Effects of Repetitive Transcranial Magnetic Stimulation at the Cerebellum on Working Memory
Abstract
1. Introduction
2. Materials and Methods
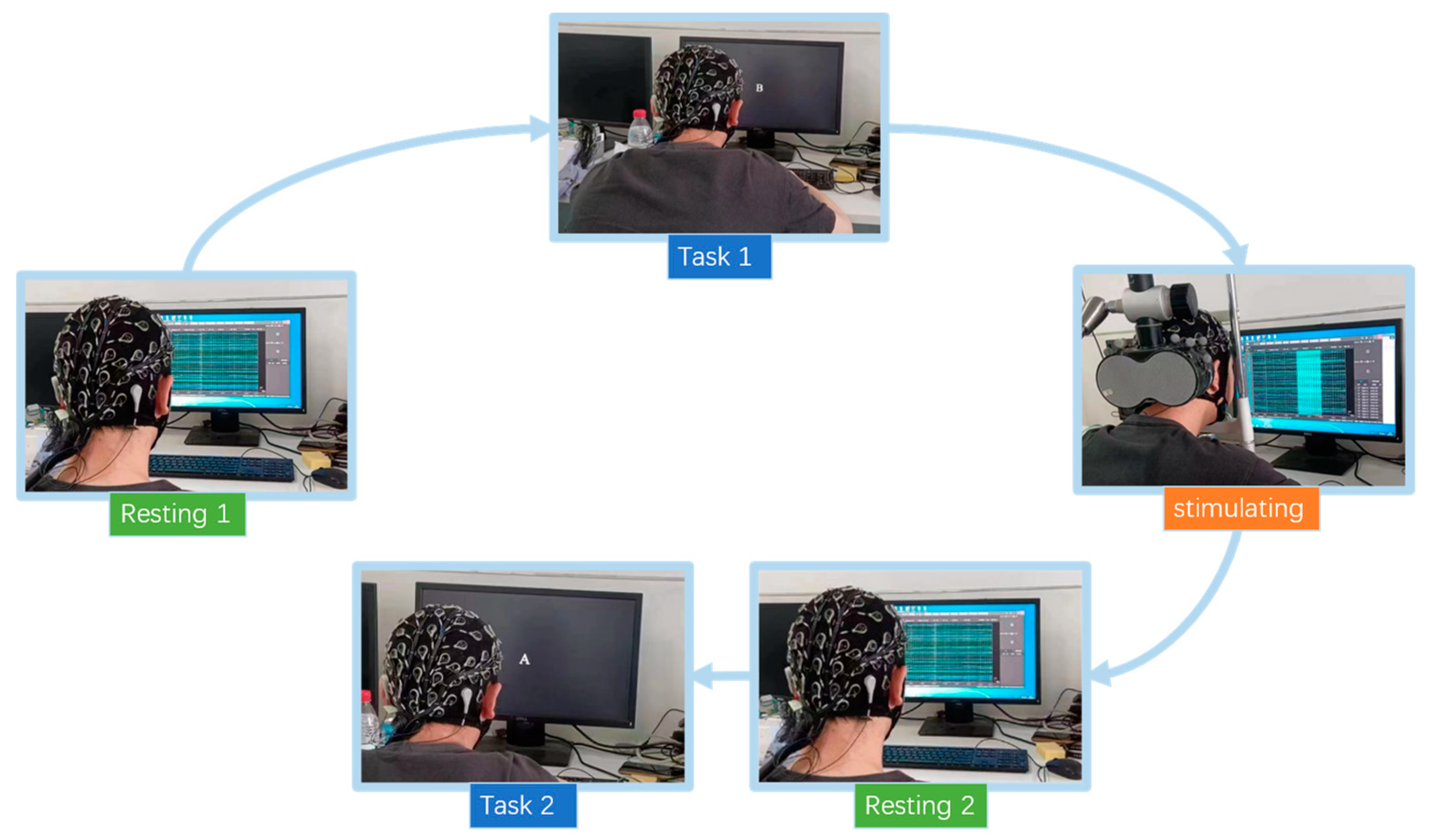
2.1. Design of Experiments
2.2. Screening of Subjects
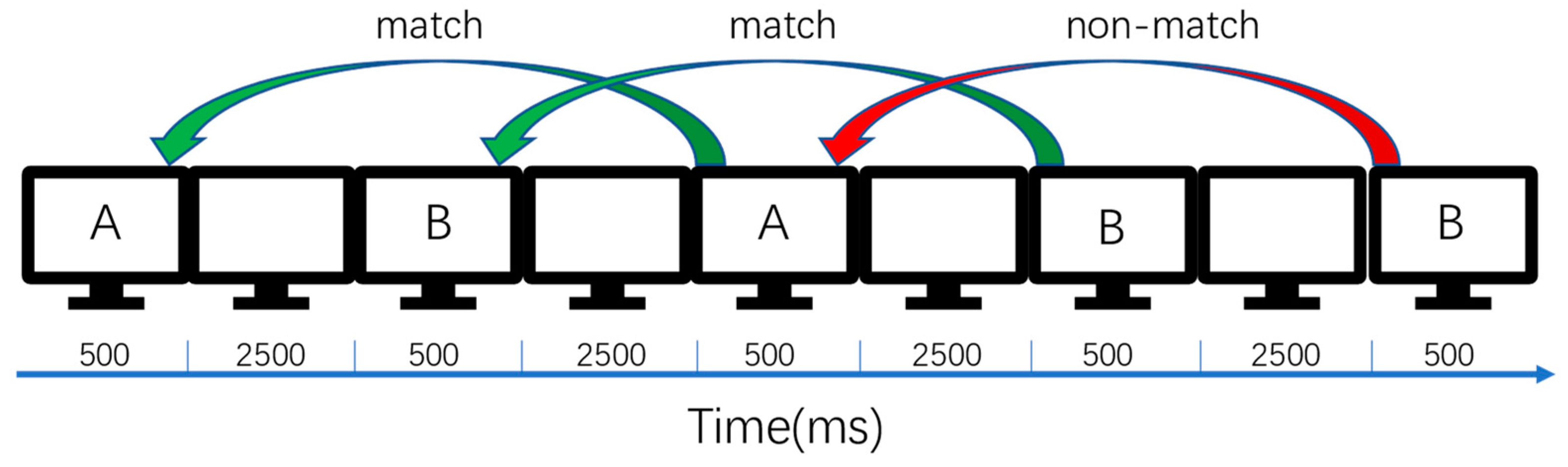
2.3. Process of Experiment
2.4. EEG Analysis Method
3. Analysis and Results
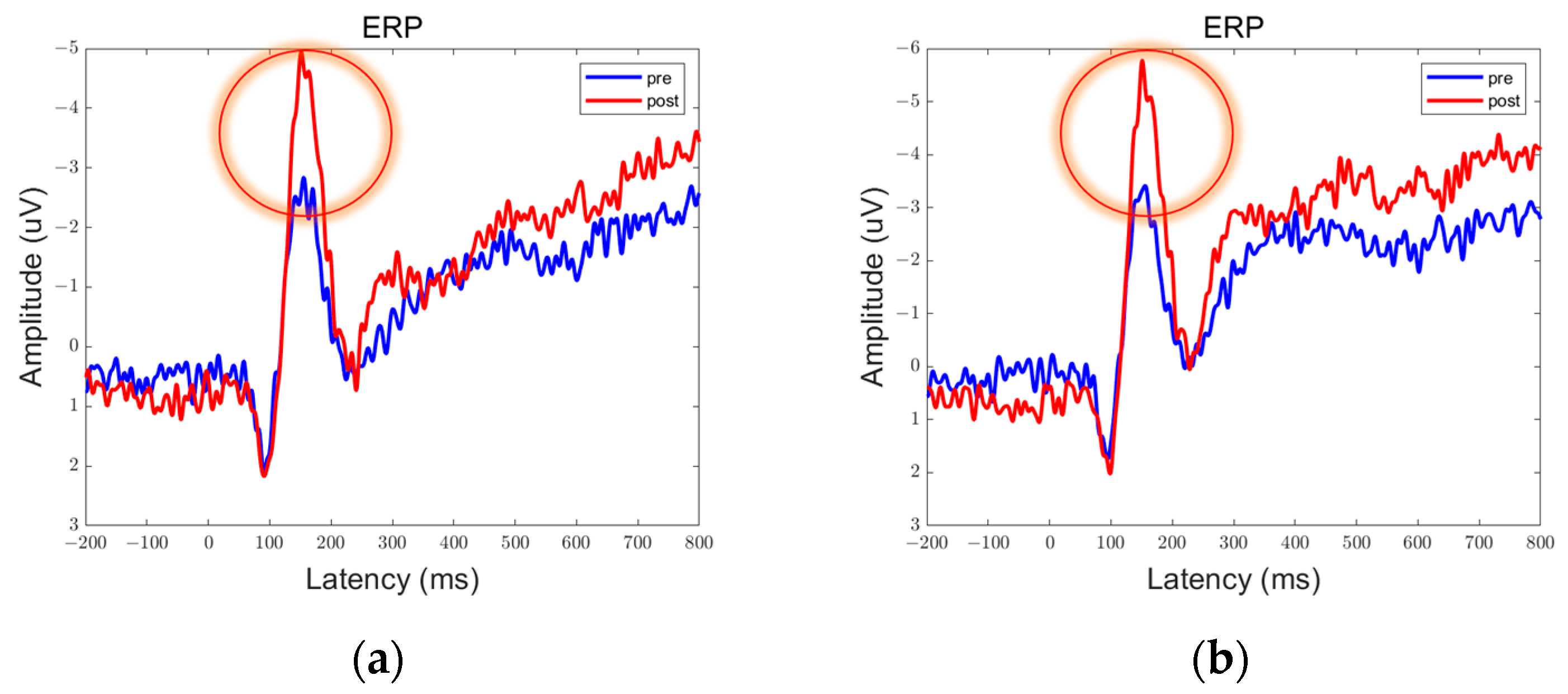
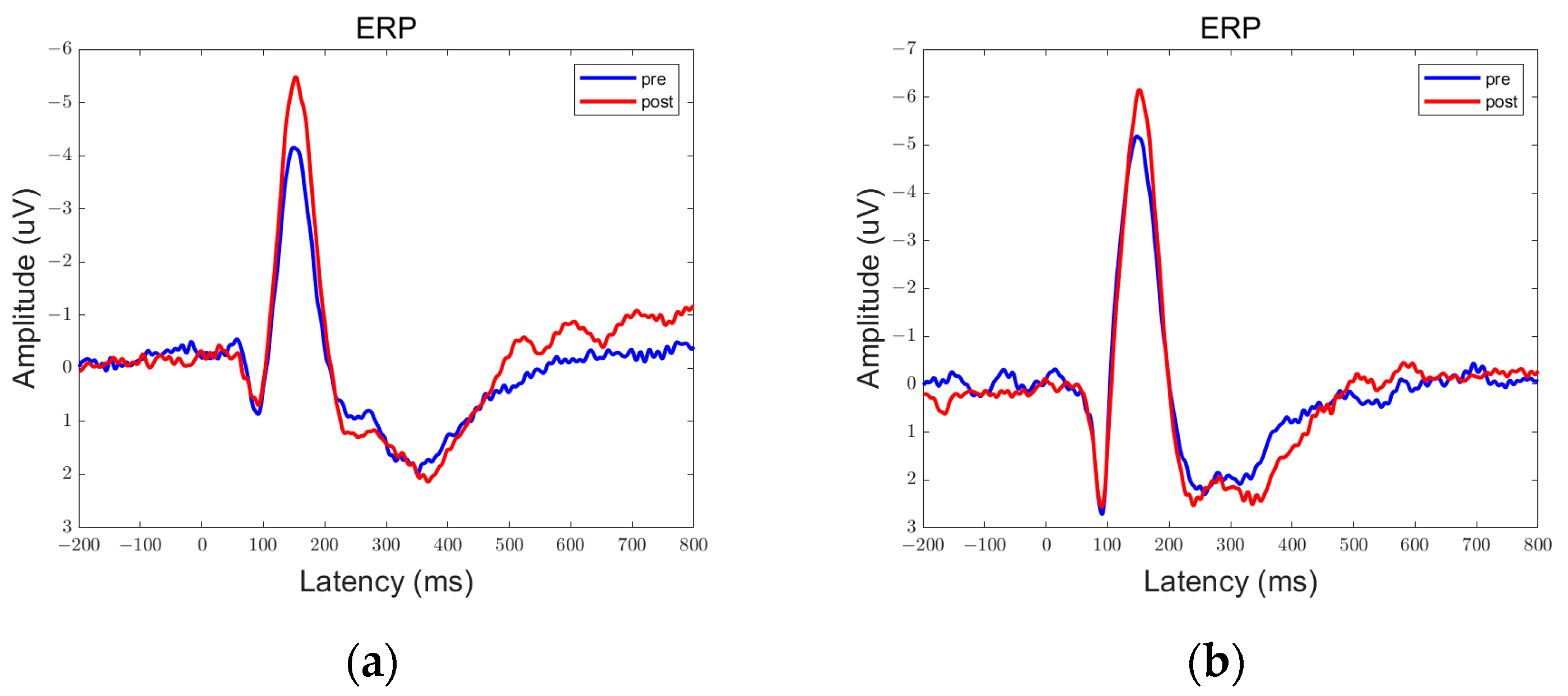
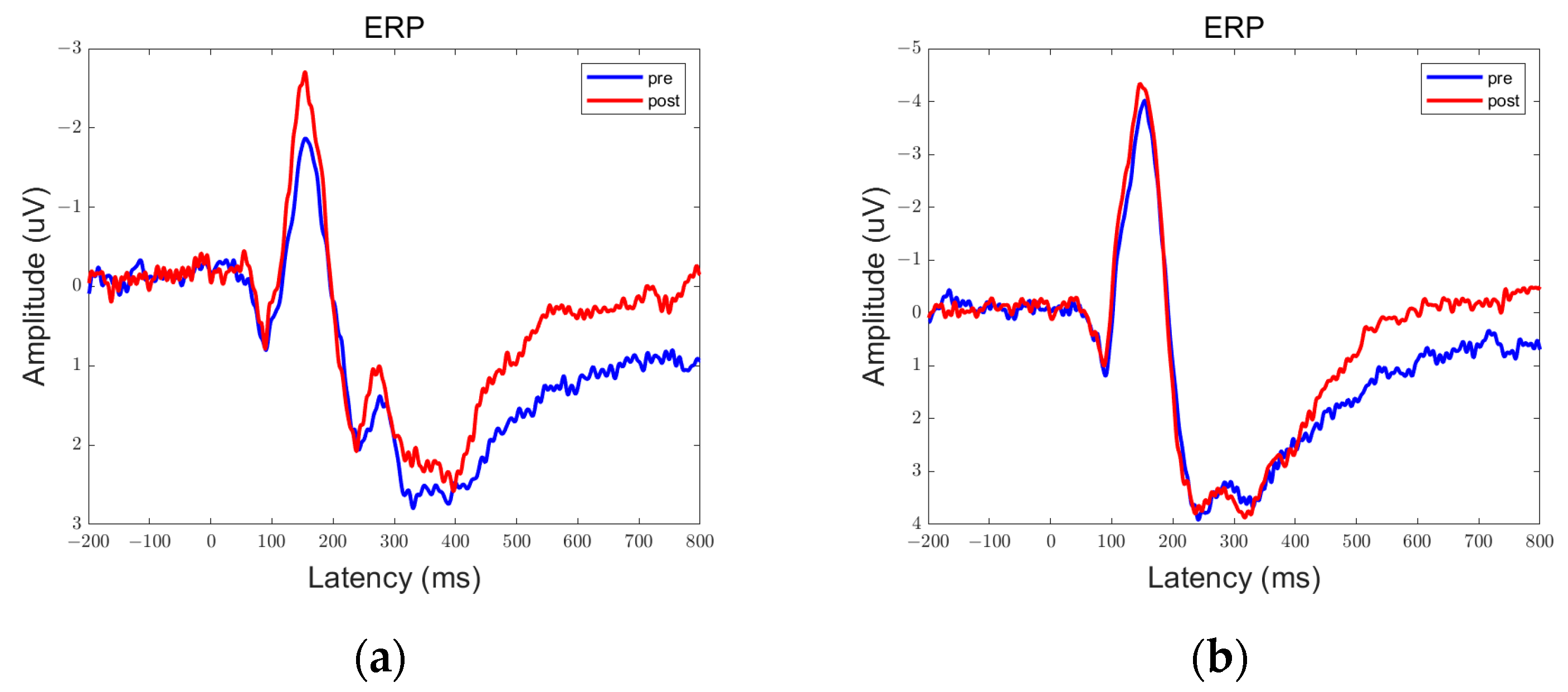
3.1. ERP Results
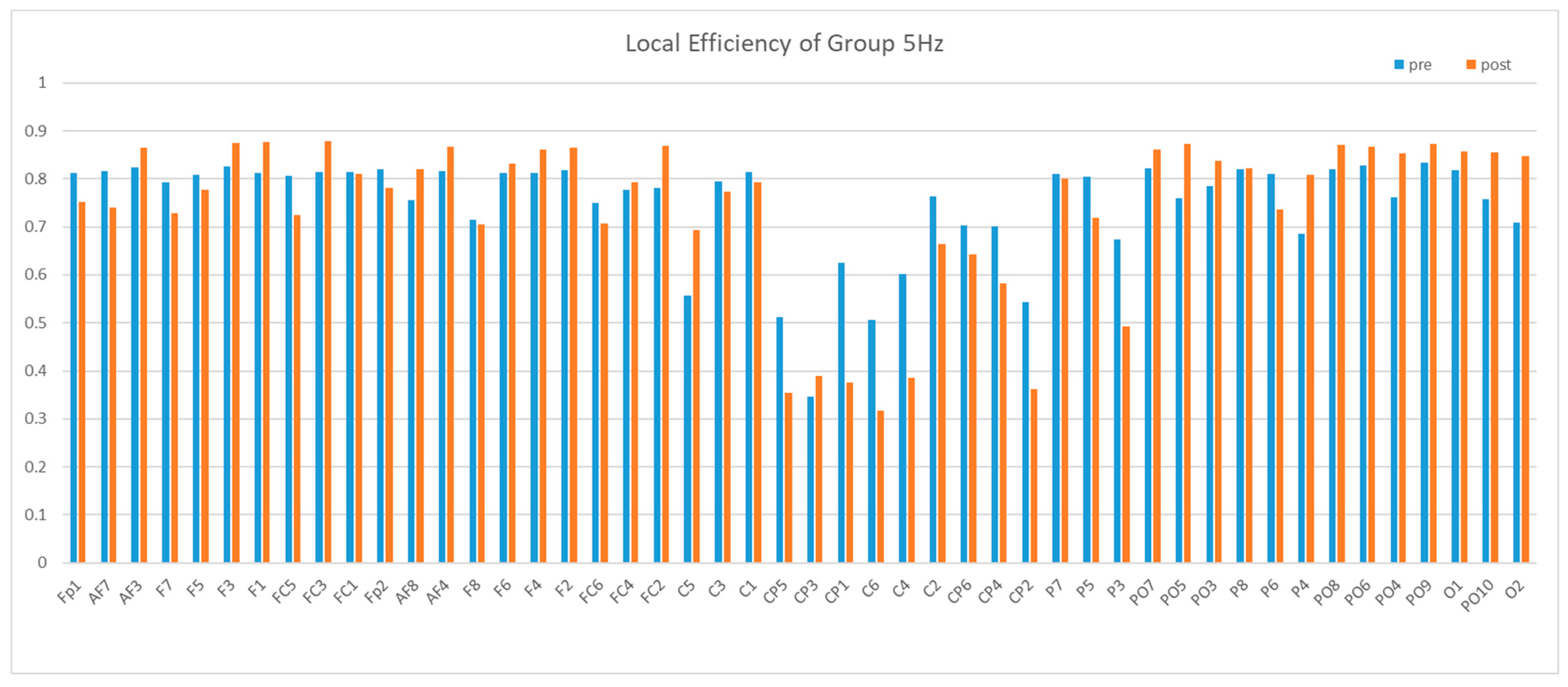
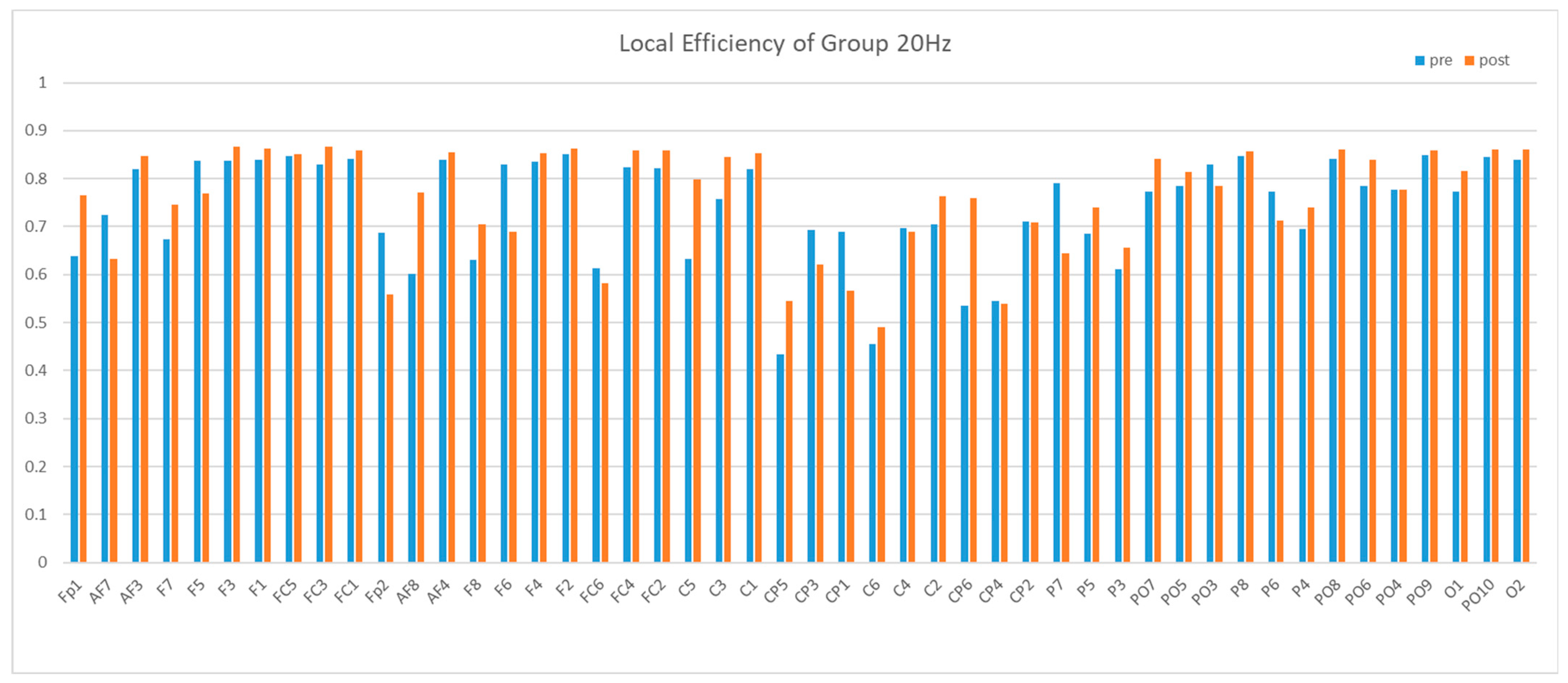
3.2. Brain Network Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baddeley, A. Working memory: Theories, models, and controversies. Annu. Rev. Psychol. 2012, 63, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Bagattini, C.; Zanni, M.; Barocco, F.; Caffarra, P.; Brignani, D.; Miniussi, C.; Defanti, C.A. Enhancing cognitive training effects in Alzheimer’s disease: rTMS as an add-on treatment. Brain Stimul. 2020, 13, 1655–1664. [Google Scholar] [CrossRef]
- Wu, X.; Ji, G.J.; Geng, Z.; Zhou, S.; Yan, Y.; Wei, L.; Wang, K. Strengthened theta-burst transcranial magnetic stimulation as an adjunctive treatment for Alzheimer’s disease: An open-label pilot study. Brain Stimul. 2020, 13, 484–486. [Google Scholar] [CrossRef]
- Parkin, B.L.; Ekhtiari, H.; Walsh, V.F. Non-invasive Human Brain Stimulation in Cognitive Neuroscience: A Primer. Neuron 2015, 87, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Marron, E.M.; Viejo-Sobera, R.; Quintana, M.; Redolar-Ripoll, D.; Rodríguez, D.; Garolera, M. Transcranial magnetic stimulation intervention in Alzheimer’s disease: A research proposal for a randomized controlled trial. BMC Res. Notes 2018, 11, 648. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Rogasch, N.C.; Hoy, K.E.; Fitzgerald, P.B. The effect of single and repeated prefrontal intermittent theta burst stimulation on cortical reactivity and working memory. Brain Stimul. 2018, 11, 566–574. [Google Scholar] [CrossRef]
- Mangano, G.R.; Oliveri, M.; Smirni, D.; Tarantino, V.; Turriziani, P. Transcranial Magnetic Stimulation Trains at 1 Hz Frequency of the Right Posterior Parietal Cortex Facilitate Recognition Memory. Front. Hum. Neurosci. 2021, 15, 696793. [Google Scholar] [CrossRef]
