A Comparison of Functional Connectivity in the Human Brainstem and Spinal Cord Associated with Noxious and Innocuous Thermal Stimulation Identified by Means of Functional MRI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Recruitment
2.2. Sham MRI Training
2.3. Stimulation Paradigm
2.4. Functional MRI Acquisition
2.5. FMRI Data Preprocessing
2.6. FMRI Data Analysis
2.7. Structural Equation Modeling (SEM)
2.8. Analyses of Covariance (ANCOVA)
3. Results
3.1. SEM Analysis
3.2. Group-Level Comparisons with ANCOVA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heinricher, M.M.; Tavares, I.; Leith, J.L.; Lumb, B.M. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res. Rev. 2009, 60, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Keltner, J.R.; Furst, A.; Fan, C.; Redfern, R.; Inglis, B.; Fields, H.L. Isolating the modulatory effect of expectation on pain transmission: A functional magnetic resonance imaging study. J. Neurosci. 2006, 26, 4437–4443. [Google Scholar] [CrossRef] [PubMed]
- Rempe, T.; Wolff, S.; Riedel, C.; Baron, R.; Stroman, P.W.; Jansen, O.; Gierthmuhlen, J. Spinal and supraspinal processing of thermal stimuli: An fMRI study. J. Magn. Reson. Imaging 2015, 41, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Summers, P.E.; Ferraro, D.; Duzzi, D.; Lui, F.; Iannetti, G.D.; Porro, C.A. A quantitative comparison of BOLD fMRI responses to noxious and innocuous stimuli in the human spinal cord. Neuroimage 2010, 50, 1408–1415. [Google Scholar] [CrossRef]
- Ioachim, G.; Powers, J.M.; Warren, H.J.M.; Stroman, P.W. Coordinated Human Brainstem and Spinal Cord Networks during the Expectation of Pain Have Elements Unique from Resting-State Effects. Brain Sci. 2020, 10, 568. [Google Scholar] [CrossRef]
- Stroman, P.W.; Bosma, R.L.; Cotoi, A.I.; Leung, R.H.; Kornelsen, J.; Lawrence-Dewar, J.M.; Pukall, C.F.; Staud, R. Continuous Descending Modulation of the Spinal Cord Revealed by Functional MRI. PLoS ONE 2016, 11, e0167317. [Google Scholar] [CrossRef]
- Coghill, R.C. Individual differences in the subjective experience of pain: New insights into mechanisms and models. Headache 2010, 50, 1531–1535. [Google Scholar] [CrossRef]
