Abnormal and Changing Information Interaction in Adults with Attention-Deficit/Hyperactivity Disorder Based on Network Motifs
Abstract
:1. Introduction
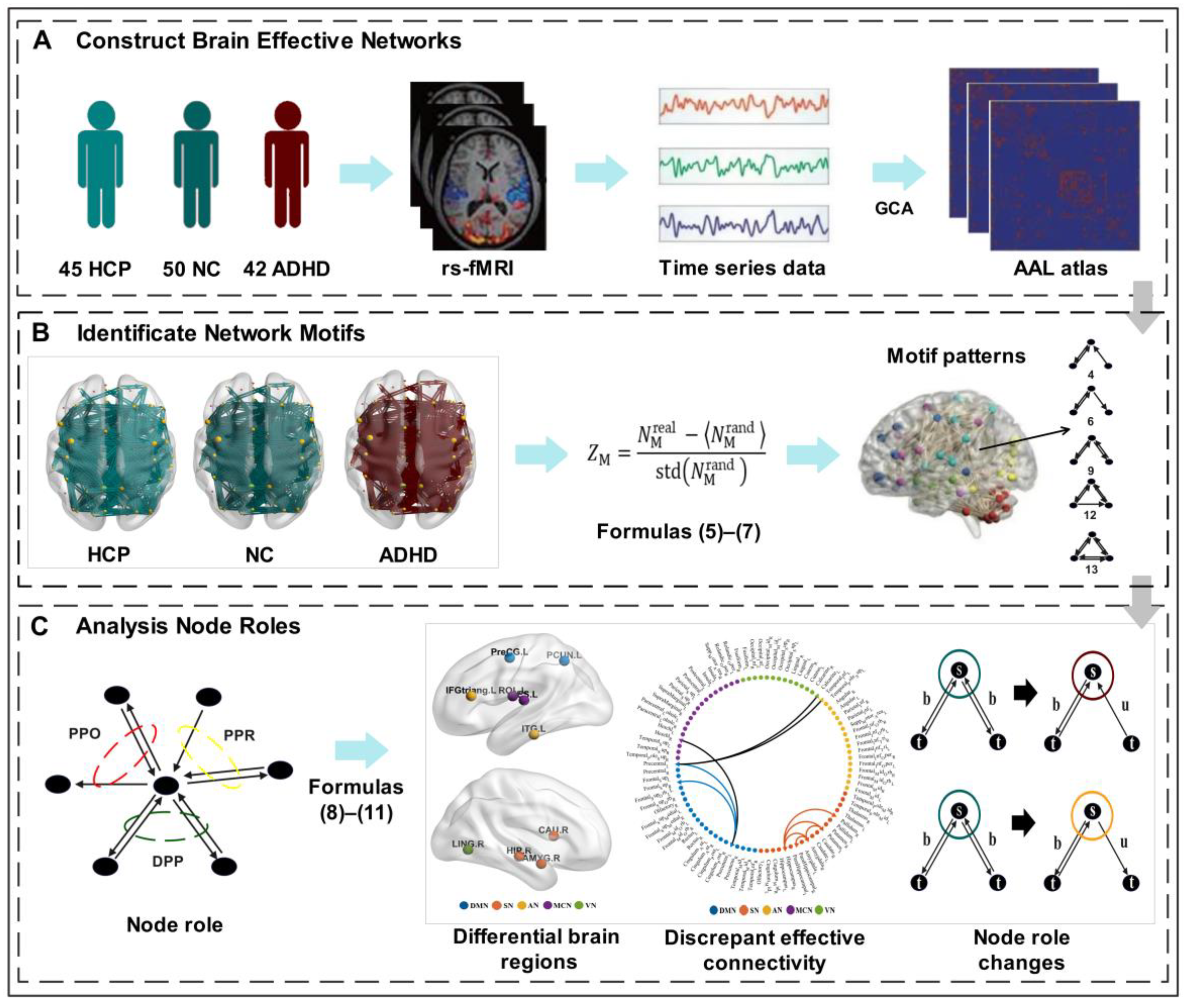
2. Materials and Methods
2.1. Participants
2.2. Data Acquisition and Preprocessing
2.3. Construction of Directed Brain Functional Networks
2.4. Identification of Network Motifs
2.5. Analysis of Motif-Based Node Roles
2.6. Statistical Analysis between Groups
3. Results
3.1. Motif Patterns within Directed Functional Brain Networks
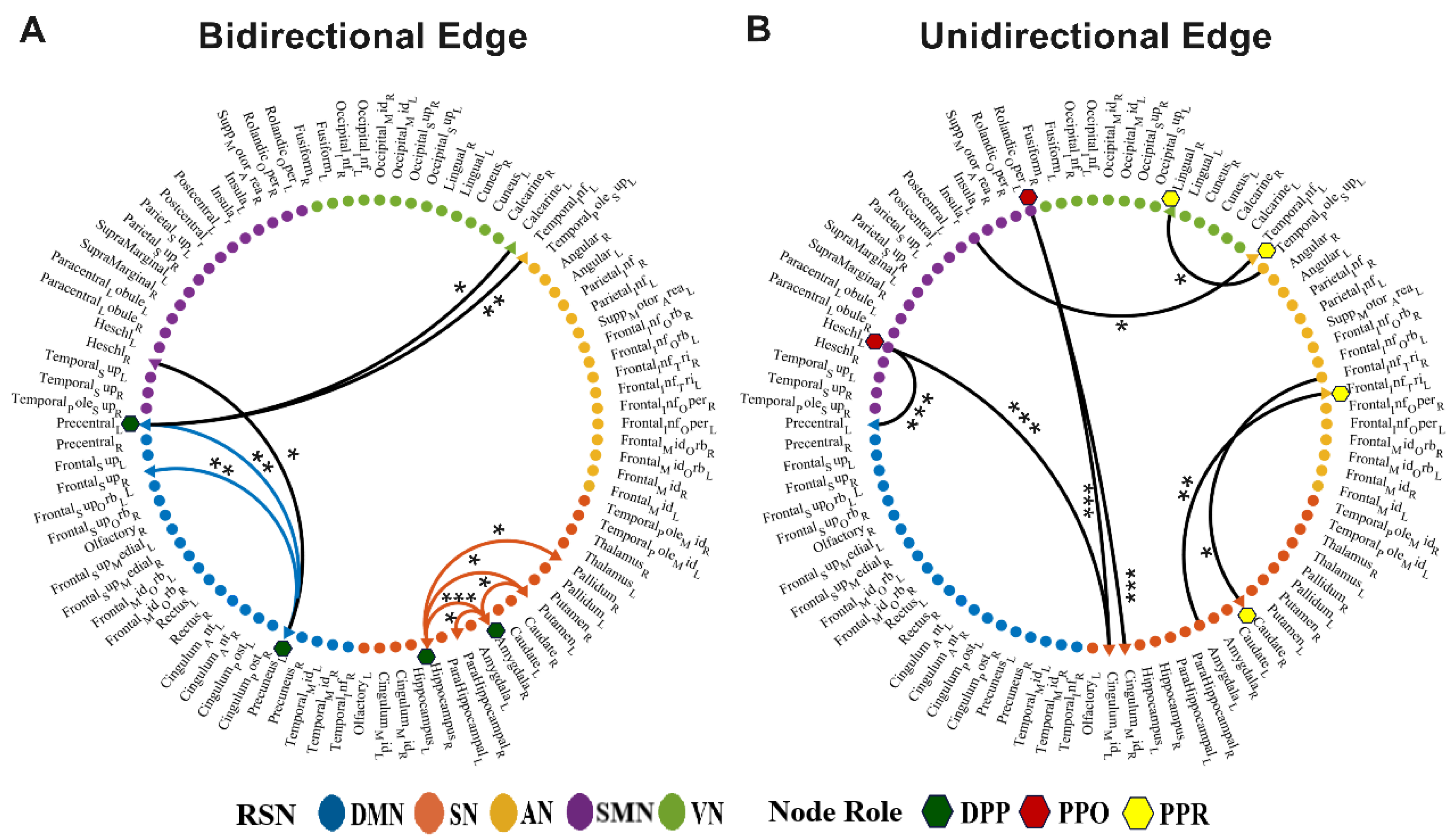
3.2. Differential Brain Regions about Node Roles between Groups
3.3. Discrepant Effective Connectivity of Node Roles between Groups
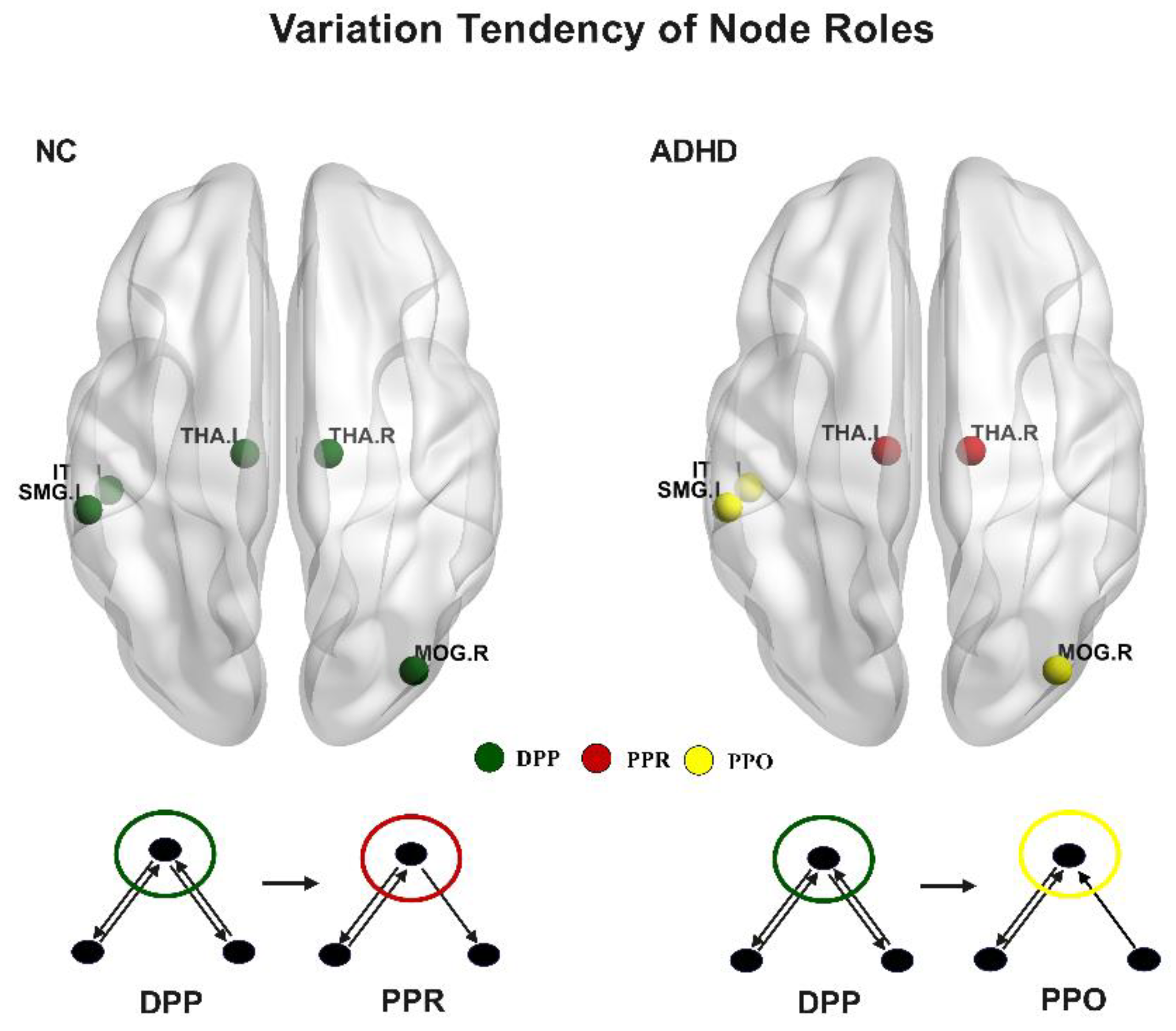
3.4. Variation Tendency of Node Roles between Groups
3.5. Correlations between Node Role-Degree and ASRS Scores
4. Discussion
4.1. Significant Motif Patterns in the Directed Functional Brain Networks
4.2. Differential Brain Regions Regarding Information Interaction Capability in ADHD Patients
4.3. Discrepant Effective Connectivity of Node Roles in ADHD Patients
4.4. Changed Information Interaction Ways of Brain Regions in ADHD Patients
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fair, D.A.; Posner, J.; Nagel, B.J.; Bathula, D.; Dias, T.G.C.; Mills, K.L.; Blythe, M.S.; Giwa, A.; Schmitt, C.F.; Nigg, J.T. Atypical Default Network Connectivity in Youth with Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2010, 68, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sroubek, A.; Kelly, M.S.; Lesser, I.; Sussman, E.; He, Y.; Branch, C.; Foxe, J.J. Atypical Pulvinar–Cortical Pathways During Sustained Attention Performance in Children with Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 1197–1207.e4. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Malek, M.; Watson, B.; Greenstein, D.; de Rossi, P.; Sharp, W. Trajectories of Cerebral Cortical Development in Childhood and Adolescence and Adult Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2013, 74, 599–606. [Google Scholar] [CrossRef]
- Wender, P. Attention-Deficit Hyperactivity Disorder in Adults; National Institutes of Health: Bethesda, MD, USA, 1998; Volume 21, pp. 761–774. [Google Scholar]
- Friedman, L.A.; Rapoport, J. Brain development in ADHD. Curr. Opin. Neurobiol. 2015, 30, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Kelly, C.; Chabernaud, C.; Proal, E.; Di Martino, A.; Milham, M.P.; Castellanos, F.X.; Ball, G.; Malpas, C.B.; Genc, S.; et al. Toward Systems Neuroscience of ADHD: A Meta-Analysis of 55 fMRI Studies. Am. J. Psychiatry 2012, 169, 1038–1055. [Google Scholar] [CrossRef] [PubMed]