- Koch, G.; Bonnì, S.; Pellicciari, M.C.; Casula, E.P.; Mancini, M.; Esposito, R.; Bozzali, M. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. NeuroImage 2018, 169, 302–311. [Google Scholar] [CrossRef]
- Chung, S.W.; Rogasch, N.C.; Hoy, K.E.; Sullivan, C.M.; Cash, R.F.; Fitzgerald, P.B. Impact of different intensities of intermittent theta burst stimulation on the cortical properties during TMS-EEG and working memory performance. Hum. Brain Mapp. 2018, 39, 783–802. [Google Scholar] [CrossRef]
- Hanslmayr, S.; Axmacher, N.; Inman, C.S. Modulating Human Memory via Entrainment of Brain Oscillations. Trends Neurosci. 2019, 42, 485–499. [Google Scholar] [CrossRef]
- Sereno, M.I.; Diedrichsen, J.; Tachrount, M.; Testa-Silva, G.; D’arceuil, H.; De Zeeuw, C. The human cerebellum has almost 80% of the surface area of the neocortex. Proc. Natl. Acad. Sci. USA 2020, 117, 19538–19543. [Google Scholar] [CrossRef]
- Oliveri, M.; Koch, G.; Torriero, S.; Caltagirone, C. Increased facilitation of the primary motor cortex following 1 Hz repetitive transcranial magnetic stimulation of the contralateral cerebellum in normal humans. Neurosci. Lett. 2005, 376, 188–193. [Google Scholar] [CrossRef]
- Dayan, M.; Olivito, G.; Molinari, M.; Cercignani, M.; Bozzali, M.; Leggio, M. Impact of cerebellar atrophy on cortical gray matter and cerebellar peduncles as assessed by voxel-based morphometry and high angular resolution diffusion imaging. Funct. Neurol. 2016, 31, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Cash, R.; Dunlop, K.; Vesia, M.; Kucyi, A.; Ghahremani, A.; Chen, R. Modulation of cognitive cerebello-cerebral functional connectivity by lateral cerebellar continuous theta burst stimulation. NeuroImage 2017, 158, 48–57. [Google Scholar] [CrossRef]
- Van Overwalle, F.; Mariën, P. Functional connectivity between the cerebrum and cerebellum in social cognition: A multi-study analysis. NeuroImage 2016, 124, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Onuki, Y.; Van Someren, E.J.; De Zeeuw, C.I.; Van der Werf, Y.D. Hippocampal-cerebellar interaction during spatio-temporal prediction. Cereb. Cortex 2015, 25, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Dickson, P.E.; Cairns, J.; Goldowitz, D.; Mittleman, G. Cerebellar contribution to higher and lower order rule learning and cognitive flexibility in mice. Neuroscience 2017, 345, 99–109. [Google Scholar] [CrossRef]