- Fillingim, R.B. Individual differences in pain responses. Curr. Rheumatol. Rep. 2005, 7, 342–347. [Google Scholar] [CrossRef]
- Stroman, P.W.; Ioachim, G.; Powers, J.M.; Staud, R.; Pukall, C. Pain processing in the human brainstem and spinal cord before, during, and after the application of noxious heat stimuli. Pain 2018, 159, 2012–2020. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J. Personal. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef]
- Spielberger, C.D. State-Trait Anxiety Inventory; Wiley Online Library: Hoboken, NJ, USA, 1970. [Google Scholar] [CrossRef]
- Crowne, D.P.; Marlowe, D. A new scale of social desirability independent of psychopathology. J. Consult. Psychol. 1960, 24, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.; Bishop, S.R.; Pivik, J. The pain catastrophizing scale: Development and validation. Psychol. Assess. 1995, 7, 524. [Google Scholar] [CrossRef]
- Vierck, C.J., Jr.; Cannon, R.L.; Fry, G.; Maixner, W.; Whitsel, B.L. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J. Neurophysiol. 1997, 78, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Staud, R.; Robinson, M.E.; Vierck, C.J., Jr.; Cannon, R.C.; Mauderli, A.P.; Price, D.D. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain 2003, 105, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Price, D.D.; Hu, J.W.; Dubner, R.; Gracely, R.H. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain 1977, 3, 57–68. [Google Scholar] [CrossRef]
- De Leener, B.; Fonov, V.S.; Collins, D.L.; Callot, V.; Stikov, N.; Cohen-Adad, J. PAM50: Unbiased multimodal template of the brainstem and spinal cord aligned with the ICBM152 space. Neuroimage 2018, 165, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Harita, S.; Stroman, P.W. Confirmation of resting-state BOLD fluctuations in the human brainstem and spinal cord after identification and removal of physiological noise. Magn. Reson. Med. 2017, 78, 2149–2156. [Google Scholar] [CrossRef]
