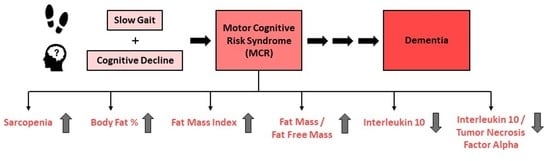
Association of Motoric Cognitive Risk Syndrome with Sarcopenia and Systemic Inflammation in Pre-Frail Older Adults
Abstract
:1. Introduction
2. Methods
2.1. Study Participants and Design
2.2. Co-Variates
2.3. Body Composition
2.4. Inflammatory Biomarkers
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Motoric Cognitive Risk Syndrome, Subjective Cognitive Decline, Slow Gait, Sarcopenia and Body Composition Associations
3.3. Motoric Cognitive Risk Syndrome, Subjective Cognitive Decline, Slow Gait and Systemic Inflammatory Biomarkers
4. Discussion
4.1. Strengths and Limitations
4.2. Study Highlights and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sathyan, S.; Ayers, E.; Gao, T.; Milman, S.; Barzilai, N.; Rockwood, K.; Verghese, J. Frailty and Risk of Incident Motoric Cognitive Risk Syndrome. J. Alzheimers Dis. 2019, 71, S85–S93. [Google Scholar] [CrossRef]
- Verghese, J.; Ayers, E.; Barzilai, N.; Bennett, D.A.; Buchman, A.S.; Holtzer, R.; Katz, M.J.; Lipton, R.B.; Wang, C. Motoric cognitive risk syndrome: Multicenter incidence study. Neurology 2014, 83, 2278–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maguire, F.J.; Killane, I.; Creagh, A.P.; Donoghue, O.; Kenny, R.A.; Reilly, R.B. Baseline Association of Motoric Cognitive Risk Syndrome With Sustained Attention, Memory, and Global Cognition. J. Am. Med. Dir. Assoc. 2018, 19, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Shimada, H.; Makizako, H.; Tsutsumimoto, K.; Verghese, J.; Suzuki, T. Motoric Cognitive Risk Syndrome: Association with Incident Dementia and Disability. J. Alzheimers Dis. 2017, 59, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Maggio, M.; Lauretani, F. Prevalence, incidence, and clinical impact of cognitive-motoric risk syndrome in Europe, USA, and Japan: Facts and numbers update 2019. J. Cachexia Sarcopenia Muscle 2019, 10, 953–955. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Sekhon, H.; Schott, A.M.; Rolland, Y.; Muir-Hunter, S.; Markle-Reid, M.; Gagne, H.; Allali, G. Motoric Cognitive Risk Syndrome and Risk for Falls, Their Recurrence, and Postfall Fractures: Results From a Prospective Observational Population-Based Cohort Study. J. Am. Med. Dir. Assoc. 2019, 20, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Navarro, S.G.; Mimenza-Alvarado, A.J.; Aguilar-Esquivel, J.E.; Yeverino-Castro, S.G.; Juarez-Cedillo, T.; Mejia-Arango, S. Motoric Cognitive Risk Syndrome: Prevalence and Risk of Cognitive Impairment in a Population Studied in the Mexican Health and Aging Study 2012-2015. J. Nutr. Health Aging 2019, 23, 227–231. [Google Scholar] [CrossRef]
- Marquez, I.; Garcia-Cifuentes, E.; Velandia, F.R.; Iragorri, A.; Saavedra, A.M.; Borda, M.G.; Osuna, M.; Ailshire, J.; Cano-Gutierrez, C.A. Motoric Cognitive Risk Syndrome: Prevalence and Cognitive Performance. A cross-sectional study. Lancet Reg. Health-Am. 2022, 8, 100162. [Google Scholar] [CrossRef]
- Merchant, R.A.; Goh, J.; Chan, Y.H.; Lim, J.Y.; Vellas, B. Slow Gait, Subjective Cognitive Decline and Motoric Cognitive Risk Syndrome: Prevalence and Associated Factors in Community Dwelling Older Adults. J. Nutr. Health Aging 2021, 25, 48–56. [Google Scholar] [CrossRef]