- Küper, M.; Kaschani, P.; Thürling, M.; Stefanescu, M.R.; Burciu, R.G.; Göricke, S.; Maderwald, S.; Ladd, M.E.; Hautzel, H.; Timmann, D. Cerebellar fMRI Activation Increases with Increasing Working Memory Demands. Cerebellum 2015, 15, 322–335. [Google Scholar] [CrossRef]
- King, M.; Hernandez-Castillo, C.R.; Poldrack, R.A.; Ivry, R.B.; Diedrichsen, J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat. Neurosci. 2019, 22, 1371–1378. [Google Scholar] [CrossRef]
- EEsterman, M.; Thai, M.; Okabe, H.; DeGutis, J.; Saad, E.; Laganiere, S.E.; Halko, M.A. Network-targeted cerebellar transcranial magnetic stimulation improves attentional control. NeuroImage 2017, 156, 190–198. [Google Scholar] [CrossRef]
- Halko, M.A.; Farzan, F.; Eldaief, M.C.; Schmahmann, J.D.; Pascual Leone, A. Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. J. Neurosci. 2014, 34, 12049–12056. [Google Scholar] [CrossRef]
- Koch, G.; Brusa, L.; Carrillo, F.; Gerfo, E.L.; Torriero, S.; Oliveri, M.; Mir, P.; Caltagirone, C.; Stanzione, P. Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson’s disease. Neurology 2009, 73, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Mori, F.; Marconi, B.; Codecà, C.; Pecchioli, C.; Salerno, S.; Torriero, S.; Gerfo, E.L.; Mir, P.; Oliveri, M.; et al. Changes in intracortical circuits of the human motor cortex following theta burst stimulation of the lateral cerebellum. Clin. Neurophysiol. 2008, 119, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, F.; Palomar, F.J.; Conde, V.; Diaz-Corrales, F.J.; Porcacchia, P.; Del-Olmo, M.; Koch, G.; Mir, P. Study of cerebello-thalamocortical pathway by transcranial magnetic stimulation in Parkinson’s disease. Brain Stimul. 2013, 6, 582–589. [Google Scholar] [CrossRef]
- Brusa, L.; Ceravolo, R.; Kiferle, L.; Monteleone, F.; Iani, C.; Schillaci, O.; Stanzione, P.; Koch, G. Metabolic changes induced by theta burst stimulation of the cerebellum in dyskinetic Parkinson’s disease patients. Park. Relat. Disord. 2012, 18, 59–62. [Google Scholar] [CrossRef]