- Stroman, P.W.; Khan, H.S.; Bosma, R.L.; Cotoi, A.I.; Leung, R.; Cadotte, D.W.; Fehlings, M.G. Changes in Pain Processing in the Spinal Cord and Brainstem after Spinal Cord Injury Characterized by Functional Magnetic Resonance Imaging. J. Neurotrauma 2016, 33, 1450–1460. [Google Scholar] [CrossRef]
- Naidich, T.P.; Duvernoy, H.M.; Delman, B.N.; Sorensen, A.G.; Kollias, S.S.; Haacke, E.M. Internal Architecture of the Brain Stem with Key Axial Sections. In Duvernoy’s Atlas of the Human Brain Stem and Cerebellum; Springer: New York, NY, USA; Wien, Austria, 2009; pp. 79–82. [Google Scholar]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- De Leener, B.; Levy, S.; Dupont, S.M.; Fonov, V.S.; Stikov, N.; Collins, D.L.; Callot, V.; Cohen-Adad, J. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage 2017, 145 Pt A, 24–43. [Google Scholar] [CrossRef]
- Lang, J.; Bartram, C.T. Fila radicularia of the ventral and dorsal radices of the human spinal cord. Gegenbaurs Morphol. Jahrb. 1982, 128, 417–462. [Google Scholar] [PubMed]
- Liebe, T.; Kaufmann, J.; Li, M.; Skalej, M.; Wagner, G.; Walter, M. In vivo anatomical mapping of human locus coeruleus functional connectivity at 3 T MRI. Hum. Brain Mapp. 2020, 41, 2136–2151. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.C.; Bowen, A.; Schier, L.A.; Tupone, D.; Uddin, O.; Heinricher, M.M. Parabrachial Complex: A Hub for Pain and Aversion. J. Neurosci. 2019, 39, 8225–8230. [Google Scholar] [CrossRef] [PubMed]
- Talairach, J.; Tournoux, P. Co-Planar Stereotaxic Atlas of the Human Brain; Thieme Medical Publishers, Inc.: New York, NY, USA, 1988. [Google Scholar]
- Leijnse, J.N.; D’Herde, K. Revisiting the segmental organization of the human spinal cord. J. Anat. 2016, 229, 384–393. [Google Scholar] [CrossRef]
- Pauli, W.M.; Nili, A.N.; Tyszka, J.M. A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci. Data 2018, 5, 180063. [Google Scholar] [CrossRef]
- Keren, N.I.; Lozar, C.T.; Harris, K.C.; Morgan, P.S.; Eckert, M.A. In vivo mapping of the human locus coeruleus. Neuroimage 2009, 47, 1261–1267. [Google Scholar] [CrossRef]
