.; Altwaijri, Y.A.; Andrade, L.; et al. Perceived helpfulness of treatment for social anxiety disorder: Findings from the WHO World Mental Health Surveys. Soc. Psychiatry Psychiatr. Epidemiol. 2022, 57, 2079–2095. [Google Scholar] [CrossRef]
- Buckner, J.D.; Zvolensky, M.J. Cannabis and related impairment: The unique roles of cannabis use to cope with social anxiety and social avoidance. Am. J. Addict. 2014, 23, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.D.; Zvolensky, M.J.; Ecker, A.H.; Jeffries, E.R.; Lemke, A.W.; Dean, K.E.; Businelle, M.S.; Gallagher, M.W. Anxiety and cannabis-related problem severity among dually diagnosed outpatients: The impact of false safety behaviors. Addict. Behav. 2017, 70, 49–53. [Google Scholar] [CrossRef]
- Buckner, J.D.; Morris, P.E.; Zvolensky, M.J. Social anxiety and risky Marijuana use: The role of underutilization of protective behavioral strategies. Addict. Behav. 2021, 123, 107078. [Google Scholar] [CrossRef] [PubMed]
- Agosti, V.; Nunes, E.; Levin, F. Rates of Psychiatric Comorbidity Among U.S. Residents with Lifetime Cannabis Dependence. Am. J. Drug Alcohol. Abus. 2002, 28, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.D.; Mallott, M.A.; Schmidt, N.B.; Taylor, J. Peer influence and gender differences in problematic cannabis use among individuals with social anxiety. J. Anxiety Disord. 2006, 20, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.D.; Schmidt, N.B.; Bobadilla, L.; Taylor, J. Social anxiety and problematic cannabis use: Evaluating the moderating role of stress reactivity and perceived coping. Behav. Res. Ther. 2006, 44, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.D.; Schmidt, N.B. Marijuana effect expectancies: Relations to social anxiety and marijuana use problems. Addict. Behav. 2008, 33, 1477–1483. [Google Scholar] [CrossRef]
- Buckner, J.D.; Schmidt, N.B. Social anxiety disorder and marijuana use problems: The mediating role of marijuana effect expectancies. Depress. Anxiety 2009, 26, 864–870. [Google Scholar] [CrossRef]
- Buckner, J.D.; Heimberg, R.G.; Matthews, R.A.; Silgado, J. Marijuana-related problems and social anxiety: The role of marijuana behaviors in social situations. Psychol. Addict. Behav. 2012, 26, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, R.M.; Blumenthal, H.; Mischel, E.R. An Examination of Social Anxiety in Marijuana and Cigarette Use Motives Among Adolescents. Subst. Use Misuse 2016, 51, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.A.; Schubert, F.T.; Hom, M.A.; Cougle, J.R. Correlates of treatment-seeking in individuals with social anxiety disorder: Findings from a nationally representative sample. J. Anxiety Disord. 2022, 91, 102616. [Google Scholar] [CrossRef]
- Single, A.; Bilevicius, E.; Ho, V.; Theule, J.; Buckner, J.D.; Mota, N.; Keough, M.T. Cannabis use and social anxiety in young adulthood: A meta-analysis. Addict. Behav. 2022, 129, 107275. [Google Scholar] [CrossRef]
- Buckner, J.D.; Heimberg, R.G.; Schneier, F.R.; Liu, S.-M.; Wang, S.; Blanco, C. The relationship between cannabis use disorders and social anxiety disorder in the National Epidemiological Study of Alcohol and Related Conditions (NESARC). Drug Alcohol. Depend. 2012, 124, 128–134. [Google Scholar] [CrossRef]