- Nijmeijer, J.S.; Minderaa, R.B.; Buitelaar, J.K.; Mulligan, A.; Hartman, C.A.; Hoekstra, P.J. Attention-deficit/hyperactivity disorder and social dysfunctioning. Clin. Psychol. Rev. 2008, 28, 692–708. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Ou, J.; Lian, Z.; Xie, L.; Li, X.; Wang, P.; Hao, Y.; Zhu, D.; Jiang, R.; Wang, Y.; Chen, Y.; et al. Atomic dynamic functional interaction patterns for characterization of ADHD. Hum. Brain Mapp. 2014, 35, 5262–5278. [Google Scholar] [CrossRef]
- Youssofzadeh, V.; Agler, W.; Tenney, J.R.; Kadis, D.S. Whole-brain MEG connectivity-based analyses reveals critical hubs in childhood absence epilepsy. Epilepsy Res. 2018, 145, 102–109. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Du, Y.; Zhai, G.; Xiong, H.; Yao, D.; Xu, P.; Gong, J.; Yin, G.; Li, F. Altered Functional Connectivity in Children with ADHD Revealed by Scalp EEG: An ERP Study. Neural Plast. 2021, 2021, 6615384. [Google Scholar] [CrossRef]
- Soman, S.M.; Vijayakumar, N.; Thomson, P.; Ball, G.; Hyde, C.; Silk, T.J. Functional and structural brain network development in children with attention deficit hyperactivity disorder. Hum. Brain Mapp. 2023, 44, 3394–3409. [Google Scholar] [CrossRef] [PubMed]
- Samea, F.; Soluki, S.; Nejati, V.; Zarei, M.; Cortese, S.; Eickhoff, S.B.; Tahmasian, M.; Eickhoff, C.R. Brain alterations in children/adolescents with ADHD revisited: A neuroimaging meta-analysis of 96 structural and functional studies. Neurosci. Biobehav. Rev. 2019, 100, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zheng, S.; Tian, Y. Resting-State fMRI Whole Brain Network Function Plasticity Analysis in Attention Deficit Hyperactivity Disorder. Neural Plast. 2022, 2022, 4714763. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, C.; He, Y.; Zang, Y.; Cao, Q.; Zhang, H.; Zhong, Q.; Wang, Y. Altered small-world brain functional networks in children with attention-deficit/hyperactivity disorder. Hum. Brain Mapp. 2009, 30, 638–649. [Google Scholar] [CrossRef]
- Lin, P.; Sun, J.; Yu, G.; Wu, Y.; Yang, Y.; Liang, M.; Liu, X. Global and local brain network reorganization in attention-deficit/hyperactivity disorder. Brain Imaging Behav. 2014, 8, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Beare, R.; Adamson, C.; Bellgrove, M.A.; Vilgis, V.; Vance, A.; Seal, M.L.; Silk, T.J. Altered structural connectivity in ADHD: A network based analysis. Brain Imaging Behav. 2017, 11, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.R.; Cohen, J.R. Dysfunctional brain network organization in neurodevelopmental disorders. In Connectomics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–100. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, D.; Li, X.; Chen, H.; Jiang, X.; Sun, L.; Cao, Q.; An, L.; Liu, T.; Wang, Y. Identifying functional connectomics abnormality in attention deficit hyperactivity disorder. In Proceedings of the 2013 IEEE 10th International Symposium on Biomedical Imaging, San Francisco, CA, USA, 7–11 April 2013. [Google Scholar] [CrossRef]