- Stroman, P.W.; Warren, H.J.M.; Ioachim, G.; Powers, J.M.; McNeil, K. A comparison of the effectiveness of functional MRI analysis methods for pain research: The new normal. PLoS ONE 2020, 15, e0243723. [Google Scholar] [CrossRef]
- Stroman, P.W. Validation of Structural Equation Modeling Methods for Functional MRI Data Acquired in the Human Brainstem and Spinal Cord. Crit. Rev. Biomed. Eng. 2016, 44, 227–241. [Google Scholar] [CrossRef]
- Leung, R.H.; Stroman, P.W. Functional Magnetic Resonance Imaging of the Human Brainstem and Cervical Spinal Cord during Cognitive Modulation of Pain. Crit. Rev. Biomed. Eng. 2016, 44, 47–71. [Google Scholar] [CrossRef]
- Martucci, K.T.; Mackey, S.C. Neuroimaging of Pain: Human Evidence and Clinical Relevance of Central Nervous System Processes and Modulation. Anesthesiology 2018, 128, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef]
- Ghazni, N.F.; Cahill, C.M.; Stroman, P.W. Tactile sensory and pain networks in the human spinal cord and brain stem mapped by means of functional MR imaging. AJNR Am. J. Neuroradiol. 2010, 31, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Borsook, D.; Edwards, R.; Elman, I.; Becerra, L.; Levine, J. Pain and analgesia: The value of salience circuits. Prog. Neurobiol. 2013, 104, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, I.; Chung, Y.A.; Kim, T.S.; Namgung, E.; Lee, S.; Oh, J.K.; Jeong, H.S.; Cho, H.; Kim, M.J.; et al. Altered attentional control over the salience network in complex regional pain syndrome. Sci. Rep. 2018, 8, 7466. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Necka, E.A.; Atlas, L.Y. Distinguishing pain from nociception, salience, and arousal: How autonomic nervous system activity can improve neuroimaging tests of specificity. Neuroimage 2020, 204, 116254. [Google Scholar] [CrossRef]
- Martins, I.; Tavares, I. Reticular Formation and Pain: The Past and the Future. Front. Neuroanat. 2017, 11, 51. [Google Scholar] [CrossRef]
- Chiang, C.Y.; Hu, J.W.; Sessle, B.J. Parabrachial area and nucleus raphe magnus-induced modulation of nociceptive and nonnociceptive trigeminal subnucleus caudalis neurons activated by cutaneous or deep inputs. J. Neurophysiol. 1994, 71, 2430–2445. [Google Scholar] [CrossRef]