- Brusa, L.; Ponzo, V.; Mastropasqua, C.; Picazio, S.; Bonnì, S.; Di Lorenzo, F.; Iani, C.; Stefani, A.; Stanzione, P.; Caltagirone, C.; et al. Theta Burst Stimulation Modulates Cerebellar-Cortical Connectivity in Patients with Progressive Supranuclear Palsy. Brain Stimul. 2014, 7, 29–35. [Google Scholar] [CrossRef]
- Kishore, A.; Popa, T.; Balachandran, A.; Backer, F.; Chandran, S.; Meunier, S. Cerebellar sensory processing alterations impact motor cortical plasticity in Parkinson’s disease: Clues from dyskinetic patients. Clin. Neurophysiol. 2013, 124, e101. [Google Scholar] [CrossRef]
- Strzalkowski, N.D.J.; Chau, A.D.; Gan, L.S.; Kiss, Z.H.T. Both 50 and 30 Hz continuous theta burst transcranial magnetic stimulation depresses the cerebellum. Cerebellum 2018, 18, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Hoffland, B.S.; Kassavetis, P.; Bologna, M.; Teo, J.T.H.; Bhatia, K.P.; Rothwell, J.C.; Edwards, M.J.; van de Warrenburg, B.P. Cerebellum-dependent associative learning deficits in primary dystonia are normalized by rTMS and practice. Eur. J. Neurosci. 2013, 38, 2166–2171. [Google Scholar] [CrossRef]
- Koch, G.; Porcacchia, P.; Ponzo, V.; Carrillo, F.; Cáceres-redondo, M.T.; Brusa, L.; Desiato, M.T.; Arciprete, F.; Di Lorenzo, F.; Pisani, A.; et al. Brain stimulation effects of two weeks of cerebellar theta burst stimulation in cervical dystonia patients. Brain Stimul. 2014, 7, 564–572. [Google Scholar] [CrossRef]
- Popa, T.; Russo, M.; Vidailhet, M.; Roze, E.; Lehéricy, S.; Bonnet, C.; Apartis, E.; Legrand, A.; Marais, L.; Meunier, S.; et al. Cerebellar rTMS stimulation may induce prolonged clinical benefits in essential tremor, and subjacent changes in functional connectivity: An open label trial. Brain Stimul. 2013, 6, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Viñas-Guasch, N.; Ng, T.H.B.; Heng, J.G.; Chan, Y.C.; Chew, E.; Desmond, J.E.; Chen, S.A. Cerebellar Transcranial Magnetic Stimulation (TMS) Impairs Visual Working Memory. Cerebellum 2022, 22, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Oldrati, V.; Schutter, D.J.L.G. Targeting the Human Cerebellum with Transcranial Direct Current Stimulation to Modulate Behavior: A Meta-Analysis. Cerebellum 2018, 17, 228–236. [Google Scholar] [CrossRef]
