Familial Transmission of Developmental Prosopagnosia: New Case Reports from an Extended Family and Identical Twins
Abstract
:1. Introduction
2. Materials and Methods
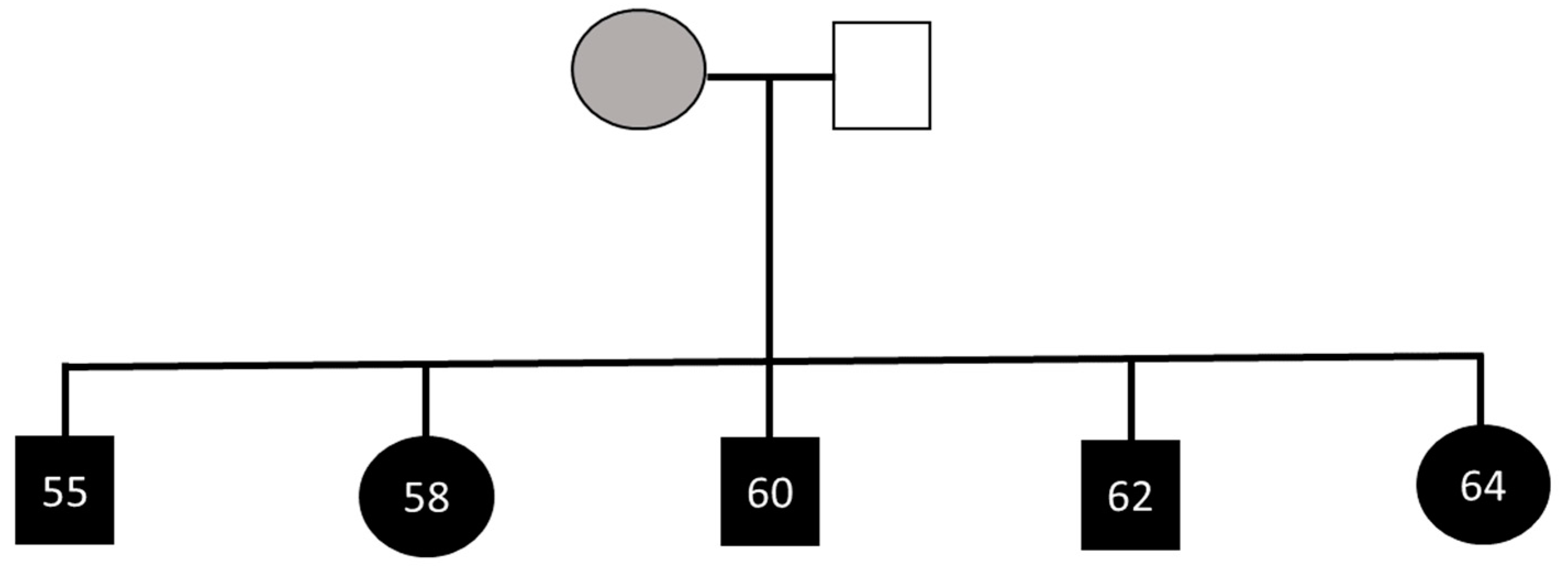
2.1. Participants
2.2. Materials
2.2.1. Face Memory Tasks
2.2.2. Face Perception Tasks
2.2.3. Object Recognition Tasks
2.3. Procedure
3. Results
3.1. Face Memory
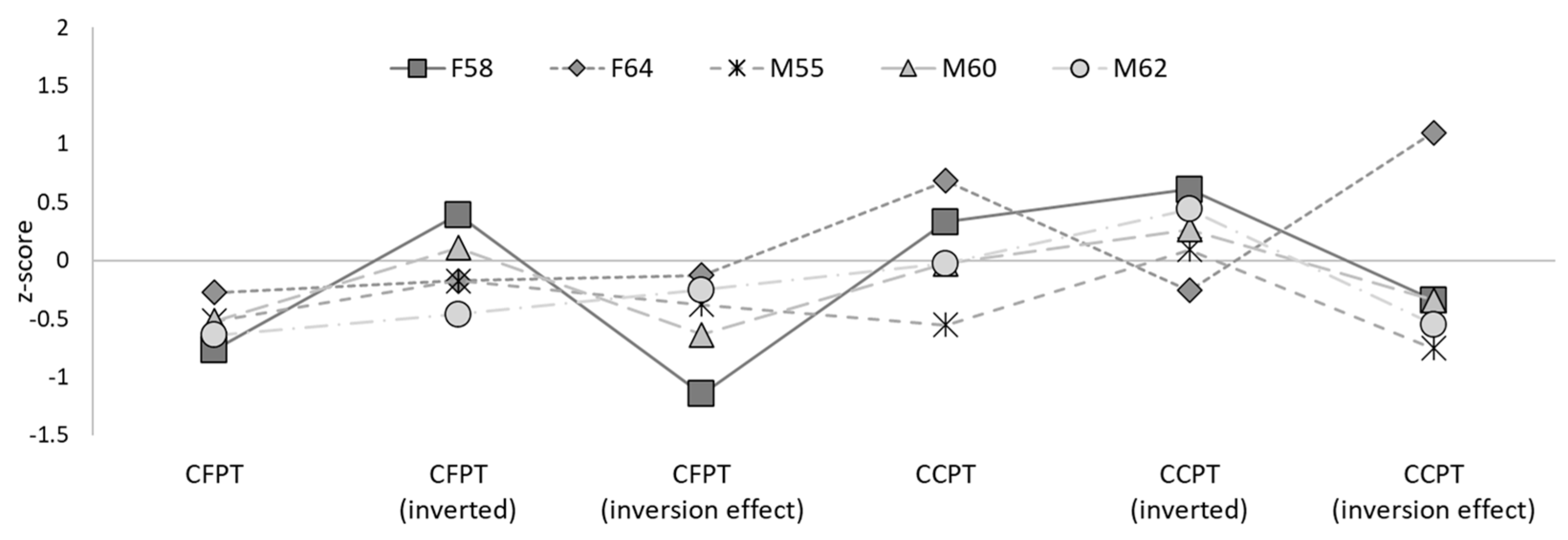
3.2. Face Perception
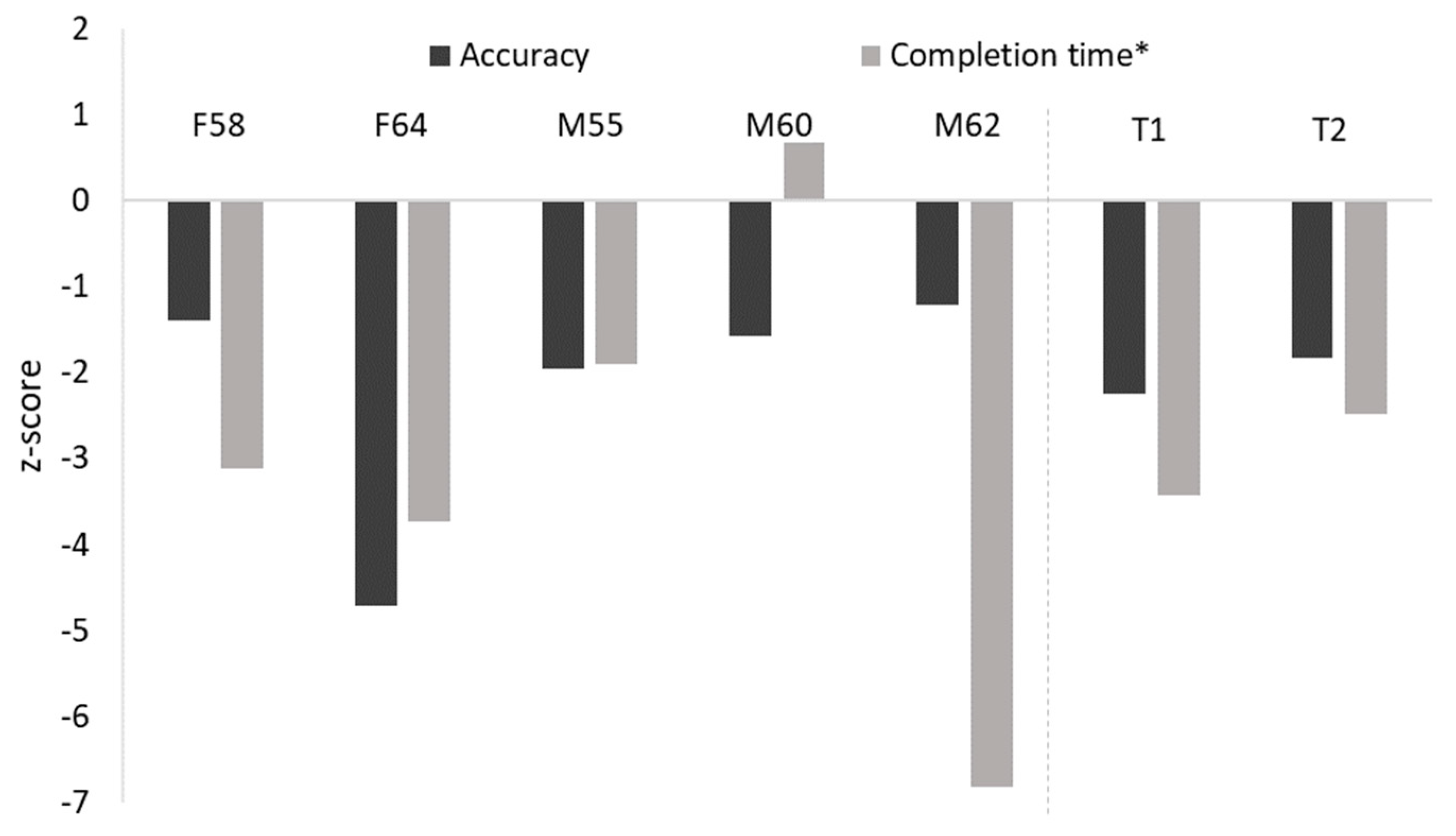
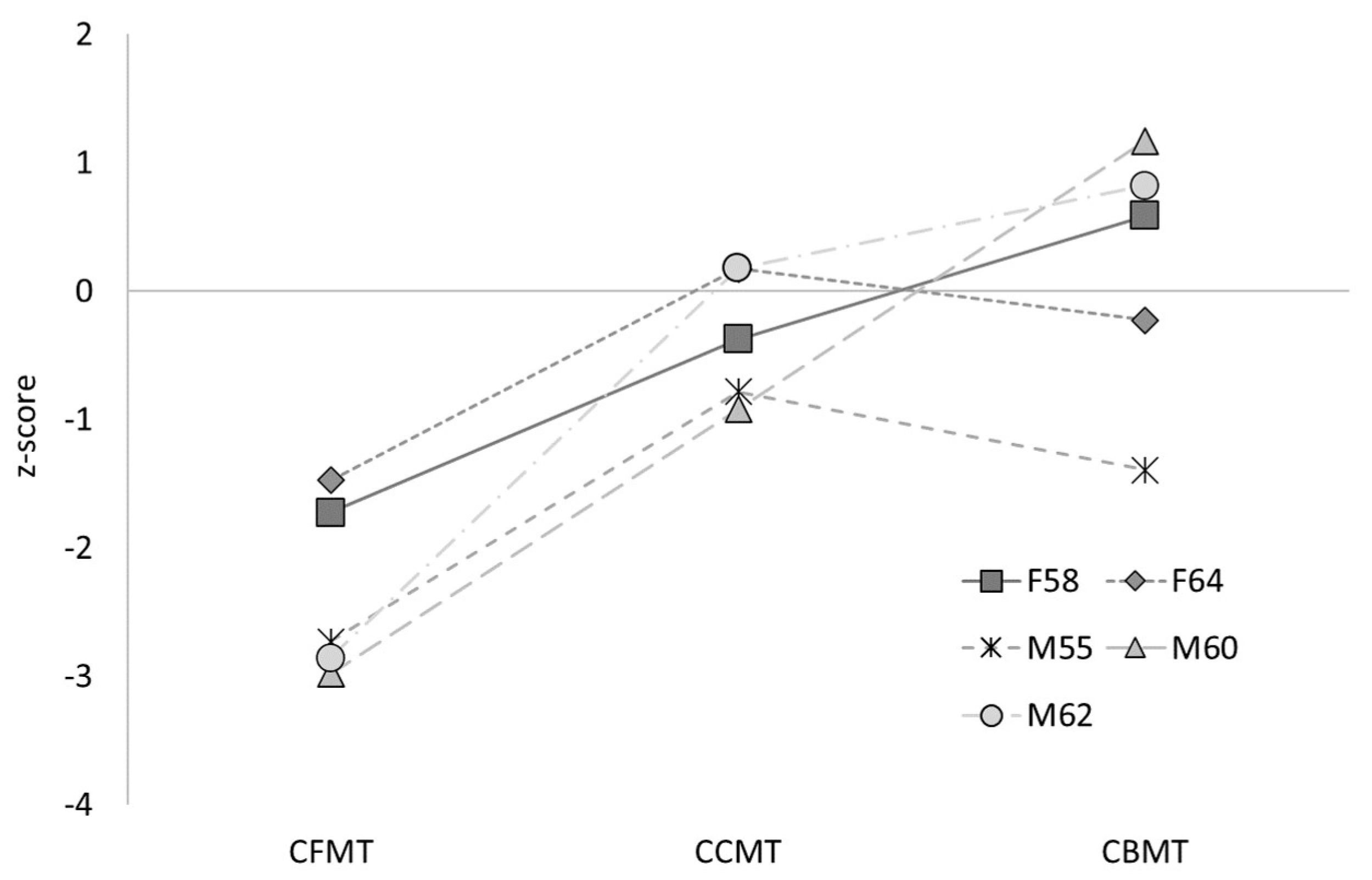
3.3. Object Recognition
3.4. Extended Family
3.5. Summary
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bate, S.; Tree, J. The definition and diagnosis of developmental prosopagnosia. Q. J. Exp. Psychol. 2017, 70, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Hills, P.; Bennetts, R.; Bate, S. Identifying hallmark symptoms of developmental prosopagnosia for non-experts. Sci. Rep. 2018, 8, 1690. [Google Scholar] [CrossRef] [PubMed]
- Susilo, T.; Duchaine, B. Advances in developmental prosopagnosia research. Curr. Opin. Neurobiol. 2013, 23, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Bennetts, R.J.; Murray, E.; Boyce, T.; Bate, S. Prevalence of face recognition deficits in middle childhood. Q. J. Exp. Psychol. 2017, 70, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Bowles, D.; McKone, E.; Dawel, A.; Duchaine, B.; Palermo, R.; Schmalzl, L.; Rivolta, D.; Wilson, E.; Yovel, G. Diagnosing prosopagnosia: Effects of ageing, sex, and participant–stimulus ethnic match on the Cambridge Face Memory Test and Cambridge Face Perception Test. Cogn. Neuropsychol. 2009, 26, 423–455. [Google Scholar] [CrossRef]
- Barton, J.; Corrow, S. The problem of being bad at faces. Neuropsychologia 2016, 89, 119–124. [Google Scholar] [CrossRef]
- Bate, S.; Dalrymple, K.; Bennetts, R. Face recognition improvements in adults and children with face recognition difficulties. Brain Commun. 2022, 4, fcac068. [Google Scholar] [CrossRef]
- Dalrymple, K.; Garrido, L.; Duchaine, B. Dissociation between face perception and face memory in adults, but not children, with developmental prosopagnosia. Dev. Cogn. Neurosci. 2014, 10, 10–20. [Google Scholar] [CrossRef]
- Schmalzl, L.; Palermo, R.; Coltheart, M. Cognitive heterogeneity in genetically based prosopagnosia: A family study. J. Neuropsychol. 2008, 2, 99–117. [Google Scholar] [CrossRef]
- Behrmann, M.; Avidan, G. Congenital prosopagnosia: Face-blind from birth. Trends Cogn. Sci. 2005, 9, 180–187. [Google Scholar] [CrossRef]