- Harper, D.E.; Ichesco, E.; Schrepf, A.; Hampson, J.P.; Clauw, D.J.; Schmidt-Wilcke, T.; Harris, R.E.; Harte, S.E. Resting Functional Connectivity of the Periaqueductal Gray Is Associated with Normal Inhibition and Pathological Facilitation in Conditioned Pain Modulation. J. Pain 2018, 19, 635.e1–635.e15. [Google Scholar] [CrossRef]
- Pereira, E.A.; Lu, G.; Wang, S.; Schweder, P.M.; Hyam, J.A.; Stein, J.F.; Paterson, D.J.; Aziz, T.Z.; Green, A.L. Ventral periaqueductal grey stimulation alters heart rate variability in humans with chronic pain. Exp. Neurol. 2010, 223, 574–581. [Google Scholar] [CrossRef]
- Makovac, E.; Venezia, A.; Hohenschurz-Schmidt, D.; Dipasquale, O.; Jackson, J.B.; Medina, S.; O’Daly, O.; Williams, S.C.R.; McMahon, S.B.; Howard, M.A. The association between pain-induced autonomic reactivity and descending pain control is mediated by the periaqueductal grey. J. Physiol. 2021, 599, 5243–5260. [Google Scholar] [CrossRef]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Adam, S.K.; Manan, N.A.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef] [PubMed]
- Kerfoot, E.C.; Chattillion, E.A.; Williams, C.L. Functional interactions between the nucleus tractus solitarius (NTS) and nucleus accumbens shell in modulating memory for arousing experiences. Neurobiol. Learn. Mem. 2008, 89, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Samuels, E.R.; Szabadi, E. Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function part II: Physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr. Neuropharmacol. 2008, 6, 254–285. [Google Scholar] [CrossRef]
- Wardach, J.; Wagner, M.; Jeong, Y.; Holden, J.E. Lateral Hypothalamic Stimulation Reduces Hyperalgesia Through Spinally Descending Orexin-A Neurons in Neuropathic Pain. West. J. Nurs. Res. 2016, 38, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, E. Cellular Mechanisms for Antinociception Produced by Oxytocin and Orexins in the Rat Spinal Lamina II-Comparison with Those of Other Endogenous Pain Modulators. Pharmaceuticals 2019, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Ab Aziz, C.B.; Ahmad, A.H. The role of the thalamus in modulating pain. Malays. J. Med. Sci. 2006, 13, 11–18. [Google Scholar] [PubMed]