- Buckner, J.D.; Bonn-Miller, M.O.; Zvolensky, M.J.; Schmidt, N.B. Marijuana use motives and social anxiety among marijuana-using young adults. Addict. Behav. 2007, 32, 2238–2252. [Google Scholar] [CrossRef]
- Foster, D.W.; Buckner, J.D.; Schmidt, N.B.; Zvolensky, M.J. Multisubstance Use Among Treatment-Seeking Smokers: Synergistic Effects of Coping Motives for Cannabis and Alcohol Use and Social Anxiety/Depressive Symptoms. Subst. Use Misuse 2016, 51, 165–178. [Google Scholar] [CrossRef]
- Metrik, J.; Jackson, K.; Bassett, S.S.; Zvolensky, M.J.; Seal, K.; Borsari, B. The mediating roles of coping, sleep, and anxiety motives in cannabis use and problems among returning veterans with PTSD and MDD. Psychol. Addict. Behav. 2016, 30, 743–754. [Google Scholar] [CrossRef]
- Morris, P.E.; Buckner, J.D. Cannabis-related problems and social anxiety: The roles of sex and cannabis use motives updated. Addict. Behav. 2023, 137, 107528. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.D.; Zvolensky, M.J.; Ecker, A.H.; Jeffries, E.R. Cannabis craving in response to laboratory-induced social stress among racially diverse cannabis users: The impact of social anxiety disorder. J. Psychopharmacol. 2016, 30, 363–369. [Google Scholar] [CrossRef]
- Buckner, J.D.; Tucker, R.P.; Morris, P.E.; Scherzer, C.R.; Crapanzano, K.A.; Pardue-Bougeois, S. Substance Misuse among a Diverse Psychiatric Inpatient Sample: Suicidal Thoughts and Behaviors and Motivation to Change. Behav. Med. 2023, 49, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Creswell, J.W.; Fetters, M.D.; Plano Clark, V.L.; Morales, A. Mixed methods intervention trials. In Mixed Methods Research for Nursing and the Health Sciences; John Wiley and Sons: Hoboken, NJ, USA, 2009; pp. 161–180. [Google Scholar]
- Guetterman, T.C.; Fetters, M.D.; Creswell, J.W. Integrating Quantitative and Qualitative Results in Health Science Mixed Methods Research Through Joint Displays. Ann. Fam. Med. 2015, 13, 554. [Google Scholar] [CrossRef]
- Hanson, W.E.; Creswell, J.W.; Clark, V.L.P.; Petska, K.S.; Creswell, J.D. Mixed methods research designs in counseling psychology. J. Couns. Psychol. 2005, 52, 224. [Google Scholar] [CrossRef]
- Liebowitz, M.R. Social phobia. Mod. Probl. Pharmacopsychiatry 1987, 22, 141–173. [Google Scholar] [PubMed]
- First, M.B.; Williams JB, W.; Karg, R.S.; Spitzer, R.L. Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV); American Psychiatric Association: Arlington, VA, USA, 2015. [Google Scholar]
- Simons, J.; Correia, C.J.; Carey, K.B.; Borsari, B.E. Validating five-factor marijuana motives measure: Relations with use, problems, and alcohol motives. J. Couns. Psychol. 1998, 45, 265–273. [Google Scholar] [CrossRef]
- Bashford, J.; Flett, R.; Copeland, J. The Cannabis Use Problems Identification Test (CUPIT): Development, reliability, concurrent and predictive validity among adolescents and adults. Addiction 2010, 105, 615–625. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar] [CrossRef]
- Baker, S.L.; Heinrichs, N.; Kim, H.-J.; Hofmann, S.G. The Liebowitz social anxiety scale as a self-report instrument: A preliminary psychometric analysis. Behav. Res. Ther. 2002, 40, 701–715. [Google Scholar] [CrossRef]