- Esins, J.; Schultz, J.; Stemper, C.; Kennerknecht, I.; Bülthoff, I. Face perception and test reliabilities in congenital prosopagnosia in seven tests. IPerception 2016, 7, 204166951562579. [Google Scholar] [CrossRef] [PubMed]
- Grueter, M.; Grueter, T.; Bell, V.; Horst, J.; Laskowski, W.; Sperling, K.; Halligan, P.W.; Ellis, H.D.; Kennerknecht, I. Hereditary prosopagnosia: The first case series. Cortex 2007, 43, 734–749. [Google Scholar] [CrossRef] [PubMed]
- Kennerknecht, I.; Grueter, T.; Welling, B.; Wentzek, S.; Horst, J.; Edwards, S.; Grueter, M. First report of prevalence of non-syndromic hereditary prosopagnosia (HPA). Am. J. Med. Genet. A 2006, 140A, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- De Haan, E.; Campbell, R. A fifteen year follow-up of a case of developmental prosopagnosia. Cortex 1992, 27, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Duchaine, B. Developmental prosopagnosia with normal configural processing. Neuroreport 2000, 11, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Duchaine, D.; Nakayama, K. Dissociations of face and object recognition in developmental prosopagnosia. J. Cogn. Neurosci. 2005, 17, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Bate, S.; Haslam, C.; Tree, J.; Hodgson, T. Evidence of an eye movement-based memory effect in congenital prosopagnosia. Cortex 2008, 44, 806–819. [Google Scholar] [CrossRef]
- Duchaine, B.; Germine, L.; Nakayama, K. Family resemblance: Ten family members with prosopagnosia and within-class object agnosia. Cogn. Neuropsychol. 2007, 24, 419–430. [Google Scholar] [CrossRef]
- Lee, Y.; Duchaine, B.; Wilson, H.; Nakayama, K. Three cases of developmental prosopagnosia from one family: Detailed neuropsychological and psychophysical investigation of face processing. Cortex 2010, 46, 949–964. [Google Scholar] [CrossRef]
- Johnen, A.; Schmukle, S.; Hüttenbrink, J.; Kischka, C.; Kennerknecht, I.; Dobel, C. A family at risk: Congenital prosopagnosia, poor face recognition and visuoperceptual deficits within one family. Neuropsychologia 2014, 58, 52–63. [Google Scholar] [CrossRef]
- Wilmer, J.B.; Germine, L.; Chabris, C.F.; Chatterjee, G.; Williams, M.; Loken, E.; Nakayama, K.; Duchaine, B. Human face recognition ability is specific and highly heritable. Proc. Natl. Acad. Sci. USA 2010, 107, 5238–5241. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Song, Y.; Hu, S.; Li, X.; Tian, M.; Zhen, Z.; Dong, Q.; Kanwisher, N.; Liu, J. Heritability of the specific cognitive ability of face perception. Curr. Biol. 2010, 20, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Richler, J.J.; Cheung, O.S.; Gauthier, I. Holistic processing predicts face recognition. Psychol. Sci. 2011, 22, 464–471. [Google Scholar] [CrossRef]