- Ferrari, C.; Fiori, F.; Suchan, B.; Plow, E.B.; Cattaneo, Z. TMS over the posterior cerebellum modulates motor cortical excitability in response to facial emotional expressions. Eur. J. Neurosci. 2021, 53, 1029–1039. [Google Scholar] [CrossRef]
- Yao, Q.; Tang, F.; Wang, Y.; Yan, Y.; Dong, L.; Wang, T.; Shi, J. Effect of cerebellum stimulation on cognitive recovery in patients with Alzheimer disease: A randomized clinical trial. Brain Stimul. 2022, 15, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yao, Q.; Yu, M.; Xiao, C.; Fan, L.; Lin, X.; Shi, J. Topological Disruption of Structural Brain Networks in Patients with Cognitive Impairment Following Cerebellar Infarction. Front Neurol. 2019, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Falkenstein, M.; Hoormann, J.; Hohnsbein, J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol. 1999, 101, 267–291. [Google Scholar] [CrossRef]
- Jaeggi, S.M.; Buschkuehl, M.; Perrig, W.J.; Meier, B. The concurrent validity of the N-back task as a working memory measure. Memory 2010, 18, 394–412. [Google Scholar] [CrossRef]
- Shi, J.; Yin, K.; Ye, X.; Du, W.; Zhao, J. A Whole-Brain Electroencephalogram Cap for Transcranial Magnetic Stimulator Therapy. CN Patent Application 114,366,127, 19 April 2022. [Google Scholar]
- Guerrero, L.; Bouazzaoui, B.; Isingrini, M.; Angel, L. Impact of working memory capacity on predominance of parietal over frontal P300 amplitude. Brain Cogn. 2023, 170, 106056. [Google Scholar] [CrossRef]
- Brush, C.J.; Kallen, A.M.; Meynadasy, M.A.; King, T.; Hajcak, G.; Sheffler, J.L. The P300, loneliness, and depression in older adults. Biol. Psychol. 2022, 171, 108339. [Google Scholar] [CrossRef] [PubMed]