- Bushnell, M.C.; Ceko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Kropotov, J.D.; Crawford, H.J.; Polyakov, Y.I. Somatosensory event-related potential changes to painful stimuli during hypnotic analgesia: Anterior cingulate cortex and anterior temporal cortex intracranial recordings. Int. J. Psychophysiol. 1997, 27, 1–8. [Google Scholar] [CrossRef]
- Bagley, E.E.; Ingram, S.L. Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology 2020, 173, 108131. [Google Scholar] [CrossRef]
- Benarroch, E.E. Locus coeruleus. Cell Tissue Res. 2018, 373, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Spisak, T.; Kincses, B.; Schlitt, F.; Zunhammer, M.; Schmidt-Wilcke, T.; Kincses, Z.T.; Bingel, U. Pain-free resting-state functional brain connectivity predicts individual pain sensitivity. Nat. Commun. 2020, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.; Erpelding, N.; Kucyi, A.; DeSouza, D.D.; Davis, K.D. Individual Differences in Temporal Summation of Pain Reflect Pronociceptive and Antinociceptive Brain Structure and Function. J. Neurosci. 2015, 35, 9689–9700. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.S.; Stroman, P.W. Inter-individual differences in pain processing investigated by functional magnetic resonance imaging of the brainstem and spinal cord. Neuroscience 2015, 307, 231–241. [Google Scholar] [CrossRef] [PubMed]

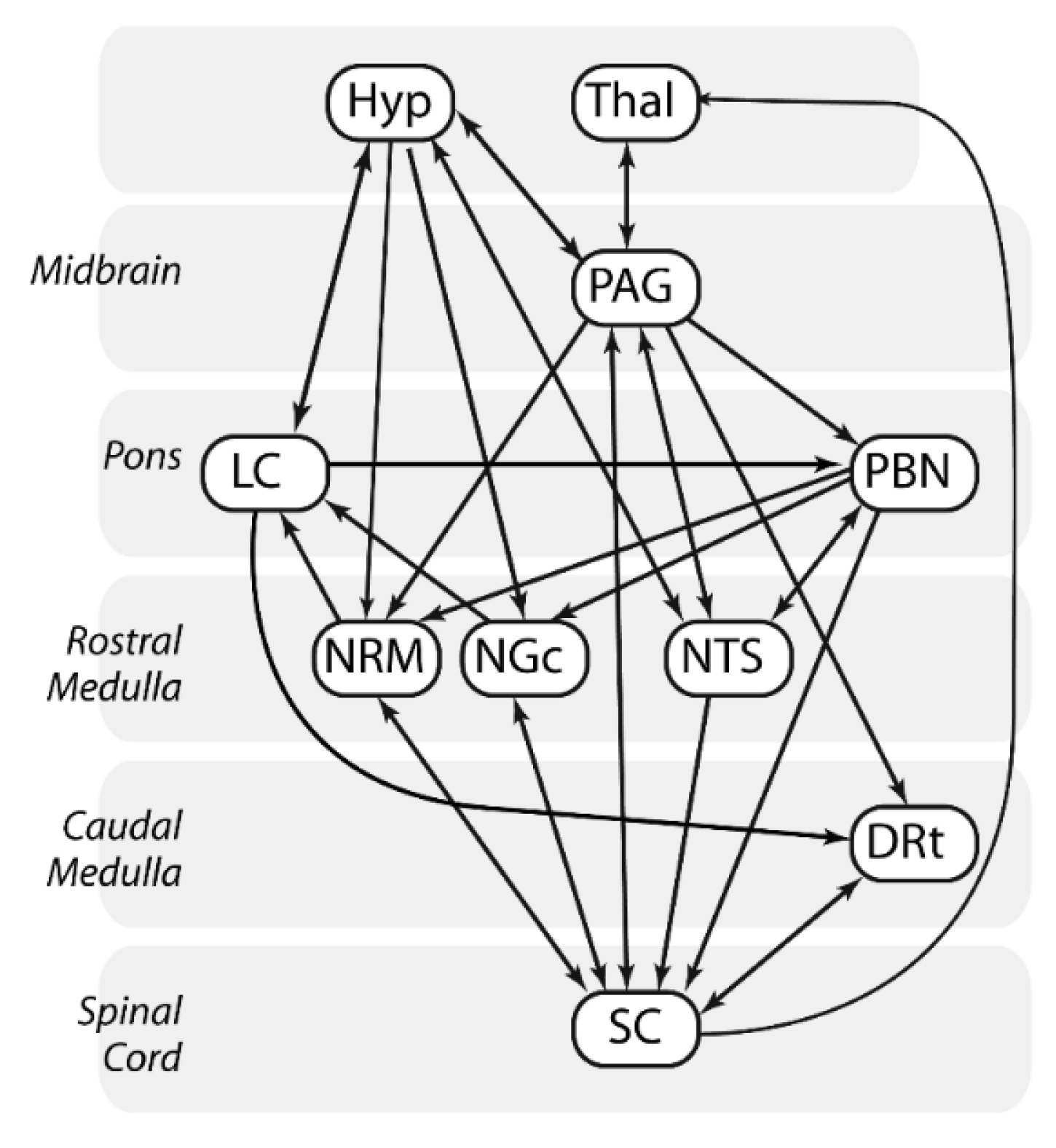
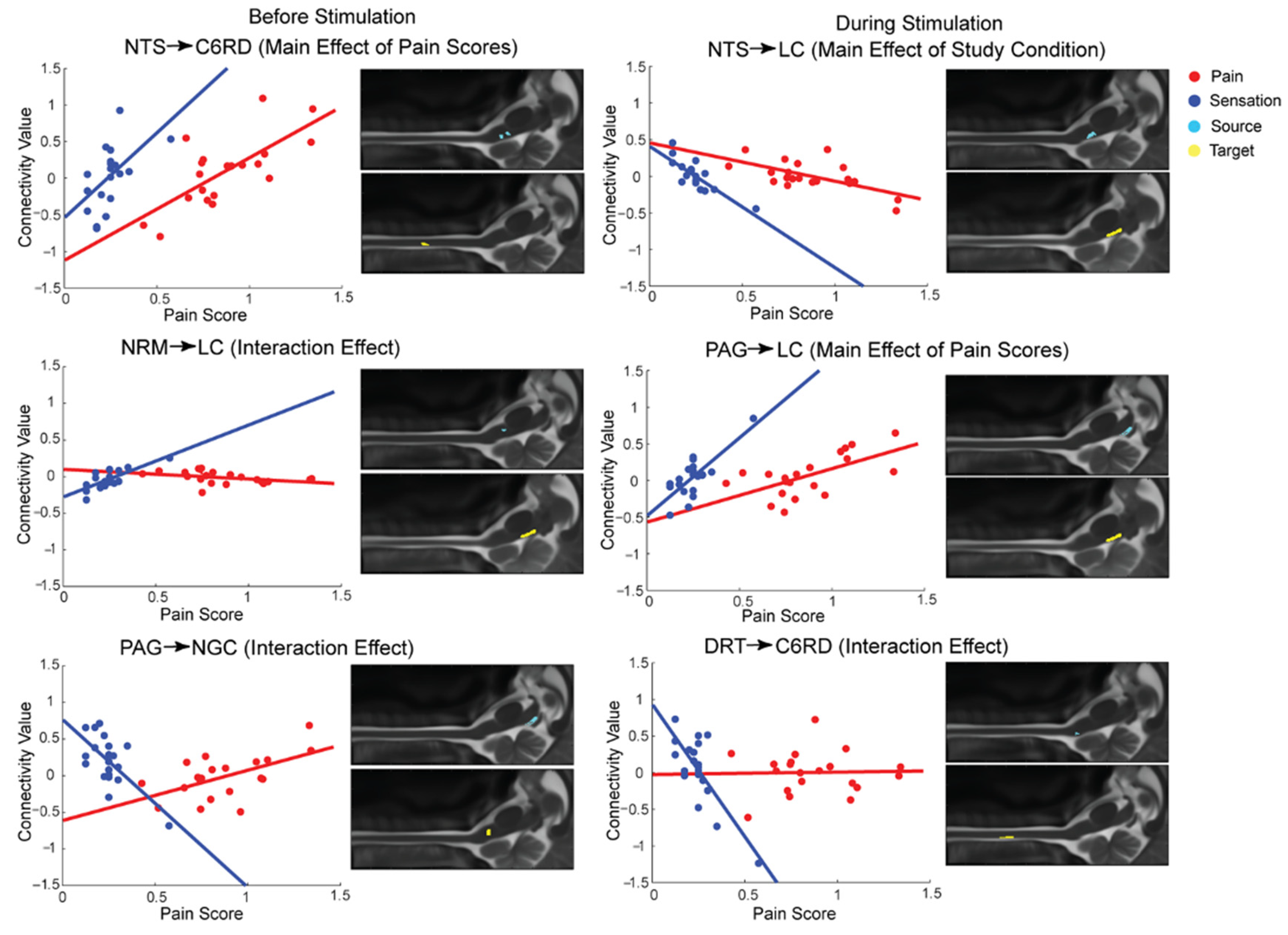
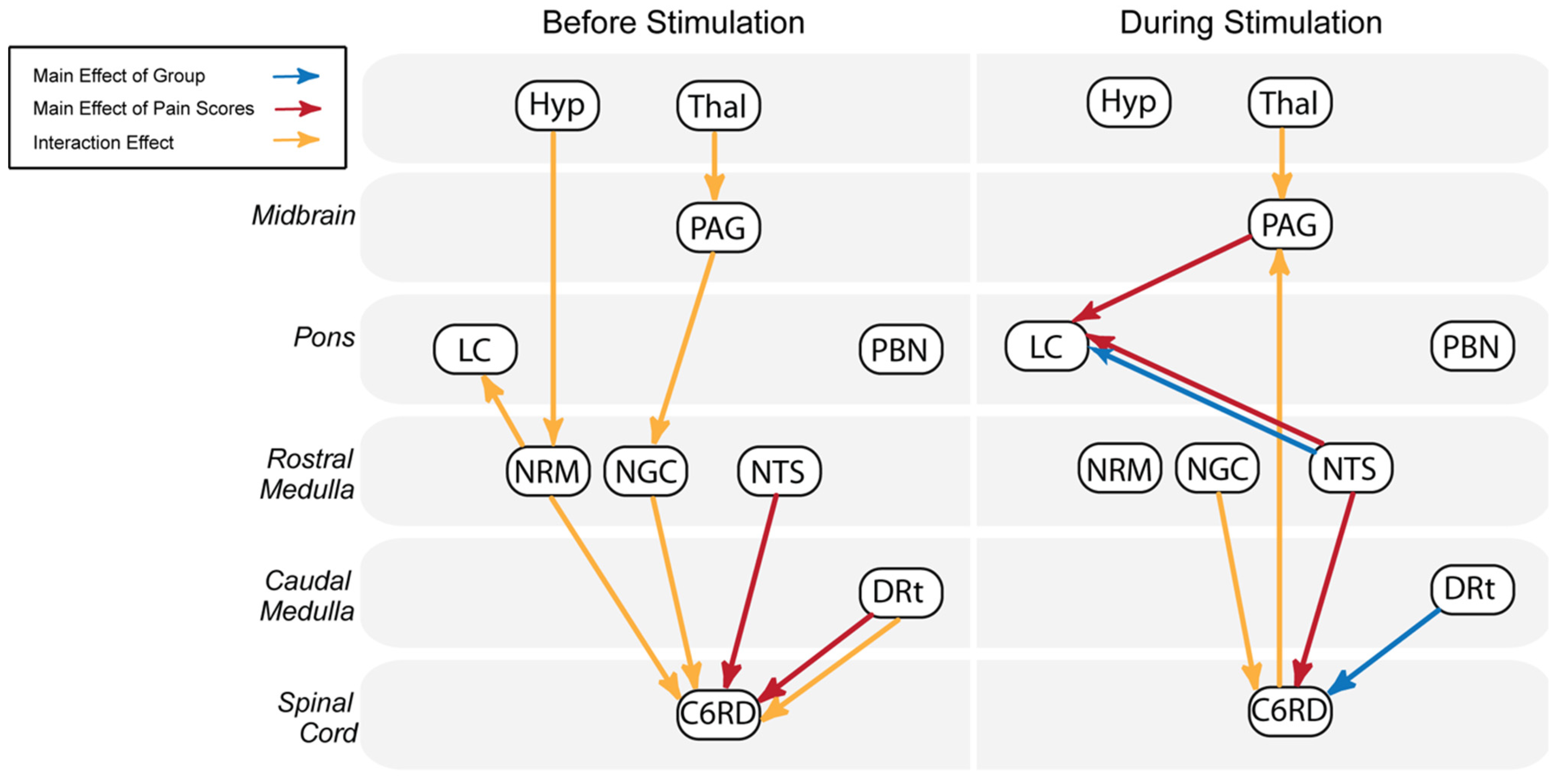
| Before Stimulation | During Stimulation | |||||||
|---|---|---|---|---|---|---|---|---|
| Connection | β | Z | T | Connection | β | Z | T | |
| Noxious | PAG ⟶ PBN | 0.16 ± 0.02 | 0.354 | 7.392 | PAG ⟶ LC | 0.61 ± 0.09 | 0.839 | 6.423 |
| PBN ⟶ NTS | 0.41 ± 0.06 | 0.598 | 6.486 | |||||
| PBN ⟶ NGc | 0.30 ± 0.04 | −0.232 | 7.899 | |||||
| Innocuous | PBN ⟶ NTS | 0.28 ± 0.04 | −0.420 | 6.615 | PAG ⟶ PBN | 0.16 ± 0.02 | 0.996 | 9.534 |