- Craddock, R.C.; Jbabdi, S.; Yan, C.-G.; Vogelstein, J.T.; Castellanos, F.X.; Di Martino, A.; Kelly, C.; Heberlein, K.; Colcombe, S.; Milham, M.P. Imaging human connectomes at the macroscale. Nat. Methods 2013, 10, 524–539. [Google Scholar] [CrossRef]
- Lan, Z.; Sun, Y.; Zhao, L.; Xiao, Y.; Kuai, C.; Xue, S.-W. Aberrant Effective Connectivity of the Ventral Putamen in Boys with Attention-Deficit/Hyperactivity Disorder. Psychiatry Investig. 2021, 18, 763–769. [Google Scholar] [CrossRef]
- Cai, W.; Griffiths, K.; Korgaonkar, M.S.; Williams, L.M.; Menon, V. Inhibition-related modulation of salience and frontoparietal networks predicts cognitive control ability and inattention symptoms in children with ADHD. Mol. Psychiatry 2021, 26, 4016–4025. [Google Scholar] [CrossRef]
- Agoalikum, E.; Klugah-Brown, B.; Wu, H.; Hu, P.; Jing, J.; Biswal, B. Structural differences among children, adolescents, and adults with attention-deficit/hyperactivity disorder and abnormal Granger causality of the right pallidum and whole-brain. Front. Hum. Neurosci. 2023, 17, 1076873. [Google Scholar] [CrossRef]
- Friston, K.J. Functional and effective connectivity: A review. Brain Connect. 2011, 1, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Milo, R.; Shen-Orr, S.; Itzkovitz, S.; Kashtan, N.; Chklovskii, D.; Alon, U. Network Motifs: Simple Building Blocks of Complex Networks. Science 2002, 298, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O.; Kötter, R. Motifs in brain networks. PLoS Biol. 2004, 2, e369. [Google Scholar] [CrossRef] [PubMed]
- Friedman, E.J.; Young, K.; Tremper, G.; Liang, J.; Landsberg, A.S.; Schuff, N.; for the Alzheimer’s Disease Neuroimaging Initiative. Directed Network Motifs in Alzheimer’s Disease and Mild Cognitive Impairment. PLoS ONE 2015, 10, e0124453. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liao, X.; Yan, C.; He, Y.; Xia, M. Identifying topological motif patterns of human brain functional networks. Hum. Brain Mapp. 2017, 38, 2734–2750. [Google Scholar] [CrossRef]
- Zou, L.; Jiao, Z.; Wang, H.; Ma, K.; Xiang, J. Directed connectivity of brain default networks in resting state using GCA and motif. Front. Biosci. 2017, 22, 1634–1643. [Google Scholar] [CrossRef]