- Chabrol, H.; Duconge, E.; Casas, C.; Roura, C.; Carey, K.B. Relations between cannabis use and dependence, motives for cannabis use and anxious, depressive and borderline symptomatology. Addict. Behav. 2005, 30, 829–840. [Google Scholar] [CrossRef]
- Cox, W.M.; Klinger, E. A motivational model of alcohol use. J. Abnorm. Psychol. 1988, 97, 168–180. [Google Scholar] [CrossRef]
- Cox, W.M.; Klinger, E. Incentive motivation, affective change, and alcohol use: A model. In Why People Drink: Parameters of Alcohol as a Reinforcer; Cox, W.M., Ed.; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Cox, W.M.; Klinger, E. Handbook of Motivational Counseling; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Cooper, M.L.; Kuntsche, E.; Levitt, A.; Barber, L.L.; Wolf, S. Motivational models of substance use: A review of theory and research on motives for using alcohol, marijuana, and tobacco. In The Oxford Handbook of Substance Use and Substance Use Disorders; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Elsaid, S.; Kloiber, S.; Le Foll, B. Effects of cannabidiol (CBD) in neuropsychiatric disorders: A review of pre-clinical and clinical findings. Prog. Mol. Biol. Transl. Sci. 2019, 167, 25–75. [Google Scholar] [PubMed]
- Elsaid, S.; Le Foll, B. The complexity of pharmacology of cannabidiol (CBD) and its implications in the treatment of brain disorders. Neuropsychopharmacology 2020, 45, 229–230. [Google Scholar] [CrossRef]
- Masataka, N. Anxiolytic effects of repeated cannabidiol treatment in teenagers with social anxiety disorders. Front. Psychol. 2019, 10, 2466. [Google Scholar] [CrossRef] [PubMed]
- Lauriola, M.; Litman, J.A.; Mussel, P.; De Santis, R.; Crowson, H.M.; Hoffman, R.R. Epistemic curiosity and self-regulation. Personal. Individ. Differ. 2015, 83, 202–207. [Google Scholar] [CrossRef]
- Szumowska, E.; Kruglanski, A.W. Curiosity as end and means. Curr. Opin. Behav. Sci. 2020, 35, 35–39. [Google Scholar] [CrossRef]
- Oyefeso, A. Personality differences among five categories of student cannabis users. Indian. J. Behav. 1991, 15, 29–34. [Google Scholar]
- Hsee, C.K.; Ruan, B. The Pandora Effect: The Power and Peril of Curiosity. Psychol. Sci. 2016, 27, 659–666. [Google Scholar] [CrossRef]
- Noordewier, M.K.; van Dijk, E. Curiosity and time: From not knowing to almost knowing. Cogn. Emot. 2017, 31, 411–421. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ravaioli, S.; Baranès, A.; Woodford, M.; Gottlieb, J. Diverse motives for human curiosity. Nat. Hum. Behav. 2019, 3, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H.; Badiani, A.; Miczek, K.A.; Müller, C.P. Non-pharmacological factors that determine drug use and addiction. Neurosci. Biobehav. Rev. 2020, 110, 3–27. [Google Scholar] [CrossRef]
- Müller, C.P.; Schumann, G. Drugs as instruments: A new framework for non-addictive psychoactive drug use. Behav. Brain Sci. 2011, 34, 293–310. [Google Scholar] [CrossRef]
- Thiel, C.M.; Müller, C.P.; Huston, J.P.; Schwarting, R.K. High versus low reactivity to a novel environment: Behavioral, pharmacological and neurochemical assessments. Neuroscience 1999, 93, 243–251. [Google Scholar] [CrossRef]