- Wang, R.; Li, J.; Fang, H.; Tian, M.; Liu, J. Individual differences in holistic processing predict face recognition ability. Psychol. Sci. 2012, 23, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Turati, C.; Bulf, H.; Simion, F. Newborns’ face recognition over changes in viewpoint. Cognition 2008, 106, 1300–1321. [Google Scholar] [CrossRef]
- Pascalis, O.; de Haan, M.; Nelson, C.A. Is face processing species-specific during the first year of life? Science 2002, 296, 1321–1323. [Google Scholar] [CrossRef] [PubMed]
- Crookes, K.; McKone, E. Early maturity of face recognition: No childhood development of holistic processing, novel face encoding, or face-space. Cognition 2009, 111, 219–247. [Google Scholar] [CrossRef]
- Bennetts, R.J.; Mole, J.; Bate, S. Super-recognition in development: A case study of an adolescent with extraordinary face recognition skills. Cogn. Neuropsychol. 2017, 34, 357–376. [Google Scholar] [CrossRef]
- McKone, E.; Palermo, R. A strong role for nature in face recognition. Proc. Natl. Acad. Sci. USA 2010, 107, 4795–4796. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Wheelwright, S.; Skinner, R.; Martin, J.; Clubley, E. The Autism-Spectrum Quotient (AQ): Evidence from Asperger Syndrome/High-Functioning Autism, Males and Females, Scientists and Mathematicians. J. Autism Dev. Disord. 2001, 31, 5–17. [Google Scholar] [CrossRef]
- Dalrymple, K.; Palermo, R. Guidelines for Studying Developmental Prosopagnosia in Adults and Children. Wiley Interdiscip. Rev. Cogn. Sci. 2016, 7, 73–87. [Google Scholar] [CrossRef] [PubMed]
- DeGutis, J.; Bahierathan, K.; Barahona, K.; Lee, E.; Evans, T.C.; Shin, H.M.; Mishra, M.; Likitlersuang, J.; Wilmer, J.B. What is the prevalence of developmental prosopagnosia? An empirical assessment of different diagnostic cutoffs. Cortex 2023, 161, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Duchaine, B.; Nakayama, K. The Cambridge Face Memory Test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia 2006, 44, 576–585. [Google Scholar] [CrossRef]