- Duclos, C.; Nadin, D.; Mahdid, Y.; Tarnal, V.; Picton, P.; Vanini, G.; Golmirzaie, G.; Janke, E.; Avidan, M.S.; Kelz, M.B.; et al. Brain network motifs are markers of loss and recovery of consciousness. Sci. Rep. 2021, 11, 3892. [Google Scholar] [CrossRef]
- Meier, J.; Märtens, M.; Hillebrand, A.; Tewarie, P.; Van Mieghem, P. Motif-based analysis of effective connectivity in brain networks. In Complex Networks & Their Applications V: Proceedings of the 5th International Workshop on Complex Networks and Their Applications (COMPLEX NETWORKS 2016), Milan, Italy, 30 November–2 December 2016; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Van Essen, D.C.; Smith, S.M.; Barch, D.M.; Behrens, T.E.J.; Yacoub, E.; Ugurbil, K. The WU-Minn Human Connectome Project: An overview. NeuroImage 2013, 80, 62–79. [Google Scholar] [CrossRef]
- Yan, C.; Zang, Y. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010, 4, 1377. [Google Scholar] [CrossRef]
- Tzoutio-Mazoyera, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Tzourio-Mazoyer, B.; Joliot, M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Bajaj, S.; Adhikari, B.M.; Friston, K.J.; Dhamala, M. Bridging the Gap: Dynamic Causal Modeling and Granger Causality Analysis of Resting State Functional Magnetic Resonance Imaging. Brain Connect. 2016, 6, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Bressler, S.L.; Seth, A.K. Wiener–Granger Causality: A well established methodology. NeuroImage 2011, 58, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Seth, A. A MATLAB toolbox for Granger causal connectivity analysis. J. Neurosci. Methods 2010, 186, 262–273. [Google Scholar] [CrossRef]
- Guye, M.; Bettus, G.; Bartolomei, F.; Cozzone, P.J. Graph theoretical analysis of structural and functional connectivity MRI in normal and pathological brain networks. Magn. Reson. Mater. Phys. Biol. Med. 2010, 23, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef]
- Achard, S.; Bullmore, E. Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 2007, 3, e17. [Google Scholar] [CrossRef] [PubMed]
- Gollo, L.L.; Mirasso, C.; Sporns, O.; Breakspear, M. Mechanisms of Zero-Lag Synchronization in Cortical Motifs. PLoS Comput. Biol. 2014, 10, e1003548. [Google Scholar] [CrossRef]
- Gollo, L.L.; Zalesky, A.; Hutchison, R.M.; Heuvel, M.v.D.; Breakspear, M. Dwelling quietly in the rich club: Brain network determinants of slow cortical fluctuations. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140165. [Google Scholar] [CrossRef]
- Gollo, L.L.; Breakspear, M. The frustrated brain: From dynamics on motifs to communities and networks. Phil. Trans. R. Soc. B 2014, 369, 20130532. [Google Scholar] [CrossRef]