- Stolerman, I. Drugs of abuse: Behavioral principles, methods and terms. Trends Pharmacol. Sci. 1992, 13, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D. Hallucinogens. Pharmacol. Ther. 2004, 101, 131–181. [Google Scholar] [CrossRef] [PubMed]
- Villarosa-Hurlocker, M.C.; Bravo, A.J.; Pearson, M.R. The Relationship Between Social Anxiety and Alcohol and Marijuana Use Outcomes Among Concurrent Users: A Motivational Model of Substance Use. Alcohol. Clin. Exp. Res. 2019, 43, 732–740. [Google Scholar] [CrossRef]
- Gobbi, G.; Atkin, T.; Zytynski, T.; Wang, S.; Askari, S.; Boruff, J.; Ware, M.; Marmorstein, N.; Cipriani, A.; Dendukuri, N. Association of cannabis use in adolescence and risk of depression, anxiety, and suicidality in young adulthood: A systematic review and meta-analysis. JAMA Psychiatry 2019, 76, 426–434. [Google Scholar] [CrossRef]
- Turna, J.; Simpson, W.; Patterson, B.; Lucas, P.; Van Ameringen, M. Cannabis use behaviors and prevalence of anxiety and depressive symptoms in a cohort of Canadian medicinal cannabis users. J. Psychiatr. Res. 2019, 111, 134–139. [Google Scholar] [CrossRef]
- Buckner, J.D.; Heimberg, R.G.; Ecker, A.H.; Vinci, C. A biopsychosocial model of social anxiety and substance use. Depress. Anxiety 2013, 30, 276–284. [Google Scholar] [CrossRef]
- Buckner, J.D.; Morris, P.E.; Abarno, C.N.; Glover, N.I.; Lewis, E.M. Biopsychosocial Model Social Anxiety and Substance Use Revised. Curr. Psychiatry Rep. 2021, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Riboldi, I.; Bachi, B.; Calabrese, A.; Moretti, F.; Crocamo, C.; Carrà, G. Efficacy of Cannabidiol for Δ-9-Tetrahydrocannabinol-Induced Psychotic Symptoms, Schizophrenia, and Cannabis Use Disorders: A Narrative Review. J. Clin. Med. 2021, 10, 1303. [Google Scholar] [CrossRef]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Bakas, T.; Van Nieuwenhuijzen, P.; Devenish, S.; McGregor, I.; Arnold, J.; Chebib, M. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol. Res. 2017, 119, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Franco, V.; Bialer, M.; Perucca, E. Cannabidiol in the treatment of epilepsy: Current evidence and perspectives for further research. Neuropharmacology 2021, 185, 108442. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C.; Gautier, C.; Price, J.C.; Kupfer, D.J.; Kinahan, P.E.; Grace, A.A.; Price, J.L.; Mathis, C.A. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol. Psychiatry 2001, 49, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.; Wang, G.-J.; Fowler, J.; Tomasi, D.; Telang, F. Addiction: Beyond dopamine reward circuitry. Proc. Natl. Acad. Sci. USA 2011, 108, 15037–15042. [Google Scholar] [CrossRef]
- Wingo, T.; Nesil, T.; Choi, J.-S.; Li, M.D. Novelty seeking and drug addiction in humans and animals: From behavior to molecules. J. Neuroimmune Pharmacol. 2016, 11, 456–470. [Google Scholar] [CrossRef]
- Zehra, A.; Burns, J.; Liu, C.K.; Manza, P.; Wiers, C.E.; Volkow, N.D.; Wang, G.-J. Cannabis addiction and the brain: A review. J. Neuroimmune Pharmacol. 2018, 13, 438–452. [Google Scholar] [CrossRef]