- Bate, S.; Bennetts, R.; Gregory, N.; Tree, J.; Murray, E.; Adams, A.; Bobak, A.K.; Penton, T.; Yang, T.; Bannisy, M.J. Objective patterns of face recognition deficits in 165 adults with self-reported developmental prosopagnosia. Brain Sci. 2019, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Biotti, F.; Gray, K.; Cook, R. Is developmental prosopagnosia best characterised as an apperceptive or mnemonic condition? Neuropsychologia 2019, 124, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Tsantani, M.; Cook, R. Normal recognition of famous voices in developmental prosopagnosia. Sci. Rep. 2020, 10, 19757. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.; Hills, P.J.; Bennetts, R.J.; Bate, S. Coping strategies for developmental prosopagnosia. Neuropsychol. Rehabil. 2020, 30, 1996–2015. [Google Scholar] [CrossRef]
- Bruce, V.; Young, A. Understanding face recognition. Br. J. Psychol. 1986, 77, 305–327. [Google Scholar] [CrossRef]
- Bennetts, R.J.; Gregory, N.J.; Tree, J.J.; Di Bernardi Luft, C.; Bannisy, M.J.; Murray, E.; Penton, T.; Bate, S. Face specific inversion effects provide evidence for two subtypes of developmental prosopagnosia. Neuropsychologia 2022, 174, 108332. [Google Scholar] [CrossRef]
- Biotti, F.; Cook, R. Impaired perception of facial emotion in developmental prosopagnosia. Cortex 2016, 81, 126–136. [Google Scholar] [CrossRef]
- Le Grand, R.; Cooper, P.; Mondloch, C.; Lewis, T.; Sagiv, N.; de Gelder, B.; Maurer, D. What aspects of face processing are impaired in developmental prosopagnosia? Brain Cogn. 2006, 61, 139–158. [Google Scholar] [CrossRef]
- Marsh, J.; Biotti, F.; Cook, R.; Gray, K. The discrimination of facial sex in developmental prosopagnosia. Sci. Rep. 2019, 9, 19079. [Google Scholar] [CrossRef] [PubMed]
- Bate, S.; Adams, A.; Bennetts, R.J.; Line, H. Developmental Prosopagnosia with concurrent topographical difficulties: A case report and virtual reality training programme. Neuropsychol. Rehabil. 2019, 29, 1290–1312. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, K.; Avidan, G.; Behrmann, M. A detailed investigation of facial expression processing in congenital prosopagnosia as compared to acquired prosopagnosia. Exp. Brain Res. 2007, 176, 356–373. [Google Scholar] [CrossRef] [PubMed]
- Susilo, T.; McKone, E.; Dennett, H.; Darke, H.; Palermo, R.; Hall, A.; Pidcock, M.; Dawel, A.; Jeffery, L.; Wilson, C.E.; et al. Face recognition impairments despite normal holistic processing and face space coding: Evidence from a case of developmental prosopagnosia. Cogn. Neuropsychol. 2010, 27, 636–664. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, K.A.; Corrow, S.; Yonas, A.; Duchaine, B. Developmental prosopagnosia in childhood. Cogn. Neuropsychol. 2012, 29, 393–418. [Google Scholar] [CrossRef]
- Murray, E.; Bennetts, R.; Tree, J.; Bate, S. An update of the Benton Facial Recognition Test. Behav. Res. Methods 2022, 54, 2318–2333. [Google Scholar] [CrossRef] [PubMed]