- González-Garrido, A.A.; López-Franco, A.L.; Gómez-Velázquez, F.R.; Ramos-Loyo, J.; Sequeira, H. Emotional content of stimuli improves visuospatial working memory. Neurosci. Lett. 2015, 585, 43–47. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Vita, A.; Gaebel, W.; Mucci, A.; Sachs, G.; Barlati, S.; Giordano, G.M.; Nibbio, G.; Nordentoft, M.; Wykes, T.; Galderisi, S. European Psychiatric Association guidance on treatment of cognitive impairment in schizophrenia. Eur. Psychiatry 2022, 65, e57. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.Y.; Tian, M.J.; Yao, Q.; Li, Q.; Tang, F.Y.; Xiao, C.Y.; Chen, J. Neuroimaging alterations in dementia with Lewy bodies and neuroimaging differences be-tween dementia with Lewy bodies and Alzheimer’s disease: An activation likelihood estimation meta-analysis. CNS Neurosci. Ther. 2022, 28, 183–205. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.; Vanhaerents, S.; Voss, J.L. Cerebellar Theta and Beta Noninvasive Stimulation Rhythms Differentially Influence Episodic Memory versus Semantic Prediction. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 7300–7310. [Google Scholar] [CrossRef] [PubMed]
- Desmond, J.E.; Chen, S.H.A.; Shieh, P.B. Cerebellar transcranial magnetic stimulation impairs verbal working memory. Ann. Neurol. 2005, 58, 553–560. [Google Scholar] [CrossRef]






| 5 Hz | 20 Hz | Sham | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | Gender | Age | No. | Gender | Age | No. | Gender | Age |
| 1 | female | 24 | 1 | male | 24 | 1 | female | 24 |
| 2 | female | 27 | 2 | female | 24 | 2 | female | 23 |
| 3 | female | 28 | 3 | male | 23 | 3 | female | 27 |
| 4 | male | 23 | 4 | female | 24 | 4 | male | 27 |
| 5 | female | 24 | 5 | male | 27 | 5 | male | 26 |
| 6 | female | 24 | 6 | male | 23 | 6 | female | 24 |
| 7 | female | 26 | 7 | male | 25 | 7 | male | 24 |
| 8 | female | 24 | 8 | female | 24 | 8 | female | 24 |
| 9 | female | 24 | 9 | female | 25 | 9 | female | 26 |
| 10 | female | 24 | 10 | male | 25 | 10 | female | 24 |
| 11 | female | 25 | 11 | male | 23 | |||
| 12 | male | 25 | 12 | male | 25 | |||
| 13 | male | 27 | ||||||
| 14 | female | 24 | ||||||
| Number of Subjects * | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 5 Hz | 1.660 | 3.789 | 0.719 | 1.807 | 2.475 | 1.792 | 1.923 | 1.400 | 2.078 | 1.671 | 1.201 | 4.948 | |
| Group 20 Hz | 1.928 | 2.412 | 1.350 | 0.640 | 0.477 | 2.161 | 0.820 | 0.843 | 0.394 | 1.219 | |||
| Group Sham | 0.481 | 1.685 | 0.650 | 1.329 | 1.007 | 0.907 | 0.999 | 1.173 | |||||