- King, P.M.; Klemmer, J.; Mansell, K.; Alcorn, J.; Mansell, H. Development of the REACH (Real education about cannabis and health) Program for Canadian youth. J. Nurs. Educ. 2020, 59, 465–469. [Google Scholar] [CrossRef]
- Onrust, S.A.; Otten, R.; Lammers, J.; Smit, F. School-based programs to reduce and prevent substance use in different age groups: What works for whom? Systematic review and meta-regression analysis. Clin. Psychol. Rev. 2016, 44, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Worley, J. Teenagers and cannabis use: Why it’s a problem and what can be done about it. J. Psychosoc. Nurs. Ment. Health Serv. 2019, 57, 11–15. [Google Scholar] [CrossRef] [PubMed]

| SAD (n = 26) | Controls (n = 26) | Statistic | Effect Size 2 | |
|---|---|---|---|---|
| Demographic Variables | ||||
| Age (M ± SD) | 27.92 ± 7.34 | 27.35 ± 6.69 | t = 0.30 | 0.08 |
| Sex (%F) | 53.8 | 50.0 | χ2 = 0.08 | 0.04 |
| Highest Level of Education (%) | χ2 = 1.95 | 0.19 | ||
| Less than university | 53.8 | 34.6 | ||
| University of higher | 46.2 | 65.4 | ||
| Years of Education (M ± SD) | 14.87 ± 1.94 | 15.62 ± 2.26 | t = −1.28 | 0.36 |
| Race (%) | χ2 = 2.58 | 0.22 | ||
| East Asian | 7.7 | 15.4 | ||
| South Asian | 3.8 | 11.5 | ||
| Black African | 7.7 | 11.5 | ||
| Caucasian | 76.9 | 57.7 | ||
| Mixed race | 3.8 | 3.8 | ||
| Race (%) | χ2 = 2.19 | 0.21 | ||
| Other | 23.1 | 42.3 | ||
| Caucasian | 76.9 | 57.7 | ||
| Race (%) | χ2 = 0.75 | 0.12 | ||
| Other | 92.3 | 84.6 | ||
| East Asian | 7.7 | 15.4 | ||
| Occupational status (%) | χ2 = 3.36 | 0.25 | ||
| Unemployed | 26.9 | 7.7 | ||
| Employed or Student | 73.1 | 92.3 | ||
| Income last year (%) | χ2 = 3.50 | 0.27 | ||
| <$50,000 | 60.0 | 33.3 | ||
| ≥$50,000 | 40.0 | 66.7 | ||
| Marital status (%) | χ2 = 0.12 | 0.05 | ||
| Single/Separated | 76.9 | 80.8 | ||
| Married/Common Law | 23.1 | 19.2 | ||
| Clinical variables | ||||
| Age of onset of cannabis use (M ± SD) | 16.63 ± 3.15 | 20.12 ± 4.74 | t = −3.12 * | 0.87 |
| Age of heaviest cannabis use (M ± SD) | 23.05 ± 8.48 | 23.02 ± 4.50 | t = 0.02 | 0.01 |
| Comorbidities (%) | ||||
| Current CUD | 57.7 | 46.2 | χ2 = 0.70 | 0.12 |
| Lifetime CUD | 80.8 | 53.8 | χ2 = 4.28 † | 0.29 |
| Past MDD | 34.6 | |||
| Current GAD | 26.9 | |||
| Other 1 | 30.8 | |||
| LSAS total scores (M ± SD) | 92.65 ± 20.82 | 9.46 ± 6.52 | t = 19.45 ‡ | 5.39 |
| CUPIT (M ± SD) | 36.54 ± 12.35 | 26.31 ± 9.80 | t = 3.31 † | 0.92 |
| Amount of cannabis use/week (g) | 7.57 ± 9.37 | 2.00 ± 1.81 | t = 2.92 † | 0.82 |
| Weekly frequency of cannabis use | 5.06 ± 2.19 | 3.96 ± 2.28 | t = 1.77 | 0.49 |
| Dependent Variables | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MMM Coping | MMM Social | MMM Enhancement | MMM Conformity | MMM Expansion | |||||||||||
| Predictor Variables | B (SE) | β | η2 | B (SE) | β | η2 | B (SE) | β | η2 | B (SE) | β | η2 | B (SE) | β | η2 |
| 1 SAD status | 3.43 (1.13) | 0.33 † | 0.16 | 3.38 (1.61) | 0.28 * | 0.04 | −0.21 (1.01) | −0.25 | 0.08 | 0.14 (0.59) | 0.03 | 0.0 | 1.96 (1.48) | 0.19 | 0.04 |
| 1 Sex | −0.86 (1.13) | −0.08 | 0.01 | −0.24 (1.61) | −0.02 | 0.0 | −1.32 (1.01) | −0.17 | 0.04 | 0.21 (0.58) | 0.05 | 0.0 | 0.45 (1.48) | 0.04 | 0.0 |