- McKone, E.; Hall, A.; Pidcock, M.; Palermo, R.; Wilkinson, R.; Rivolta, D.; Yovel, G.; David, J.M.; O’Connor, K.B. Face ethnicity and measurement reliability affect face recognition performance in developmental prosopagnosia: Evidence from the Cambridge Face Memory Test–Australian. Cogn. Neuropsychol. 2011, 28, 109–146. [Google Scholar] [CrossRef]
- Murray, E.; Bate, S. Diagnosing developmental prosopagnosia: Repeat assessment using the Cambridge Face Memory Test. R. Soc. Open Sci. 2020, 7, 200884. [Google Scholar] [CrossRef]
- Young, A.W.; Newcombe, F.; de Haan, E.H.; Small, M.; Hay, D.C. Face perception after brain injury: Selective impairments affecting identity and expression. Brain 1993, 116, 941–959. [Google Scholar] [CrossRef]
- Rezlescu, C.; Pitcher, D.; Duchaine, B. Acquired prosopagnosia with spared within-class object recognition but impaired recognition of degraded basic-level objects. Cogn. Neuropsychol. 2012, 29, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.K. Looking at upside-down faces. J. Exp. Psychol. 1969, 81, 141–145. [Google Scholar] [CrossRef]
- Yovel, G.; Kanwisher, N. Face perception: Domain specific, not process specific. Neuron 2004, 44, 889–898. [Google Scholar] [PubMed]
- Yovel, G.; Duchaine, B. Specialized face perception mechanisms extract both part and spacing information: Evidence from developmental prosopagnosia. J. Cogn. Neurosci. 2006, 18, 580–593. [Google Scholar] [CrossRef]
- Benton, A.L.; Van Allen, M.W. Impairment in facial recognition in patients with cerebral disease. Trans. Am. Neurol. Assoc. 1968, 93, 38–42. [Google Scholar] [CrossRef]
- Benton, A.L.; Sivan, A.B.; Hamsher, K.D.S.; Varney, N.R.; Spreen, O. Facial recognition: Stimulus and multiple choice pictures. In Contributions to Neuropsychological Assessment; Benton, A.L., Sivan, A.B., Hamsher, K.D.S., Varney, N.R., Spreen, O., Eds.; Oxford University Press: Oxford, UK, 1983; pp. 30–40. [Google Scholar]
- Bate, S.; Bennetts, R.J.; Tree, J.J.; Adams, A.; Murray, E. The domain-specificity of face matching impairments in 40 cases of developmental prosopagnosia. Cognition 2019, 192, e104031. [Google Scholar] [CrossRef]
- Geskin, J.; Behrmann, M. Congenital prosopagnosia without object agnosia? A literature review. Cogn. Neuropsychol. 2017, 35, 4–54. [Google Scholar] [CrossRef]
- Kanwisher, N. Functional specificity in the human brain: A window into the functional architecture of the mind. Proc. Natl. Acad. Sci. USA 2010, 107, 11163–11170. [Google Scholar] [CrossRef]