| NRM ⟶ C6RD | 0.24 ± 0.09 | 4.843 | 2.751 | PAG ⟶ Hyp | 0.15 ± 0.02 | 0.849 | 6.988 | |
| PAG ⟶ NGc | 0.23 ± 0.04 | 0.688 | 6.349 | |||||
| NRM ⟶ C6RD | 0.04 ± 0.03 | 4.971 | 1.212 | |||||
| NTS ⟶ LC | 0.01 ± 0.05 | −4.699 | 0.251 | |||||
| Before Stimulation | During Stimulation | |||||
|---|---|---|---|---|---|---|
| Connection | p-Value | F-Value | Connection | p-Value | F-Value | |
| Main Effect of Study Condition | DRT ⟶ C6RD | 2.24 × 10−5 | 23.68 | |||
| NTS ⟶ LC | 3.09 × 10−5 | 22.67 | ||||
| Main Effect of Pain Scores | NTS ⟶ C6RD | 1.48 × 10−5 | 25.04 | NTS ⟶ LC | 3.24 × 10−6 | 30.25 |
| DRT ⟶ C6RD | 2.69 × 10−5 | 23.14 | PAG ⟶ LC | 1.18 × 10−5 | 25.77 | |
| NTS ⟶ C6RD | 4.47 × 10−5 | 21.54 | ||||
| Interaction Effect | DRT ⟶ C6RD | 6.46 × 10−7 | 36.27 | Tha ⟶ PAG | 6.92 × 10−6 | 27.62 |
| NRM ⟶ C6RD | 1.23 × 10−6 | 33.85 | NGC ⟶ C6RD | 1.45 × 10−5 | 25.11 | |
| NRM ⟶ LC | 2.29 × 10−6 | 31.47 | DRT ⟶ C6RD | 2.29 × 10−5 | 23.66 | |
| Tha ⟶ PAG | 7.08 × 10−6 | 27.52 | ||||
| Hyp ⟶ NRM | 3.02 × 10−5 | 22.79 | ||||
| NGC ⟶ C6RD | 3.47 × 10−5 | 22.33 | ||||
| PAG ⟶ NGC | 4.47 × 10−5 | 21.56 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koning, E.; Powers, J.M.; Ioachim, G.; Stroman, P.W. A Comparison of Functional Connectivity in the Human Brainstem and Spinal Cord Associated with Noxious and Innocuous Thermal Stimulation Identified by Means of Functional MRI. Brain Sci. 2023, 13, 777. https://doi.org/10.3390/brainsci13050777
Koning E, Powers JM, Ioachim G, Stroman PW. A Comparison of Functional Connectivity in the Human Brainstem and Spinal Cord Associated with Noxious and Innocuous Thermal Stimulation Identified by Means of Functional MRI. Brain Sciences. 2023; 13(5):777. https://doi.org/10.3390/brainsci13050777
Chicago/Turabian StyleKoning, Elena, Jocelyn M. Powers, Gabriela Ioachim, and Patrick W. Stroman. 2023. "A Comparison of Functional Connectivity in the Human Brainstem and Spinal Cord Associated with Noxious and Innocuous Thermal Stimulation Identified by Means of Functional MRI" Brain Sciences 13, no. 5: 777. https://doi.org/10.3390/brainsci13050777