- Allali, G.; Ayers, E.I.; Verghese, J. Motoric Cognitive Risk Syndrome Subtypes and Cognitive Profiles. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 378–384. [Google Scholar] [CrossRef] [Green Version]
- Chhetri, J.K.; Han, C.; Dan, X.; Ma, L.; Chan, P. Motoric Cognitive Risk Syndrome in a Chinese Older Adult Population: Prevalence and Associated Factors. J. Am. Med. Dir. Assoc. 2020, 21, 136–137. [Google Scholar] [CrossRef] [Green Version]
- Verghese, J.; Wang, C.; Lipton, R.B.; Holtzer, R. Motoric cognitive risk syndrome and the risk of dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Tian, Q.; Resnick, S.M.; Mielke, M.M.; Yaffe, K.; Launer, L.J.; Jonsson, P.V.; Grande, G.; Welmer, A.K.; Laukka, E.J.; Bandinelli, S.; et al. Association of Dual Decline in Memory and Gait Speed With Risk for Dementia Among Adults Older Than 60 Years: A Multicohort Individual-Level Meta-analysis. JAMA Netw. Open 2020, 3, e1921636. [Google Scholar] [CrossRef] [PubMed]
- Kumai, K.; Meguro, K.; Kasai, M.; Nakamura, K.; Nakatsuka, M. Neuroepidemiologic and Neurobehavioral Characteristics of Motoric Cognitive Risk Syndrome in an Old-Old Population: The Kurihara Project. Dement. Geriatr. Cogn. Disord. Extra 2016, 6, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, A.; Darweesh, S.K.L.; Dommershuijsen, L.J.; Vernooij, M.W.; Ikram, M.K.; Wolters, F.J.; Ikram, M.A. Risk factors, neuroimaging correlates and prognosis of the motoric cognitive risk syndrome: A population-based comparison with mild cognitive impairment. Eur. J. Neurol. 2022, 29, 1587–1599. [Google Scholar] [CrossRef]
- Xu, W.; Bai, A.; Liang, Y.; Lin, Z. Association between depression and motoric cognitive risk syndrome among community-dwelling older adults in China: A 4-year prospective cohort study. Eur. J. Neurol. 2022, 29, 1377–1384. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Ismail, Z.; Livingston, G. One third of dementia cases can be prevented within the next 25 years by tackling risk factors. The case “for” and “against”. Alzheimers Res. Ther. 2020, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Merchant, R.A.; Chan, Y.H.; Hui, R.J.Y.; Tsoi, C.T.; Kwek, S.C.; Tan, W.M.; Lim, J.Y.; Sandrasageran, S.; Wong, B.L.L.; Chen, M.Z.; et al. Motoric cognitive risk syndrome, physio-cognitive decline syndrome, cognitive frailty and reversibility with dual-task exercise. Exp. Gerontol. 2021, 150, 111362. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Woodhouse, L.; Rodriguez-Manas, L.; Fried, L.P.; Woo, J.; Aprahamian, I.; Sanford, A.; Lundy, J.; et al. Physical Frailty: ICFSR International Clinical Practice Guidelines for Identification and Management. J. Nutr. Health Aging 2019, 23, 771–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, S.; Zeng, X.; Xu, L.; Chen, L.; Liu, Z.; Chu, J.; Yang, Y.; Wu, X.; Chen, X. Association between motoric cognitive risk syndrome and frailty among older Chinese adults. BMC Geriatr. 2020, 20, 110. [Google Scholar] [CrossRef] [Green Version]