- McKone, E.; Robbins, R. Are faces special? In The Oxford Handbook of Face Perception; Calder, A.J., Rhodes, G., Johnson, M.H., Haxby, J.V., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 149–176. [Google Scholar]
- Barton, J.J.S.; Albonico, A.; Susilo, T.; Duchaine, B.; Corrow, S.L. Object recognition in acquired and developmental prosopagnosia. Cogn. Neuropsychol. 2019, 36, 54–84. [Google Scholar] [CrossRef]
- Duchaine, B.C.; Yovel, G.; Butterworth, E.J.; Nakayama, K. Prosopagnosia as an impairment to face-specific mechanisms: Elimination of the alternative hypotheses in a developmental case. Cogn. Neuropsychol. 2006, 23, 714–747. [Google Scholar] [CrossRef]
- Fry, R.; Wilmer, J.; Xie, I.; Verfaeille, M.; DeGutis, J. Evidence for normal novel object recognition abilities in developmental prosopagnosia. R. Soc. Open Sci. 2020, 7, 200988. [Google Scholar] [CrossRef] [PubMed]
- Behrmann, M.; Avidan, G.; Marotta, J.J.; Kimchi, R. Detailed exploration of face-related processing in congenital prosopagnosia: 1. Behavioral findings. J. Cogn. Neurosci. 2005, 17, 1130–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, J.; Liu, X.; Song, Y.; Wang, R.; Yang, Z.; Liu, J. Altered spontaneous neural activity in the occipital face area reflects behavioral deficits in developmental prosopagnosia. Neuropsychologia 2016, 89, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Dennett, H.; McKone, E.; Tavashmi, R.; Hall, A.; Pidcock, M.; Edwards, M.; Duchaine, B. The Cambridge Car Memory Test: A task matched in format to the Cambridge Face Memory Test, with norms, reliability, sex differences, dissociations from face memory, and expertise effects. Beh. Res. Methods 2012, 44, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Penton, T.; Leman Koybasi, S.; Banissy, M.J. Social perception and aging: The relationship between aging and the perception of subtle changes in facial happiness and identity. Acta Psychol. 2017, 179, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Corrow, S.L.; Davies-Thompson, J.; Fletcher, K.; Hills, C.; Corrow, J.C.; Barton, J.J.S. Training face perception in developmental prosopagnosia through perceptual learning. Neuropsychologia 2019, 134, 107196. [Google Scholar] [CrossRef]
- Stantić, M.; Pounder, Z.; Bate, S.; Susilo, T.; Catmur, C.; Bird, G. Individuals with developmental prosopagnosia show independent impairments in face perception, face memory and face matching. Cortex 2022, 157, 266–273. [Google Scholar] [CrossRef]
