Dynamic Changes in Neuroglial Reaction and Tissue Repair after Photothrombotic Stroke in Neonatal Mouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Photothrombotic Stroke
2.3. Staining 2,3,5-Triphenyltetrazolium Chloride (TTC)
2.4. Brain Tissue Processing
2.5. Immunohistochemistry and Imaging
2.6. Statistical Analyses
3. Results
3.1. Ischemic Infarction Evaluated by TTC Staining
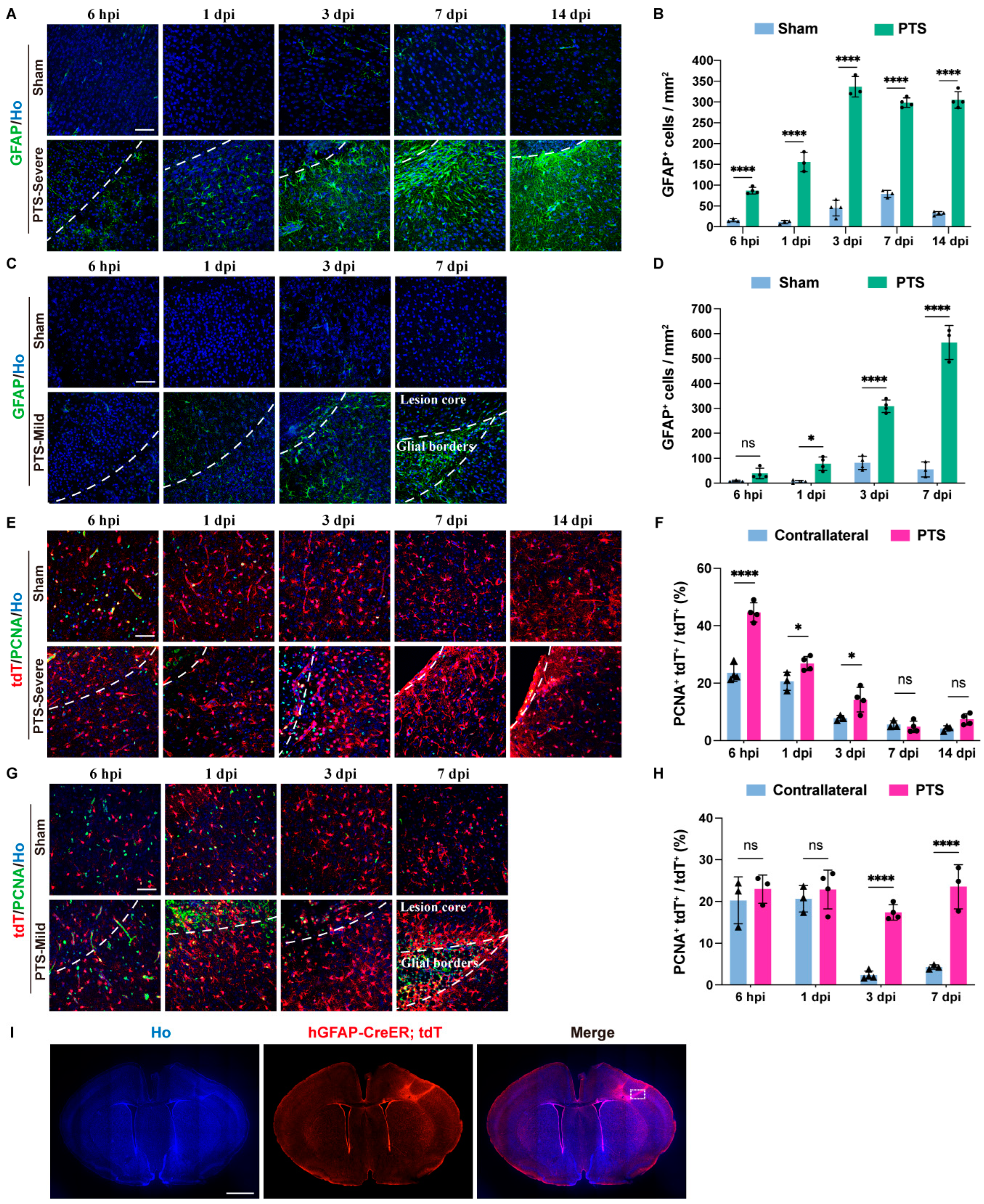
3.2. Reactivity and Proliferation of Immature Astrocytes after Ischemic Infarction
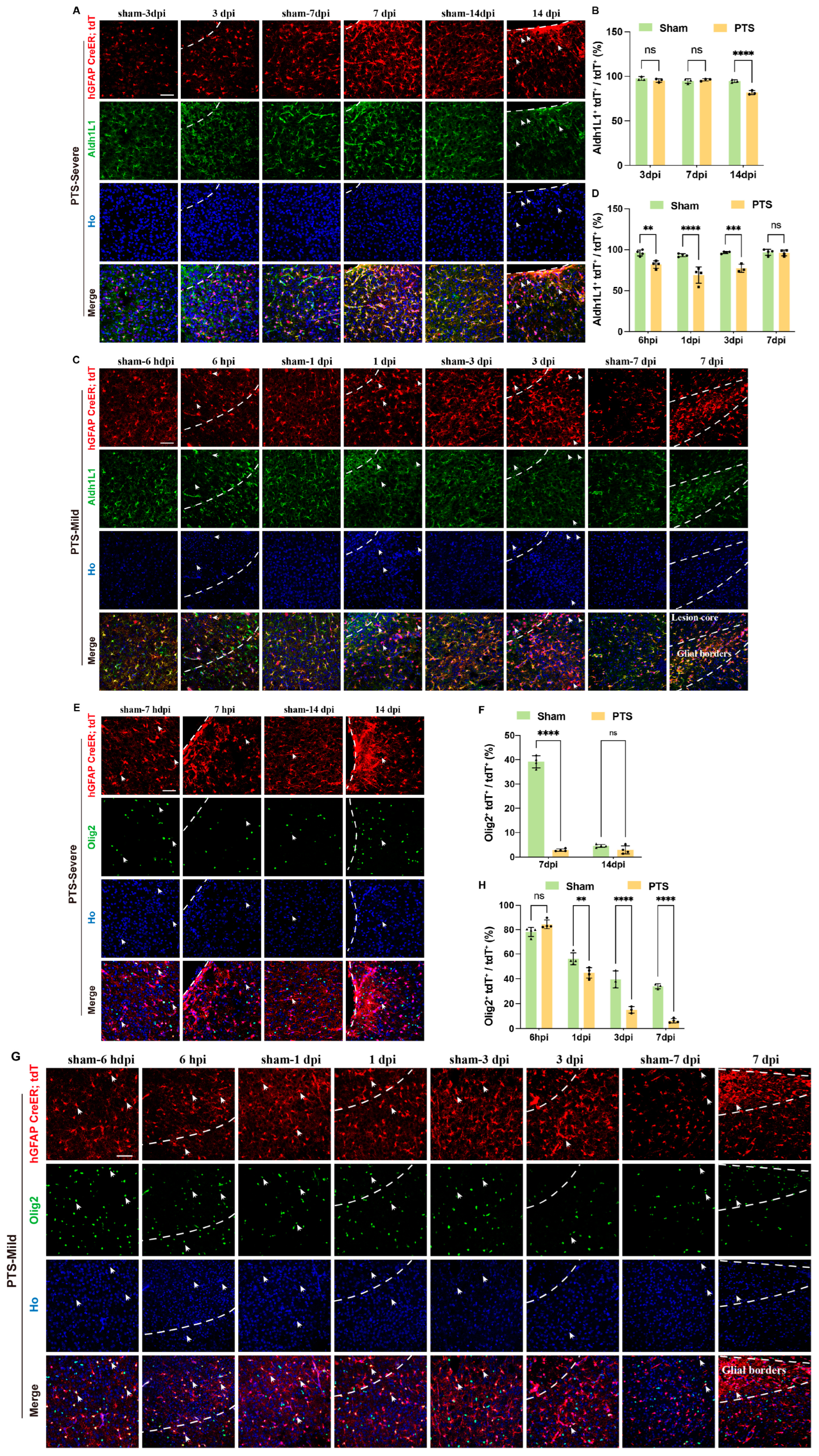
3.3. Dynamic Changes in Immature Astrocytes after Ischemic Infarction
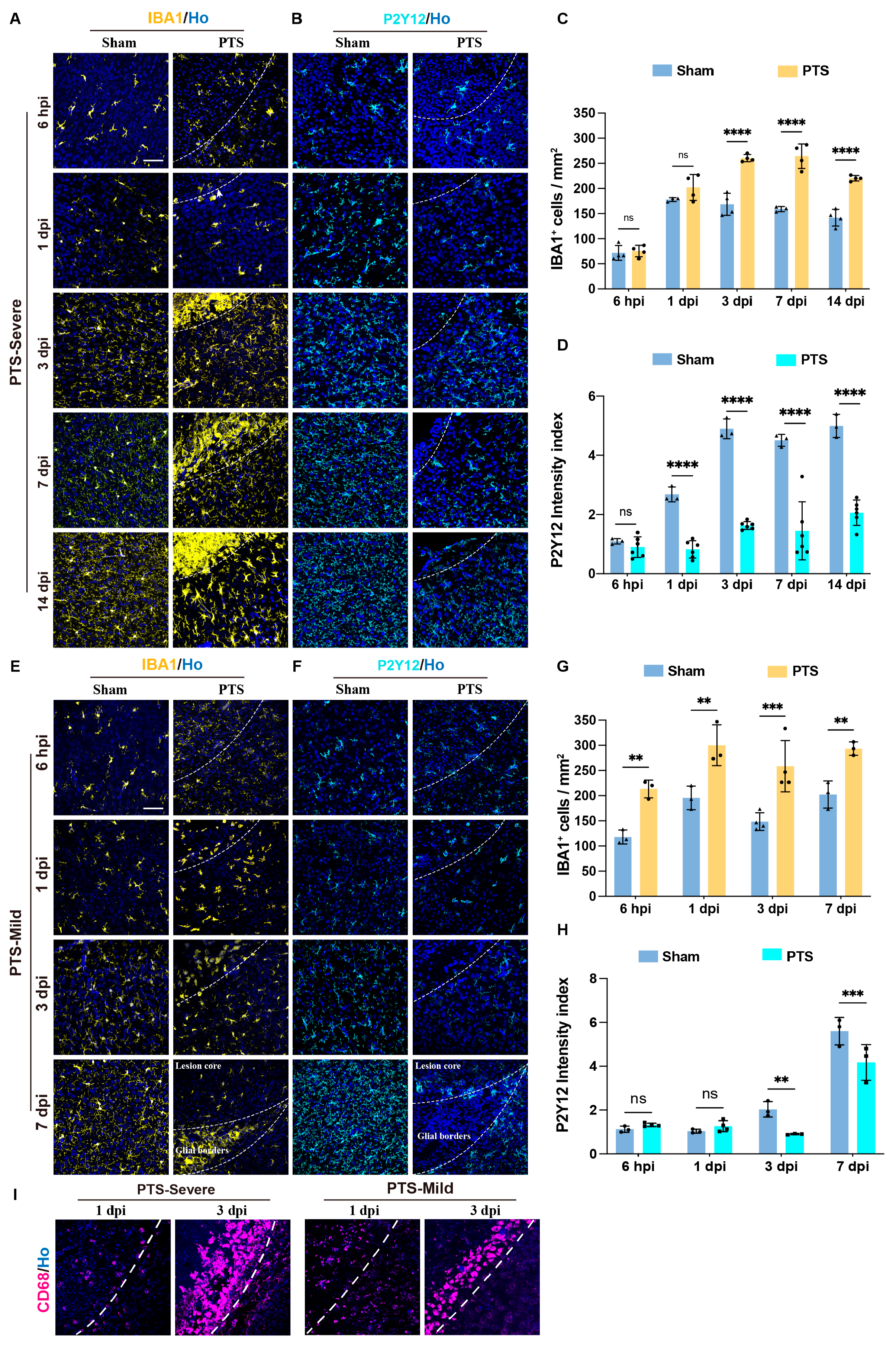
3.4. Dynamic Activation of Microglia Cells after Early Postnatal PTS
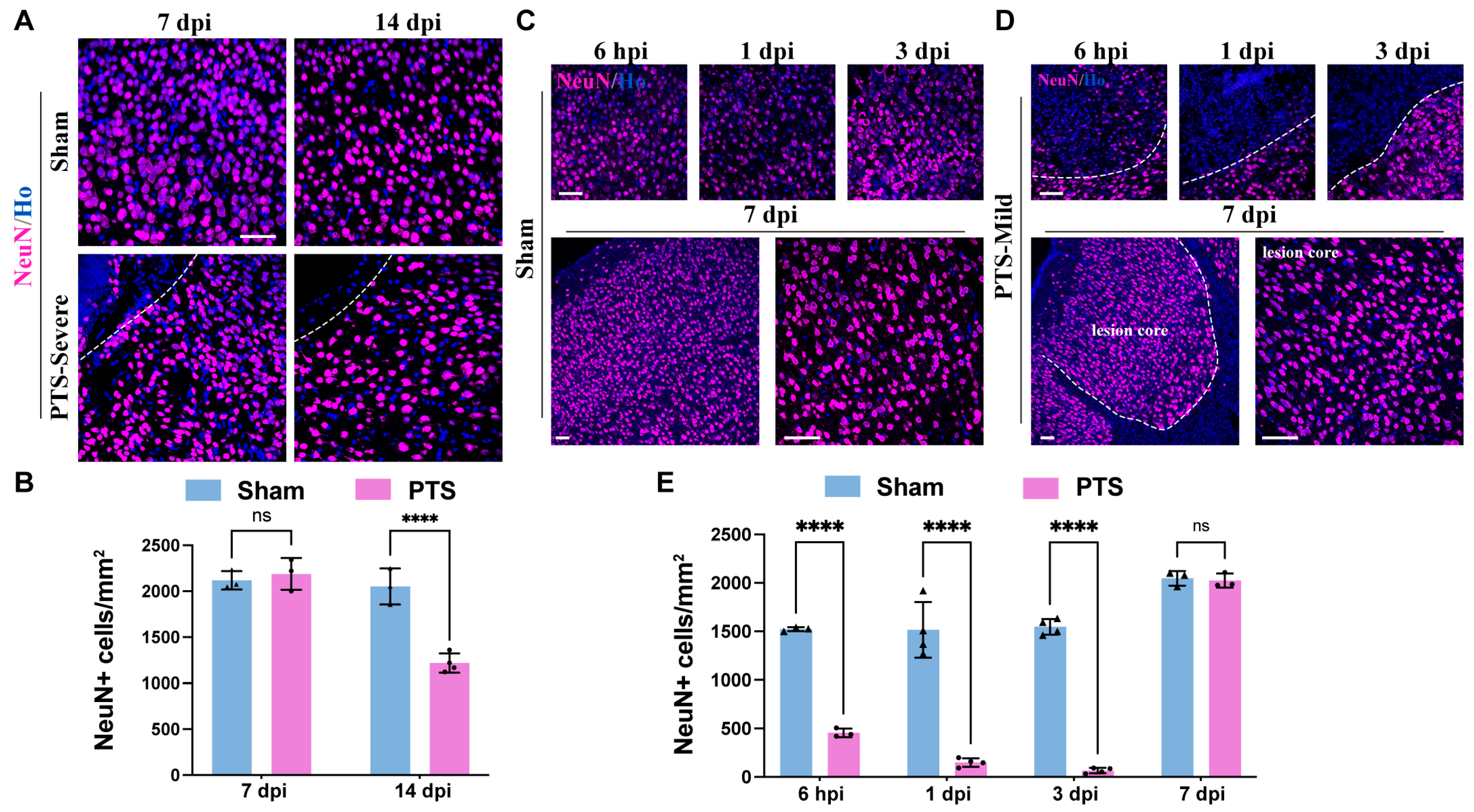
3.5. Neuronal Regeneration and Tissue Repair around the Ischemic Area