- Shen, K.; Bezgin, G.; Hutchison, R.M.; Gati, J.S.; Menon, R.S.; Everling, S.; McIntosh, A.R. Information Processing Architecture of Functionally Defined Clusters in the Macaque Cortex. J. Neurosci. 2012, 32, 17465–17476. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Smallwood, J.; Spreng, R.N. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014, 1316, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Hoogman, M.; Bralten, J.; Hibar, D.P.; Mennes, M.; Zwiers, M.P.; Schweren, L.S.J.; van Hulzen, K.J.E.; Medland, S.E.; Shumskaya, E.; Jahanshad, N.; et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: A cross-sectional mega-analysis. Lancet Psychiatry 2017, 4, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Mooney, M.A.; Bhatt, P.; Hermosillo, R.J.M.; Ryabinin, P.; Nikolas, M.; Faraone, S.V.; Fair, D.A.; Wilmot, B.; Nigg, J.T. Smaller total brain volume but not subcortical structure volume related to common genetic risk for ADHD. Psychol. Med. 2021, 51, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Köhler, S.; Crane, J.; Milner, B. Differential contributions of the parahippocampal place area and the anterior hippocampus to human memory for scenes. Hippocampus 2002, 12, 718–723. [Google Scholar] [CrossRef]
- Burgess, N.; Maguire, E.A.; O’Keefe, J. The Human Hippocampus and Spatial and Episodic Memory. Neuron 2002, 35, 625–641. [Google Scholar] [CrossRef]
- Dehue, T.; Bijl, D.; de Winter, M.; Scheepers, F.; Vanheule, S.; van Os, J.; Verhaeghe, P.; Verhoeff, B. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults. Lancet Psychiatry 2017, 4, 438–439. [Google Scholar] [CrossRef]
- Dalgleish, T. The emotional brain. Nat. Rev. Neurosci. 2004, 5, 583–589. [Google Scholar] [CrossRef]
- Posner, J.; Nagel, B.J.; Maia, T.V.; Mechling, A.; Oh, M.; Wang, Z.; Peterson, B.S. Abnormal Amygdalar Activation and Connectivity in Adolescents with Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 828–837.e3. [Google Scholar] [CrossRef]
- Braver, T.S.; Barch, D.M.; Gray, J.R.; Molfese, D.L.; Snyder, A. Anterior cingulate cortex and response conflict: Effects of frequency, inhibition and errors. Cerebral Cortex 2001, 11, 825–836. [Google Scholar] [CrossRef]
- Valera, E.M.; Faraone, S.V.; Murray, K.E.; Seidman, L.J. Meta-Analysis of Structural Imaging Findings in Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2007, 61, 1361–1369. [Google Scholar] [CrossRef]