- Xue, Q.-L.; Bandeen-Roche, K.; Tian, J.; Kasper, J.D.; Fried, L.P. Progression of Physical Frailty and the Risk of All-Cause Mortality: Is There a Point of No Return? J. Am. Geriatr. Soc. 2021, 69, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Merchant, R.A.; Chen, M.Z.; Tan, L.W.L.; Lim, M.Y.; Ho, H.K.; van Dam, R.M. Singapore Healthy Older People Everyday (HOPE) Study: Prevalence of Frailty and Associated Factors in Older Adults. J. Am. Med. Dir. Assoc. 2017, 18, 734.e9–734.e14. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Morley, J.E. Frailty and cognition: Linking two common syndromes in older persons. J. Nutr. Health Aging 2013, 17, 723–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falsarella, G.R.; Gasparotto, L.P.R.; Barcelos, C.C.; Coimbra, I.B.; Moretto, M.C.; Pascoa, M.A.; Ferreira, T.C.B.R.; Coimbra, A.M.V. Body composition as a frailty marker for the elderly community. Clin. Interv. Aging 2015, 10, 1661–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saedi, A.A.; Feehan, J.; Phu, S.; Duque, G. Current and emerging biomarkers of frailty in the elderly. Clin. Interv. Aging 2019, 14, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Cui, C.; Mackey, R.H.; Shaaban, C.E.; Kuller, L.H.; Lopez, O.L.; Sekikawa, A. Associations of body composition with incident dementia in older adults: Cardiovascular Health Study-Cognition Study. Alzheimers Dement. J. Alzheimers Assoc. 2020, 16, 1402–1411. [Google Scholar] [CrossRef]
- Semba, R.D.; Tian, Q.; Carlson, M.C.; Xue, Q.-L.; Ferrucci, L. Motoric cognitive risk syndrome: Integration of two early harbingers of dementia in older adults. Ageing Res. Rev. 2020, 58, 101022. [Google Scholar] [CrossRef]
- Jayaraman, A.; Htike, T.T.; James, R.; Picon, C.; Reynolds, R. TNF-mediated neuroinflammation is linked to neuronal necroptosis in Alzheimer’s disease hippocampus. Acta Neuropathol. Commun. 2021, 9, 159. [Google Scholar] [CrossRef]
- Porro, C.; Cianciulli, A.; Panaro, M.A. The Regulatory Role of IL-10 in Neurodegenerative Diseases. Biomolecules 2020, 10, 1017. [Google Scholar] [CrossRef]
- Rhinn, H.; Tatton, N.; McCaughey, S.; Kurnellas, M.; Rosenthal, A. Progranulin as a therapeutic target in neurodegenerative diseases. Trends Pharmacol. Sci. 2022, 43, 641–652. [Google Scholar] [CrossRef]
- Conte, M.; Giuliani, C.; Chiariello, A.; Iannuzzi, V.; Franceschi, C.; Salvioli, S. GDF15, an emerging key player in human aging. Ageing Res. Rev. 2022, 75, 101569. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Dias, F.; Teixeira, A.L.; Guimarães, H.C.; Barbosa, M.T.; Resende, E.P.F.; Beato, R.G.; Carmona, K.C.; Caramelli, P. Accuracy of the 15-item Geriatric Depression Scale (GDS-15) in a community-dwelling oldest-old sample: The Pietà Study. Trends Psychiatry Psychother. 2017, 39, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Vartiainen, P.; Mäntyselkä, P.; Heiskanen, T.; Hagelberg, N.; Mustola, S.; Forssell, H.; Kautiainen, H.; Kalso, E. Validation of EQ-5D and 15D in the assessment of health-related quality of life in chronic pain. Pain 2017, 158, 1577–1585. [Google Scholar] [CrossRef]