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirton, A.; Deveber, G. Life after perinatal stroke. Stroke 2013, 44, 3265–3271. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.B.; Lynch, J.K. Stroke in newborn infants. Lancet. Neurol. 2004, 3, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, M.; Kirton, A. Perinatal stroke: Mechanisms, management, and outcomes of early cerebrovascular brain injury. Lancet. Child Adolesc. Health 2018, 2, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.B. Perinatal ischemic stroke. Stroke 2007, 38, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Raju, T.N.; Nelson, K.B.; Ferriero, D.; Lynch, J.K. Ischemic perinatal stroke: Summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics 2007, 120, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Yager, J.Y.; Ashwal, S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatr. Neurol. 2009, 40, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, D.; Natarajan, N.; Ashwal, S.; Vexler, Z.S. Mechanisms of perinatal arterial ischemic stroke. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2014, 34, 921–932. [Google Scholar] [CrossRef]
- Dietz, R.M.; Dingman, A.L.; Herson, P.S. Cerebral ischemia in the developing brain. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2022, 42, 1777–1796. [Google Scholar] [CrossRef]
- Maxwell, K.A.; Dyck, R.H. Induction of reproducible focal ischemic lesions in neonatal mice by photothrombosis. Dev. Neurosci. 2005, 27, 121–126. [Google Scholar] [CrossRef]
- Uzdensky, A.B. Photothrombotic Stroke as a Model of Ischemic Stroke. Transl. Stroke Res. 2018, 9, 437–451. [Google Scholar] [CrossRef]
- Labat-gest, V.; Tomasi, S. Photothrombotic ischemia: A minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. J. Vis. Exp. 2013, 76, e50370. [Google Scholar] [CrossRef]
- Sims, N.R.; Yew, W.P. Reactive astrogliosis in stroke: Contributions of astrocytes to recovery of neurological function. Neurochem. Int. 2017, 107, 88–103. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Stetler, A.R.; Leak, R.K.; Hu, X.; Chen, J. Phagocytic microglia and macrophages in brain injury and repair. CNS Neurosci. Ther. 2022, 28, 1279–1293. [Google Scholar] [CrossRef]