- Rubia, K.; Halari, R.; Cubillo, A.; Mohammad, A.-M.; Scott, S.; Brammer, M. Disorder-specific inferior prefrontal hypofunction in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure conduct disorder during cognitive flexibility. Hum. Brain Mapp. 2010, 31, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, W.; Hou, H.; Chen, X.; Zhang, J.; Liu, J.; Guo, Z.; Bai, G. Decreased functional connectivity between the dorsal anterior cingulate cortex and lingual gyrus in Alzheimer’s disease patients with depression. Behav. Brain Res. 2017, 326, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qiao, L.; Chen, Q.; Yang, W.; Xu, M.; Yao, X.; Qiu, J.; Yang, D. Gray Matter Volume of the Lingual Gyrus Mediates the Relationship between Inhibition Function and Divergent Thinking. Front. Psychol. 2016, 7, 1532. [Google Scholar] [CrossRef] [PubMed]
- Mechelli, A.; Humphreys, G.W.; Mayall, K.; Olson, A.; Price, C.J. Differential effects of word length and visual contrast in the fusiform and lingual gyri during. Proc. R. Soc. B Boil. Sci. 2000, 267, 1909–1913. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Young, I.M.; Conner, A.K.; Glenn, C.A.; Chakraborty, A.R.; Nix, C.E.; Bai, M.Y.; Dhanaraj, V.; Fonseka, R.D.; Hormovas, J.; et al. Anatomy and White Matter Connections of the Inferior Temporal Gyrus. World Neurosurg. 2020, 143, e656–e666. [Google Scholar] [CrossRef]
- Conway, B.R. The organization and operation of inferior temporal cortex. Annu. Rev. Vis. Sci. 2018, 4, 381–402. [Google Scholar] [CrossRef]
- Nobre, A.C.; Allison, T.; McCarthy, G. Word recognition in the human inferior temporal lobe. Nature 1994, 372, 260–263. [Google Scholar] [CrossRef]
- Mehta, T.R.; Monegro, A.; Nene, Y.; Fayyaz, M.; Bollu, P.C. Neurobiology of ADHD: A Review. Curr. Dev. Disord. Rep. 2019, 6, 235–240. [Google Scholar] [CrossRef]
- Glenn, A.L.; Raine, A.; Yaralian, P.S.; Yang, Y. Increased Volume of the Striatum in Psychopathic Individuals. Biol. Psychiatry 2010, 67, 52–58. [Google Scholar] [CrossRef]
- Gold, M.S.; Blum, K.; Oscar–Berman, M.; Braverman, E.R. Low Dopamine Function in Attention Deficit/Hyperactivity Disorder: Should Genotyping Signify Early Diagnosis in Children? Postgrad. Med. 2014, 126, 153–177. [Google Scholar] [CrossRef]
- Banich, M.T.; Burgess, G.C.; Depue, B.E.; Ruzic, L.; Bidwell, L.C.; Hitt-Laustsen, S.; Du, Y.P.; Willcutt, E.G. The neural basis of sustained and transient attentional control in young adults with ADHD. Neuropsychologia 2009, 47, 3095–3104. [Google Scholar] [CrossRef]
- Carmona, S.; Proal, E.; Hoekzema, E.A.; Gispert, J.-D.; Picado, M.; Moreno, I.; Soliva, J.C.; Bielsa, A.; Rovira, M.; Hilferty, J.; et al. Ventro-Striatal Reductions Underpin Symptoms of Hyperactivity and Impulsivity in Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2009, 66, 972–977. [Google Scholar] [CrossRef]