- Wallace, M.; Shelkey, M. Katz Index of Independence in Activities of Daily Living (ADL). Nurs. Clin. N. Am. 2007, 27, 93–94. [Google Scholar]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Lee, W.Y.; Basri, N.A.; Collinson, S.L.; Merchant, R.A.; Venketasubramanian, N.; Chen, C.L. The Montreal Cognitive Assessment is superior to the Mini-Mental State Examination in detecting patients at higher risk of dementia. Int. Psychogeriatr. 2012, 24, 1749–1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- Topolski, T.D.; LoGerfo, J.; Patrick, D.L.; Williams, B.; Walwick, J.; Patrick, M.B. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev. Chronic Dis. 2006, 3, A118. [Google Scholar]
- Pratt, J.; De Vito, G.; Narici, M.; Boreham, C. Neuromuscular Junction Aging: A Role for Biomarkers and Exercise. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 576–585. [Google Scholar] [CrossRef]
- Wang, T. Searching for the link between inflammaging and sarcopenia. Ageing Res. Rev. 2022, 77, 101611. [Google Scholar] [CrossRef]
- Rai, M.; Demontis, F. Muscle-to-Brain Signaling Via Myokines and Myometabolites. Brain Plast. 2022, 8, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Pierzchalski, P.; Szczepanik, M.; Bonior, J.; Zoladz, J.A. Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines. Cells 2022, 11, 160. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Siervo, M.; Prado, C.M.; Mire, E.; Broyles, S.; Wells, J.C.; Heymsfield, S.; Katzmarzyk, P.T. Body composition indices of a load-capacity model: Gender- and BMI-specific reference curves. Public Health Nutr. 2015, 18, 1245–1254. [Google Scholar] [CrossRef] [Green Version]
- Merchant, R.A.; Seetharaman, S.; Au, L.; Wong, M.W.K.; Wong, B.L.L.; Tan, L.F.; Chen, M.Z.; Ng, S.E.; Soong, J.T.Y.; Hui, R.J.Y.; et al. Relationship of Fat Mass Index and Fat Free Mass Index With Body Mass Index and Association With Function, Cognition and Sarcopenia in Pre-Frail Older Adults. Front. Endocrinol. 2021, 12, 765415. [Google Scholar] [CrossRef]
- Correa-de-Araujo, R.; Addison, O.; Miljkovic, I.; Goodpaster, B.H.; Bergman, B.C.; Clark, R.V.; Elena, J.W.; Esser, K.A.; Ferrucci, L.; Harris-Love, M.O.; et al. Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging. Front. Physiol. 2020, 11, 963. [Google Scholar] [CrossRef]
- Qi, J.Y.; Yang, L.K.; Wang, X.S.; Wang, M.; Li, X.B.; Feng, B.; Wu, Y.M.; Liu, S.B.; Zhang, K. Mechanism of CNS regulation by irisin, a multifunctional protein. Brain Res. Bull. 2022, 188, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.; Kim, M.; Won, C.W. Motoric cognitive risk syndrome is associated with processing speed and executive function, but not delayed free recall memory: The Korean frailty and aging cohort study (KFACS). Arch. Gerontol. Geriatr. 2020, 87, 103990. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.J.; Wing, S.S.; Rahme, E.; Morais, J.A.; Chevalier, S. Association of Low Muscle Mass With Cognitive Function During a 3-Year Follow-up Among Adults Aged 65 to 86 Years in the Canadian Longitudinal Study on Aging. JAMA Netw. Open 2022, 5, e2219926. [Google Scholar] [CrossRef]
- Salinas-Rodríguez, A.; Palazuelos-González, R.; Rivera-Almaraz, A.; Manrique-Espinoza, B. Longitudinal association of sarcopenia and mild cognitive impairment among older Mexican adults. J. Cachexia Sarcopenia Muscle 2021, 12, 1848–1859. [Google Scholar] [CrossRef]