- DeGutis, J.; Cohan, S.; Mercado, R.; Wilmer, J.; Nakayama, K. Holistic processing of the mouth but not the eyes in developmental prosopagnosia. Cogn. Neuropsychol. 2012, 29, 419–446. [Google Scholar] [CrossRef]
- DeGutis, J.; Cohan, S.; Nakayama, K. Holistic face training enhances face processing in developmental prosopagnosia. Brain 2014, 137, 1781–1798. [Google Scholar] [CrossRef]
- Palermo, R.; Rossion, B.; Rhodes, G.; Laguesse, R.; Tez, T.; Hall, B.; Albonico, A.; Malaspina, M.; Daini, R.; Irons, J.; et al. Do People Have Insight into their Face Recognition Abilities? Q. J. Exp. Psychol. 2017, 70, 218–233. [Google Scholar] [CrossRef]
- White, D.; Rivolta, D.; Burton, A.M.; Al-Janabi, S.; Palermo, R. Face matching impairment in developmental prosopagnosia. Q. J. Exp. Psychol. 2017, 70, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S.; Blacker, D.; Blazer, D.G.; Ganguli, M.; Jeste, D.V.; Paulsen, J.S.; Petersen, R.C. Classifying neurocognitive disorders: The DSM-5 approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Garthwaite, P.; Porter, S. Point and interval estimates of effect sizes for the case-controls design in neuropsychology: Rationale, methods, implementations, and proposed reporting standards. Cogn. Neuropsychol. 2010, 27, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Fry, R.; Saad, E.; Arizpe, J.; Ohashi, Y.; DeGutis, J. Comparing the sensitivity of face matching assessments to detect face perception impairments. Neuropsychologia 2021, 163, 108067. [Google Scholar] [CrossRef] [PubMed]
- Biotti, F.; Wu, E.; Yang, H.; Jiahui, G.; Duchaine, B.; Cook, R. Normal composite face effects in developmental prosopagnosia. Cortex 2017, 95, 63–76. [Google Scholar] [CrossRef]
- Klargaard, S.; Starrfelt, R.; Gerlach, C. Inversion effects for faces and objects in developmental prosopagnosia: A case series analysis. Neuropsychologia 2018, 113, 52–60. [Google Scholar] [CrossRef]
- Burns, E.; Martin, J.; Chan, A.; Xu, H. Impaired processing of facial happiness, with or without awareness, in developmental prosopagnosia. Neuropsychologia 2017, 102, 217–228. [Google Scholar] [CrossRef]

| CFMT Raw Score (% Correct) | Famous Faces (%) | |
|---|---|---|
| F58 | 44 (61.11) * | 40.43 ** |
| F64 | 46 (63.89) * | 28.57 ** |
| M55 | 36 (50.00) ** | 31.67 ** |
| M60 | 34 (47.22) ** | 33.33 ** |
| M62 | 35 (48.61) ** | 32.14 ** |
| Controls | 57.45 (SD = 8.38) | 89.97 (SD = 7.76) |
| CFMT Raw Score (% Correct) | CFMT-Aus Raw Score (% Correct) | Famous Faces (%) | |
|---|---|---|---|
| T1 | 57 (79.17) | 33 (45.83) ** | 39.22 ** |
| T2 | 38 (52.78) ** | 43 (59.72) ** | 57.41 ** |