| P (5 Hz and Sham) | 0.005 | ||||||||||||
| P (20 Hz and Sham) | 0.897 | ||||||||||||
| Group 5 Hz | 2.521 | 0.989 | 1.320 | 0.112 | 6.361 | 1.009 | 0.224 | 2.871 | 0.670 | 0.347 | 2.613 | ||
| Group 20 Hz | 2.699 | 1.429 | 1.464 | 1.351 | 1.481 | 2.055 | 9.102 | 2.243 | 2.128 | 1.801 | |||
| Group Sham | 0.615 | 1.475 | 0.243 | 1.110 | 0.566 | 1.102 | 0.735 | 1.350 | |||||
| P (5 Hz and Sham) | 0.657 | ||||||||||||
| P (20 Hz and Sham) | 0.0003 |
| Number of Subjects * | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 5 Hz | 0.949 | 1.158 | 1.533 | 1.551 | 0.969 | 1.189 | 1.568 | 0.811 | 1.170 | 1.077 | 1.473 | |
| Group 20 Hz | 1.073 | 1.104 | 0.895 | 1.175 | 1.640 | 1.026 | 1.153 | 1.128 | 1.382 | 1.053 | 0.930 | |
| Group Sham | 0.727 | 1.246 | 0.836 | 1.005 | 1.474 | 0.950 | 1.042 | 1.454 | 1.085 | |||
| P (5 Hz and Sham) | 0.288 | |||||||||||
| P (20 Hz and Sham) | 0.543 | |||||||||||
| Group 5 Hz | 1.158 | 1.071 | 1.145 | 1.155 | 2.084 | 1.833 | 1.009 | 1.057 | 1.216 | 1.172 | 0.677 | |
| Group 20 Hz | 1.354 | 1.535 | 2.733 | 1.189 | 1.004 | 1.222 | 0.973 | 3.457 | 1.316 | 1.213 | 1.011 | |
| Group Sham | 0.898 | 0.881 | 1.475 | 0.905 | 0.605 | 0.918 | 1.104 | 2.271 | 1.192 | |||
| P (5 Hz and Sham) | 0.323 | |||||||||||
| P (20 Hz and Sham) | 0.058 |
| No. | Group 5 Hz | Group 20 Hz | Group Sham | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| 1 | 0.594 | 0.619 | 0.480 | 0.539 | 0.713 | 0.605 |
| 2 | 0.498 | 0.600 | 0.699 | 0.500 | 0.549 | 0.521 |
| 3 | 0.538 | 0.503 | 0.505 | 0.623 | 0.509 | 0.478 |
| 4 | 0.552 | 0.649 | 0.544 | 0.507 | 0.478 | 0.484 |
| 5 | 0.487 | 0.490 | 0.552 | 0.526 | 0.573 | 0.636 |
| 6 | 0.573 | 0.683 | 0.617 | 0.640 | 0.527 | 0.757 |
| 7 | 0.492 | 0.518 | 0.556 | 0.532 | 0.504 | 0.512 |
| 8 | 0.508 | 0.506 | 0.477 | 0.516 | 0.458 | 0.503 |
| 9 | 0.482 | 0.612 | 0.520 | 0.507 | 0.508 | 0.505 |
| 10 | 0.495 | 0.481 | 0.487 | 0.738 | 0.469 | 0.471 |
| 11 | 0.504 | 0.576 | 0.552 | 0.551 | ||
| 12 | 0.496 | 0.661 | 0.475 | 0.470 | ||
| 13 | 0.478 | 0.545 | ||||
| 14 | 0.566 | 0.510 | ||||
| Ave = 0.519 | Ave = 0.568 | Ave = 0.646 | Ave = 0.665 | Ave = 0.529 | Ave = 0.547 | |
| P = 0.0366 | P = 0.1939 | P = 0.7337 | ||||