- Martone, A.M.; Marzetti, E.; Calvani, R.; Picca, A.; Tosato, M.; Santoro, L.; Di Giorgio, A.; Nesci, A.; Sisto, A.; Santoliquido, A.; et al. Exercise and Protein Intake: A Synergistic Approach against Sarcopenia. BioMed Res. Int. 2017, 2017, 2672435. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Won, C.W. Sarcopenia Is Associated with Cognitive Impairment Mainly Due to Slow Gait Speed: Results from the Korean Frailty and Aging Cohort Study (KFACS). Int. J. Environ. Res. Public Health 2019, 16, 1491. [Google Scholar] [CrossRef] [Green Version]
- Sekhon, H.; Allali, G.; Launay, C.P.; Barden, J.; Szturm, T.; Liu-Ambrose, T.; Chester, V.L.; Wong, C.H.; Beauchet, O. Motoric cognitive risk syndrome, incident cognitive impairment and morphological brain abnormalities: Systematic review and meta-analysis. Maturitas 2019, 123, 45–54. [Google Scholar] [CrossRef]
- Chou, M.Y.; Nishita, Y.; Nakagawa, T.; Tange, C.; Tomida, M.; Shimokata, H.; Otsuka, R.; Chen, L.K.; Arai, H. Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr. 2019, 19, 186. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chung, C.P.; Lee, P.L.; Chou, K.H.; Chang, L.H.; Lin, S.Y.; Lee, Y.J.; Lin, C.P.; Wang, P.N. The Flexibility of Physio-Cognitive Decline Syndrome: A Longitudinal Cohort Study. Front. Public Health 2022, 10, 820383. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Westwater, M.L.; Noble, S.; Rosenblatt, M.; Dai, W.; Qi, S.; Sui, J.; Calhoun, V.D.; Scheinost, D. Associations between grip strength, brain structure, and mental health in >40,000 participants from the UK Biobank. BMC Med. 2022, 20, 286. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef]
- Lobo-Silva, D.; Carriche, G.M.; Castro, A.G.; Roque, S.; Saraiva, M. Balancing the immune response in the brain: IL-10 and its regulation. J. Neuroinflamm. 2016, 13, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, T.; Rogers, V.E.; Henriksen, V.T.; Trawick, R.H.; Momberger, N.G.; Lynn Rasmussen, G. Circulating IL-10 is compromised in patients predisposed to developing and in patients with severe knee osteoarthritis. Sci. Rep. 2021, 11, 1812. [Google Scholar] [CrossRef]
- Ortí-Casañ, N.; Wu, Y.; Naudé, P.J.W.; De Deyn, P.P.; Zuhorn, I.S.; Eisel, U.L.M. Targeting TNFR2 as a Novel Therapeutic Strategy for Alzheimer’s Disease. Front. Neurosci. 2019, 13, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, C.; Fillmore, N.R.; Ramos-Cejudo, J.; Brophy, M.; Osorio, R.; Gurney, M.E.; Qiu, W.Q.; Au, R.; Perry, G.; Dubreuil, M.; et al. Potential long-term effect of tumor necrosis factor inhibitors on dementia risk: A propensity score matched retrospective cohort study in US veterans. Alzheimers Dement. 2022, 18, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Decourt, B.; Lahiri, D.K.; Sabbagh, M.N. Targeting Tumor Necrosis Factor Alpha for Alzheimer’s Disease. Curr. Alzheimer Res. 2017, 14, 412–425. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Facciorusso, A.; Singh, A.G.; Casteele, N.V.; Zarrinpar, A.; Prokop, L.J.; Grunvald, E.L.; Curtis, J.R.; Sandborn, W.J. Obesity and response to anti-tumor necrosis factor-α agents in patients with select immune-mediated inflammatory diseases: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0195123. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.-H.; Tao, L.-L.; Su, D.-Q.; Guo, C.-J.; Liu, H. Diagnostic utility of GDF15 in neurodegenerative diseases: A systematic review and meta-analysis. Brain Behav. 2022, 12, e2502. [Google Scholar] [CrossRef]