| Controls | 58.43 (SD = 8.03) a | 57.73 (SD = 7.34) b | 94.37 (SD = 5.58) a |
| CFPT % Correct (SD) a | BFRT-r (SD) b | ||||
|---|---|---|---|---|---|
| Upright | Inverted | Inversion Effect | Accuracy (%) | Task Completion Time (sec) | |
| F58 | 59.72 | 55.56 | 4.16 | 64.81 | 859.39 * |
| F64 | 65.28 | 50.00 | 15.28 | 31.48 * | 951.63 * |
| M55 | 62.50 | 50.00 | 12.50 | 59.26 * | 679.61 |
| M60 | 62.50 | 52.78 | 9.72 | 62.96 | 298.60 |
| M62 | 61.11 | 47.22 | 13.89 | 66.67 | 1410.94 * |
| Controls | 68.36 (11.30) | 51.71 (9.78) | 16.64 (10.91) | 78.79 (10.03) | 397.98 (148.43) |
| CFPT % Correct (SD) a | BFRT-r (SD) b | ||||
|---|---|---|---|---|---|
| Upright | Inverted | Inversion Effect | Accuracy (%) | Task Completion Time (sec) | |
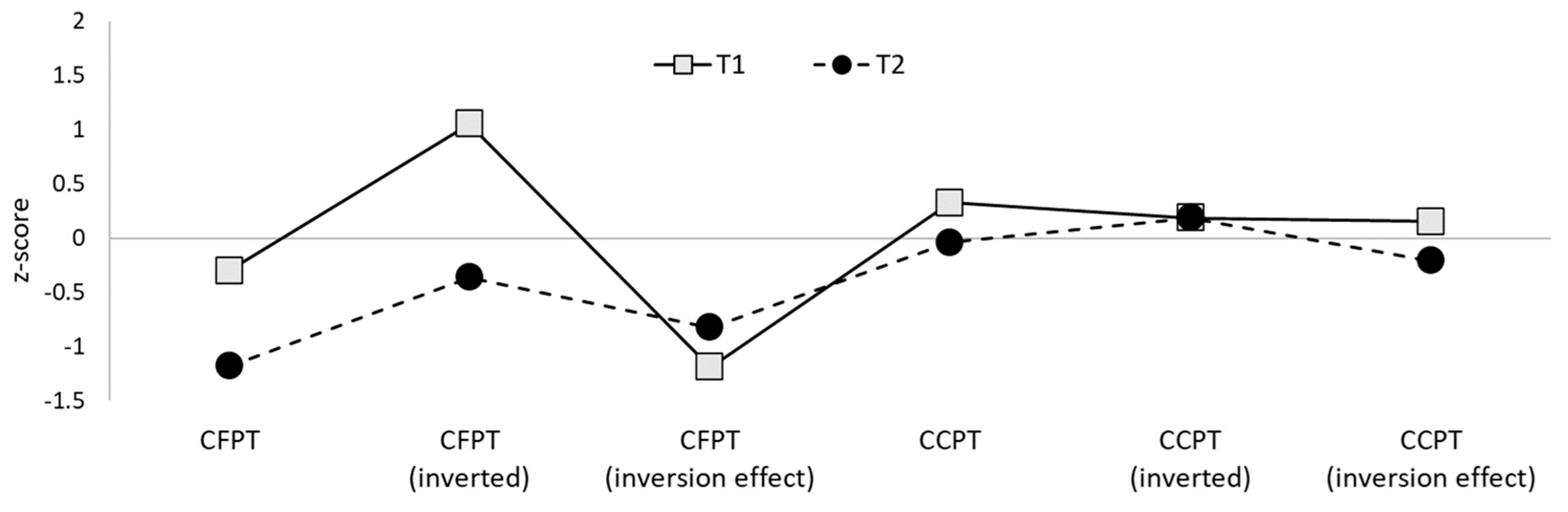
| T1 | 69.44 | 63.89 | 5.55 | 59.26 * | 701.93 * |
| T2 | 59.72 | 50.00 | 9.72 | 62.96 * | 577.99 * |
| Controls | 72.74 (11.03) | 53.55 (9.81) | 19.19 (11.46) | 78.83 (8.72) | 253.75 (130.97) |
| CCPT % Correct (SD) a | Object Memory % Correct (SD) | ||||
|---|---|---|---|---|---|
| Upright | Inverted | Inversion Effect | CCMT b | CBMT a | |
| F58 | 65.08 | 68.25 | −3.17 | 63.89 | 86.11 |
| F64 | 68.25 | 60.32 | 7.94 | 69.44 | 76.39 |
| M55 | 57.14 | 63.49 | −6.35 | 59.72 | 62.50 |
| M60 | 61.90 | 65.08 | −3.17 | 58.33 | 93.06 |
| M62 | 61.90 | 66.67 | −4.76 | 69.44 | 88.89 |
| Controls | 62.11 (8.98) | 62.66 (9.14) | −0.55 (7.72) | 67.69 (10.17) | 79.12 (11.93) |
| CCPT % Correct (SD) a | Object Memory % Correct (SD) | ||||
|---|---|---|---|---|---|
| Upright | Inverted | Inversion Effect | CCMT b | CBMT a | |
| T1 | 66.67 | 63.49 | 3.17 | 65.28 | 77.78 |
| T2 | 63.49 | 63.49 | 0.00 | 72.22 | 83.33 |
| Controls | 63.84 (8.69) | 62.02 (7.83) | 1.82 (8.74) | 65.68% (12.56) | 77.05% (14.65) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bate, S.; Murray, E.; Bennetts, R.J. Familial Transmission of Developmental Prosopagnosia: New Case Reports from an Extended Family and Identical Twins. Brain Sci. 2024, 14, 49. https://doi.org/10.3390/brainsci14010049
Bate S, Murray E, Bennetts RJ. Familial Transmission of Developmental Prosopagnosia: New Case Reports from an Extended Family and Identical Twins. Brain Sciences. 2024; 14(1):49. https://doi.org/10.3390/brainsci14010049
Chicago/Turabian StyleBate, Sarah, Ebony Murray, and Rachel J. Bennetts. 2024. "Familial Transmission of Developmental Prosopagnosia: New Case Reports from an Extended Family and Identical Twins" Brain Sciences 14, no. 1: 49. https://doi.org/10.3390/brainsci14010049