- Zhao, D.; Zheng, S.; Yang, L.; Tian, Y. Causal connectivity abnormalities of regional homogeneity in children with attention deficit hyperactivity disorder: A rest-state fMRI study. ADMET DMPK 2017, 5, 242–252. [Google Scholar] [CrossRef]
- Braun, U.; Schäfer, A.; Walter, H.; Erk, S.; Romanczuk-Seiferth, N.; Haddad, L.; Schweiger, J.I.; Grimm, O.; Heinz, A.; Tost, H.; et al. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc. Natl. Acad. Sci. USA 2015, 112, 11678–11683. [Google Scholar] [CrossRef]
- Adcock, R.A.; Constable, R.T.; Gore, J.C.; Goldman-Rakic, P.S. Functional neuroanatomy of executive processes involved in dual-task performance. Proc. Natl. Acad. Sci. USA 2000, 97, 3567–3572. [Google Scholar] [CrossRef] [PubMed]
- Barkley, R.A.; Fischer, M. The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 503–513. [Google Scholar] [PubMed]
- Chronis-Tuscano, A.; Molina, B.S.G.; Pelham, W.E.; Applegate, B.; Dahlke, A.; Overmyer, M.; Lahey, B.B. Very Early Predictors of Adolescent Depression and Suicide Attempts in Children with Attention-Deficit/Hyperactivity Disorder. Arch. Gen. Psychiatry 2010, 67, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.C.; Samanez-Larkin, G.R.; Young, J.S.; Cowan, R.L.; Kessler, R.M.; Zald, D.H. Caudate asymmetry is related to attentional impulsivity and an objective measure of ADHD-like attentional problems in healthy adults. Anat. Embryol. 2016, 221, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Matheus, F.C.; Rial, D.; Real, J.I.; Lemos, C.; Ben, J.; Guaita, G.O.; Pita, I.R.; Sequeira, A.C.; Pereira, F.C.; Walz, R.; et al. Decreased synaptic plasticity in the medial prefrontal cortex underlies short-term memory deficits in 6-OHDA-lesioned rats. Behav. Brain Res. 2016, 301, 43–54. [Google Scholar] [CrossRef]
- Ma, I.; van Holstein, M.; Mies, G.W.; Mennes, M.; Buitelaar, J.; Cools, R.; Cillessen, A.H.; Krebs, R.M.; Scheres, A. Ventral striatal hyperconnectivity during rewarded interference control in adolescents with ADHD. Cortex 2016, 82, 225–236. [Google Scholar] [CrossRef]
- Kieling, C.; Goncalves, R.R.; Tannock, R.; Castellanos, F.X. Neurobiology of Attention Deficit Hyperactivity Disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2008, 17, 285–307. [Google Scholar] [CrossRef] [PubMed]
- Scheidegger, M. Comparative Phenomenology and Neurobiology of Meditative and Psychedelic States of Consciousness. In Handbook of Medical Hallucinogens; The Guilford Press: New York, NY, USA, 2021; p. 395. [Google Scholar]
- Shenton, M.E.; Dickey, C.C.; Frumin, M.; McCarley, R.W. A review of MRI findings in schizophrenia. Schizophr. Res. 2001, 49, 1–52. [Google Scholar] [CrossRef]