- Bateman, A.; Cheung, S.T.; Bennett, H.P.J. A Brief Overview of Progranulin in Health and Disease. In Progranulin: Methods and Protocols; Bateman, A., Bennett, H.P.J., Cheung, S.T., Eds.; Springer: New York, NY, USA, 2018; pp. 3–15. [Google Scholar] [CrossRef]
- Kaur, J.; Mukheja, S.; Varma, S.; Kalra, H.S.; Khosa, B.S.; Vohra, K. Serum progranulin/tumor necrosis factor-α ratio as independent predictor of systolic blood pressure in overweight hypertensive patients: A cross-sectional study. Egypt. Heart J. 2020, 72, 25. [Google Scholar] [CrossRef] [PubMed]
- Ofori-Asenso, R.; Chin, K.L.; Mazidi, M.; Zomer, E.; Ilomaki, J.; Zullo, A.R.; Gasevic, D.; Ademi, Z.; Korhonen, M.J.; LoGiudice, D.; et al. Global Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e198398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzzan, S.; Azab, A.N. Anti-TNF-α Compounds as a Treatment for Depression. Molecules 2021, 26, 2368. [Google Scholar] [CrossRef]

| Healthy | Subjective Cognitive Decline | Slow Gait | Motoric Cognitive Risk Syndrome | p Value | |
|---|---|---|---|---|---|
| n = 116 (29.2%) | n = 47 (11.8%) | n = 175 (44.1%) | n = 59 (14.9%) | ||
| Demographics | |||||
| Age, years | 70.2 ± 4.9 a | 71.6 ± 5.5 | 73.9 ± 5.6 | 74.4 ± 6.4 a | <0.001 |
| Gender | 0.033 | ||||
| Male | 50 (43.1) | 19 (40.4) | 79 (45.1) | 14 (23.7) | |
| Female | 66 (56.9) | 28 (59.6) | 96 (54.9) | 45 (76.3) | |
| Ethnicity | 0.047 | ||||
| Chinese | 109 (94.0) | 41 (87.2) | 138 (78.9) | 50 (84.7) | |
| Malay | 2 (1.7) | 2 (4.3) | 18 (10.3) | 3 (5.1) | |
| Indian | 5 (4.3) | 4 (8.5) | 16 (9.1) | 6 (10.2) | |
| Others | 0 (0.0) | 0 (0.0) | 3 (1.7) | 0 (0.0) | |
| BMI, kg/m2 | 24.6 ± 4.1 a,b | 24.7 ± 3.8 c,d | 26.0 ± 4.9 a,c | 26.0 ± 5.2 b,d | 0.031 |
| Waist circumference (cm) * | 89.0 ± 12.7 | 88.3 ± 10.0 | 94.5 ± 13.4 | 92.5 ± 13.6 | 0.006 |
| Education, years | 9.4 ± 4.1 a | 8.3 ± 5.2 | 7.1 ± 4.1 | 6.6 ± 4.0 a | <0.001 |
| Chronic Disease | |||||
| Hypertension | 77 (66.4) | 29 (61.7) | 122 (69.7) | 41 (69.5) | 0.692 |
| Hyperlipidemia | 86 (74.1) | 34 (72.3) | 136 (77.7) | 44 (74.6) | 0.869 |
| Diabetes | 45 (38.8) | 19 (40.4) | 91 (52.0) | 26 (44.1) | 0.138 |
| Stroke | 8 (6.9) | 3 (6.4) | 17 (9.7) | 4 (6.8) | 0.767 |
| Multi-morbidity | 86 (74.1) | 32 (68.1) | 144 (82.3) | 44 (74.6) | 0.132 |
| Polypharmacy | 24 (20.7) | 12 (25.5) | 59 (33.7) | 16 (27.1) | 0.100 |
| Perceived Health Rating | 71.8 ± 14.3 a | 71.5 ± 11.3 | 68.7 ± 14.4 | 64.8 ± 15.3 a | 0.012 |
| RAPA Total | 3.5 ± 1.5 a | 3.2 ± 1.6 | 3.3 ± 1.6 | 2.8 ± 1.5 a | 0.031 |
| Rare/Light Activity | 64 (55.2) | 31 (66.0) | 109 (62.3) | 44 (74.6) | 0.086 |
| Moderate/Vigorous Activity | 52 (44.8) | 16 (34.0) | 66 (37.7) | 15 (25.4) | |