| No. | Group 5 Hz | Group 20 Hz | Group Sham | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| 1 | 0.574 | 0.660 | 0.498 | 0.596 | 0.663 | 0.587 |
| 2 | 0.488 | 0.498 | 0.518 | 0.625 | 0.506 | 0.522 |
| 3 | 0.611 | 0.553 | 0.473 | 0.535 | 0.622 | 0.537 |
| 4 | 0.608 | 0.672 | 0.569 | 0.586 | 0.516 | 0.478 |
| 5 | 0.539 | 0.538 | 0.530 | 0.702 | 0.576 | 0.573 |
| 6 | 0.684 | 0.535 | 0.514 | 0.540 | 0.577 | 0.527 |
| 7 | 0.620 | 0.716 | 0.537 | 0.508 | 0.603 | 0.582 |
| 8 | 0.599 | 0.581 | 0.478 | 0.507 | 0.486 | 0.546 |
| 9 | 0.605 | 0.618 | 0.541 | 0.559 | 0.554 | 0.540 |
| 10 | 0.620 | 0.538 | 0.519 | 0.635 | 0.626 | 0.627 |
| 11 | 0.468 | 0.498 | 0.500 | 0.584 | ||
| 12 | 0.566 | 0.605 | 0.545 | 0.506 | ||
| 13 | 0.486 | 0.551 | ||||
| 14 | 0.511 | 0.509 | ||||
| Ave = 0.570 | Ave = 0.577 | Ave = 0.622 | Ave = 0.688 | Ave = 0.573 | Ave = 0.552 | |
| P = 0.9817 | P = 0.0226 | P = 0.4727 | ||||
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pre | 0.767 | 0.699 | 0.643 | 0.588 | 0.559 | 0.517 | 0.678 | 0.638 | 0.669 | 0.629 | 0.731 | 0.565 | 0.686 | 0.558 |
| post | 0.457 | 0.424 | 0.462 | 0.375 | 0.611 | 0.391 | 0.682 | 0.507 | 0.516 | 0.601 | 0.557 | 0.149 | 0.696 | 0.749 |
| sum | pre | 8.715 | P = 0.0159 | |||||||||||
| post | 7.388 | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Song, B.; Shi, J.; Yin, K.; Du, W. Effects of Repetitive Transcranial Magnetic Stimulation at the Cerebellum on Working Memory. Brain Sci. 2023, 13, 1158. https://doi.org/10.3390/brainsci13081158
Yao J, Song B, Shi J, Yin K, Du W. Effects of Repetitive Transcranial Magnetic Stimulation at the Cerebellum on Working Memory. Brain Sciences. 2023; 13(8):1158. https://doi.org/10.3390/brainsci13081158
Chicago/Turabian StyleYao, Jiangnan, Bo Song, Jingping Shi, Kuiying Yin, and Wentao Du. 2023. "Effects of Repetitive Transcranial Magnetic Stimulation at the Cerebellum on Working Memory" Brain Sciences 13, no. 8: 1158. https://doi.org/10.3390/brainsci13081158
APA StyleYao, J., Song, B., Shi, J., Yin, K., & Du, W. (2023). Effects of Repetitive Transcranial Magnetic Stimulation at the Cerebellum on Working Memory. Brain Sciences, 13(8), 1158. https://doi.org/10.3390/brainsci13081158