- Bailey, T.; Joyce, A. The Role of the Thalamus in ADHD Symptomatology and Treatment. Appl. Neuropsychol. Child 2015, 4, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Bertram; Edward, H. Exploring the Thalamus and Its Role in Cortical Function, 2nd Edition. J. Neuro-Ophthalmol. 2010, 30, 110–111. [Google Scholar] [CrossRef]
- Pruim, R.H.; Beckmann, C.F.; Oldehinkel, M.; Oosterlaan, J.; Heslenfeld, D.; Hartman, C.A.; Hoekstra, P.J.; Faraone, S.V.; Franke, B.; Buitelaar, J.K.; et al. An Integrated Analysis of Neural Network Correlates of Categorical and Dimensional Models of Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Kaboodvand, N.; Iravani, B.; Fransson, P. Dynamic synergetic configurations of resting-state networks in ADHD. NeuroImage 2020, 207, 116347. [Google Scholar] [CrossRef] [PubMed]
- Iravani, B.; Arshamian, A.; Fransson, P.; Kaboodvand, N. Whole-brain modelling of resting state fMRI differentiates ADHD subtypes and facilitates stratified neuro-stimulation therapy. NeuroImage 2021, 231, 117844. [Google Scholar] [CrossRef]
- Petrovic, P.; Castellanos, F.X. Top-Down Dysregulation—From ADHD to Emotional Instability. Front. Behav. Neurosci. 2016, 10, 70. [Google Scholar] [CrossRef]




| Group | HCP | ADHD | NC | p-Value |
|---|---|---|---|---|
| Number | 45 | 42 | 50 | -- |
| Age (mean ± SD) | 30.6 ± 3.17 | 32.71 ± 10.47 | 32 ± 8.96 | 0.728 a |
| Sex (M/F) | 14/31 | 21/21 | 27/23 | 0.702 b |
| ASRS | -- | 15.43 ± 3.80 | 7.94 ± 2.89 | 0.000 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Guo, Y.; Xue, J.; Dong, Y.; Sun, Y.; Wang, B.; Xiang, J.; Liu, Y. Abnormal and Changing Information Interaction in Adults with Attention-Deficit/Hyperactivity Disorder Based on Network Motifs. Brain Sci. 2023, 13, 1331. https://doi.org/10.3390/brainsci13091331
Wu X, Guo Y, Xue J, Dong Y, Sun Y, Wang B, Xiang J, Liu Y. Abnormal and Changing Information Interaction in Adults with Attention-Deficit/Hyperactivity Disorder Based on Network Motifs. Brain Sciences. 2023; 13(9):1331. https://doi.org/10.3390/brainsci13091331
Chicago/Turabian StyleWu, Xubin, Yuxiang Guo, Jiayue Xue, Yanqing Dong, Yumeng Sun, Bin Wang, Jie Xiang, and Yi Liu. 2023. "Abnormal and Changing Information Interaction in Adults with Attention-Deficit/Hyperactivity Disorder Based on Network Motifs" Brain Sciences 13, no. 9: 1331. https://doi.org/10.3390/brainsci13091331
APA StyleWu, X., Guo, Y., Xue, J., Dong, Y., Sun, Y., Wang, B., Xiang, J., & Liu, Y. (2023). Abnormal and Changing Information Interaction in Adults with Attention-Deficit/Hyperactivity Disorder Based on Network Motifs. Brain Sciences, 13(9), 1331. https://doi.org/10.3390/brainsci13091331