| MoCA, total | 27.2 ± 2.6 a,b | 26.0 ± 4.0 c,d | 24.8 ± 4.1 a,c | 23.5 ± 4.9 b,d | <0.001 |
| ≥1 Fall in 12 months | 27 (23.3) | 9 (19.1) | 36 (20.6) | 22 (37.3) | 0.085 |
| Depression | 20 (17.2) | 14 (29.8) | 44 (25.1) | 31 (52.5) | <0.001 |
| At least moderate pain | 12 (10.3) | 5 (10.6) | 31 (17.7) | 10 (16.9) | 0.273 |
| ≥1 ADL impairment | 9 (7.8) | 9 (19.1) | 42 (24.0) | 20 (33.9) | <0.001 |
| ≥1 IADL impairment | 17 (14.7) | 9 (19.1) | 56 (32.0) | 25 (42.4) | <0.001 |
| MNA Total | 12.8 ± 1.5 | 12.8 ± 1.7 | 12.8 ± 1.5 | 12.5 ± 1.6 | 0.444 |
| Nutrition Status | 0.113 | ||||
| Malnourished | 0 (0.0) | 1 (2.1) | 0 (0.0) | 1 (1.7) | |
| At Risk | 22 (19.0) | 4 (8.5) | 28 (16.0) | 14 (23.7) | |
| Normal | 94 (81.0) | 42 (89.4) | 147 (84.0) | 44 (74.6) | |
| Physical Performance | |||||
| Max gait speed, m/s | 1.2 ± 0.2 a,b | 1.2 ± 0.2 c,d | 0.8 ± 0.2 a,c | 0.8 ± 0.2 b,d | <0.001 |
| Max handgrip strength, kg | 23.4 ± 7.0 a | 23.6 ± 8.2 b | 21.5 ± 6.8 c | 18.2 ± 4.3 a,b,c | <0.001 |
| Low grip strength 1 | 52 (44.8) | 18 (38.3) | 104 (59.4) | 39 (66.1) | 0.002 |
| SPPB, total | 11.0 ± 1.3 a,b | 10.7 ± 1.5 c,d | 9.0 ± 2.2 a,c | 8.4 ± 2.3 b,d | <0.001 |
| 5× STS time (s) | 11.2 ± 2.9 a,b | 12.1 ± 3.0 c | 13.8 ± 4.9 a,d | 16.3 ± 7.1 b,c,d | <0.001 |
| Body Composition | |||||
| ASMI (kg/m2) | 7.0 ± 2.4 | 7.0 ± 2.2 | 7.3 ± 2.7 | 6.5 ± 2.0 | 0.220 |
| Body fat percentage (%) | 31.1 ± 8.8 a | 31.9 ± 7.3 | 33.7 ± 9.4 | 36.2 ± 9.3 a | 0.007 |
| Fat Mass Index | 7.9 ± 3.2 a | 8.0 ± 2.7 | 9.0 ± 3.8 | 9.7 ± 4.1 a | 0.008 |
| Fat Free Mass Index | 16.8 ± 2.4 | 16.6 ± 1.9 | 16.8 ± 2.4 | 16.1 ± 1.7 | 0.205 |
| Fat Mass to Fat Free Mass Ratio | 0.5 ± 0.2 a | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.2 a | 0.003 |
| Visceral fat area (cm2) * | 94.1 ± 39.0 | 92.1 ± 32.9 | 105.9 ± 47.7 | 110.1 ± 51.2 | 0.095 |
| Sarcopenia (AWGS) 2 | 1 (0.9) | 1 (2.1) | 36 (20.6) | 17 (28.8) | <0.001 |
| Subjective Cognitive Decline | Slow Gait | Motoric Cognitive Risk Syndrome | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted # | Unadjusted | Adjusted # | Unadjusted | Adjusted # | |
| Waist Circumference (cm) | 1.01 (1.03) | 1.02 (0.96–1.04) | 1.04 (1.01–1.06) | 1.04 (1.01–1.07) | 1.02 (0.99–1.05) | 1.03 (0.99–1.07) |
| Body Fat Percentage | 1.01 (0.97–1.05) | 1.00 (0.95–1.05) | 1.03 (1.00–1.06) | 1.03 (0.99–1.07) | 1.07 (1.03–1.11) | 1.06 (1.01–1.12) |
| Fat Mass Index | 1.02 (0.91–1.13) | 0.99 (0.87–1.13) | 1.10 (1.02–1.19) | 1.10 (0.99–1.21) | 1.15 (1.05–1.27) | 1.16 (1.02–1.30) |
| Fat Free Mass Index | 0.96 (0.82–1.12) | 0.98 (0.81–1.19) | 1.02 (0.91–1.13) | 1.09 (0.95–1.26) | 0.87 (0.74–1.01) | 0.99 (0.81–1.21) |
| Fat Mass to Fat Free Mass ratio | 1.28 (0.22–3.55) | 0.78 (0.08–2.99) | 1.61 (1.33–2.09) | 1.26 (0.68–1.83) | 1.44 (1.04–4.41) | 2.56 (1.62–3.33) |
| ASMI (kg/m2) | 0.99 (0.85–1.16) | 1.01 (0.83–1.23) | 1.05 (0.95–1.17) | 1.09 (0.95–1.25) | 0.85 (0.69–1.06) | 0.98 (0.78–1.24) |
| Sarcopenia ^ | 2.35 (2.00–5.60) | 2.83 (0.16–5.29) | 2.25 (1.68–3.02) | 1.90 (1.14–2.40) | 3.16 (2.04–4.75) | 2.62 (1.46–3.17) |
| Healthy | Subjective Cognitive Decline | Slow Gait | Motoric Cognitive Risk Syndrome | p-Value | |
|---|---|---|---|---|---|