| Age | −0.12 (0.08) | −0.15 | 0.04 | −0.16 (0.12) | −0.18 | 0.04 | −0.13 (0.07) | −0.23 | 0.07 | −0.04 (0.04) | −0.13 | 0.02 | −0.11 (0.11) | −0.14 | 0.02 |
| 1 Current CUD | 5.45 (1.15) | 0.52 ‡ | 0.33 | 2.98 (1.63) | 0.25 | 0.07 | 2.57 (1.02) | 0.32 * | 0.12 | 0.95 (0.60) | 0.23 | 0.05 | 0.73 (1.51) | 0.07 | 0.01 |
| (n = 26) | Age | Sex 1 | Current CUD 1 | Lifetime MDD 1 | Current GAD 1 | Educ. 1 | Race (W) 1 | Race (EA) 1 | Income 1 | Occ. Status 1 | Marital Status 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total LSAS scores | −0.16 | −0.06 | 0.28 | 0.19 | 0.02 | −0.14 | 0.18 | −0.03 | −0.29 | 0.26 | −0.25 |
| Weekly cannabis use frequency | 0.14 | 0.21 | 0.60 † | 0.06 | 0.28 | −0.39 * | 0.55 † | 0.34 | −0.28 | 0.07 | −0.24 |
| The weekly amount of cannabis use (g) | 0.13 | 0.23 | 0.42 * | −0.02 | 0.46 * | −0.59 † | −0.24 | 0.18 | −0.35 | 0.09 | −0.14 |
| CUPIT | 0.13 | 0.16 | 0.65 † | −0.02 | 0.41 * | −0.43 * | −0.27 | 0.20 | −0.55 † | 0.13 | −0.20 |
| MMM Total | −0.55 † | 0.20 | 0.51 † | 0.14 | 0.11 | 0.07 | −0.13 | 0.15 | −0.34 | −0.11 | −0.46 * |
| MMM Coping | −0.51 † | 0.11 | 0.60 † | 0.17 | 0.27 | 0.14 | 0.06 | 0.11 | −0.43 * | 0.02 | −0.32 |
| MMM Social | −0.38 | 0.08 | 0.32 | −0.03 | 0.24 | −0.11 | −0.11 | 0.06 | −0.12 | −0.29 | −0.32 |
| MMM Enhancement | −0.32 | −0.06 | 0.38 | 0.24 | 0.07 | 0.21 | 0.00 | 0.13 | −0.43 * | −0.20 | −0.54 † |
| MMM Conformity | −0.27 | 0.22 | 0.38 | −0.19 | −0.02 | 0.12 | 0.05 | 0.16 | 0.05 | −0.23 | −0.24 |
| MMM Expansion | −0.33 | 0.33 | 0.10 | 0.19 | −0.26 | 0.01 | −0.31 | 0.09 | −0.19 | 0.29 | −0.16 |
| (n = 26) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Total LSAS scores | |||||||||
| 2. Weekly cannabis use frequency | 0.16 | ||||||||
| 3. The weekly amount of cannabis use (g) | 0.28 | 0.56 † | |||||||
| 4. CUPIT | 0.37 | 0.70 † | 0.78 † | ||||||
| 5. MMM Total | 0.43 * | 0.12 | 0.26 | 0.23 | |||||
| 6. MMM Coping | 0.49 * | 0.22 | 0.21 | 0.36 | 0.80 † | ||||
| 7. MMM Social | 0.50 † | 0.31 | 0.18 | 0.17 | 0.82 † | 0.59 † | |||
| 8. MMM Enhancement | 0.27 | −0.17 | 0.05 | 0.16 | 0.69 † | 0.53 † | 0.39 * | ||
| 9. MMM Conformity | 0.13 | 0.12 | 0.07 | 0.05 | 0.41 * | 0.33 | 0.36 | 0.17 | |
| 10. MMM Expansion | −0.06 | −0.16 | 0.26 | −0.01 | 0.50 * | 0.16 | 0.15 | 0.25 | −0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsaid, S.; Wang, R.; Kloiber, S.; Le Foll, B.; Hassan, A.N. Motivations for Cannabis Use in Individuals with Social Anxiety Disorder (SAD). Brain Sci. 2023, 13, 1698. https://doi.org/10.3390/brainsci13121698
Elsaid S, Wang R, Kloiber S, Le Foll B, Hassan AN. Motivations for Cannabis Use in Individuals with Social Anxiety Disorder (SAD). Brain Sciences. 2023; 13(12):1698. https://doi.org/10.3390/brainsci13121698
Chicago/Turabian StyleElsaid, Sonja, Ruoyu Wang, Stefan Kloiber, Bernard Le Foll, and Ahmed N. Hassan. 2023. "Motivations for Cannabis Use in Individuals with Social Anxiety Disorder (SAD)" Brain Sciences 13, no. 12: 1698. https://doi.org/10.3390/brainsci13121698
APA StyleElsaid, S., Wang, R., Kloiber, S., Le Foll, B., & Hassan, A. N. (2023). Motivations for Cannabis Use in Individuals with Social Anxiety Disorder (SAD). Brain Sciences, 13(12), 1698. https://doi.org/10.3390/brainsci13121698