| n = 36 (33.6%) | n = 22 (20.6%) | n = 33 (30.8%) | n = 16 (15.0%) | ||
| GDF-15 (pg./mL) | 715.6 ± 315.4 | 981.8 ± 506.4 | 1100.5 ± 565.0 | 1132.4 ± 602.5 | 0.076 |
| Interleukin 6 (pg./mL) | 2.7 ± 0.8 | 2.8 ± 0.8 | 3.1 ± 1.2 | 2.9 ± 0.7 | 0.404 |
| Interleukin 10 (IL-10) (ng/mL) | 2.4 ± 0.9 | 2.4 ± 0.9 | 2.3 ± 1.0 | 2.3 ± 1.0 | 0.968 |
| Progranulin (ng/mL) | 69.3 ± 11.8 | 68.8 ± 12.7 | 67.2 ± 14.1 | 67.5 ± 13.7 | 0.915 |
| Tumor Necrosis-α (TNF-α) (pg/mL) | 7.0 ± 1.7 a | 7.1 ± 1.4 b | 8.5 ± 2.8 | 9.5 ± 2.6 a,b | <0.001 |
| IL-10/TNF-α | 396.9 ± 295.1 a | 306.2 ± 85.7 | 307.7 ± 184.6 | 222.6 ± 117.8 a | 0.045 |
| Progranulin/TNF-α | 10,124.4 ± 4018.4 a | 9563.2 ± 2461.7 b | 8744.7 ± 4006.7 | 6869.2 ± 2235.4 a,b | 0.022 |
| Subjective Cognitive Decline | Slow Gait | Motor Cognitive Risk Syndrome | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| GDF-15 | 1.00 (0.99–1.01) | 1.00 (0.98–1.02) | 1.00 (0.97–1.03) | 1.01 (1.00–1.02) | 1.01 (0.99–1.02) | 1.01 (0.99–1.03) | 1.01 (1.00–1.02) | 1.01 (0.99–1.03) | 1.02 (0.99–1.04) |
| IL-6 | 1.23 (0.80–1.87) | 0.88 (0.36–2.10) | 0.69 (0.22–2.16) | 1.26 (0.86–1.85) | 0.58 (0.23–1.42) | 0.19 (0.04–1.08) | 1.19 (0.74–1.90) | 0.79 (0.33–1.91) | 0.57 (0.11–2.87) |
| IL-10 | 1.02 (0.95–1.09) | 1.06 (0.93–1.20) | 1.03 (0.89–1.18) | 1.02 (0.96–1.09) | 1.07 (0.94–1.21) | 1.04 (0.91–1.20) | 0.87 (0.53–1.44) | 0.68 (0.27–1.75) | 0.22 (0.05–0.98) |
| PRGN | 0.99 (0.96–1.04) | 0.96 (0.88–1.04) | 0.96 (0.88–1.05) | 0.99 (0.95–1.03) | 0.96 (0.90–1.02) | 0.96 (0.89–1.04) | 0.99 (0.94–1.04) | 0.97 (0.90–1.04) | 0.99 (0.91–1.09) |
| TNF-α | 0.97 (0.80–1.18) | 0.72 (0.41–1.26) | 0.87 (0.45–1.69) | 1.09 (0.94–1.28) | 0.99 (0.73–1.35) | 1.23 (0.79–1.93) | 1.23 (1.04–1.46) | 1.37 (1.01–1.87) | 1.55 (0.99–2.43) |
| IL-10/TNF-α | 0.98 (0.95–1.01) | 0.99 (0.98–1.08) | 0.99 (0.97–1.01) | 0.99 (0.95–1.01) | 0.99 (0.98–1.04) | 0.99 (0.98–1.04) | 0.98 (0.97–0.99) | 0.98 (0.97–0.99) | 0.98 (0.97–0.99) |
| PRGN/ TNF-α | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 0.98 (0.92–0.99) | 0.98 (0.96–0.99) | 1.00 (0.99–1.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merchant, R.A.; Chan, Y.H.; Anbarasan, D.; Aprahamian, I. Association of Motoric Cognitive Risk Syndrome with Sarcopenia and Systemic Inflammation in Pre-Frail Older Adults. Brain Sci. 2023, 13, 936. https://doi.org/10.3390/brainsci13060936
Merchant RA, Chan YH, Anbarasan D, Aprahamian I. Association of Motoric Cognitive Risk Syndrome with Sarcopenia and Systemic Inflammation in Pre-Frail Older Adults. Brain Sciences. 2023; 13(6):936. https://doi.org/10.3390/brainsci13060936
Chicago/Turabian StyleMerchant, Reshma Aziz, Yiong Huak Chan, Denishkrshna Anbarasan, and Ivan Aprahamian. 2023. "Association of Motoric Cognitive Risk Syndrome with Sarcopenia and Systemic Inflammation in Pre-Frail Older Adults" Brain Sciences 13, no. 6: 936. https://doi.org/10.3390/brainsci13060936
APA StyleMerchant, R. A., Chan, Y. H., Anbarasan, D., & Aprahamian, I. (2023). Association of Motoric Cognitive Risk Syndrome with Sarcopenia and Systemic Inflammation in Pre-Frail Older Adults. Brain Sciences, 13(6), 936. https://doi.org/10.3390/brainsci13060936