- Ganat, Y.M.; Silbereis, J.; Cave, C.; Ngu, H.; Anderson, G.M.; Ohkubo, Y.; Ment, L.R.; Vaccarino, F.M. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 8609–8621. [Google Scholar] [CrossRef]
- Madisen, L.; Zwingman, T.A.; Sunkin, S.M.; Oh, S.W.; Zariwala, H.A.; Gu, H.; Ng, L.L.; Palmiter, R.D.; Hawrylycz, M.J.; Jones, A.R.; et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010, 13, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Nakano-Doi, A.; Sakuma, R.; Matsuyama, T.; Nakagomi, T. Ischemic stroke activates the VE-cadherin promoter and increases VE-cadherin expression in adult mice. Histol. Histopathol. 2018, 33, 507–521. [Google Scholar] [CrossRef]
- Sakuma, R.; Kawahara, M.; Nakano-Doi, A.; Takahashi, A.; Tanaka, Y.; Narita, A.; Kuwahara-Otani, S.; Hayakawa, T.; Yagi, H.; Matsuyama, T.; et al. Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J. Neuroinflamm. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hong, W.; Gong, P.; Qi, G.; Wang, X.; Kang, S.; Tang, H.; Qin, S. Specific knockout of Sox2 in astrocytes reduces reactive astrocyte formation and promotes recovery after early postnatal traumatic brain injury in mouse cortex. Glia 2023, 71, 602–615. [Google Scholar] [CrossRef]
- Hong, W.; Gong, P.; Pan, X.; Ren, Z.; Liu, Y.; Qi, G.; Li, J.L.; Sun, W.; Ge, W.P.; Zhang, C.L.; et al. Temporal-spatial Generation of Astrocytes in the Developing Diencephalon. Neurosci. Bull. 2024, 40, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Chun, H.; Shin, J.I.; Lee, C.J. Astrocyte Specificity and Coverage of hGFAP-CreERT2 [Tg(GFAP-Cre/ERT2)13Kdmc] Mouse Line in Various Brain Regions. Exp. Neurobiol. 2018, 27, 508–525. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.A.; Novitch, B.G.; Goldman, J.E. Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 7289–7298. [Google Scholar] [CrossRef]
- Tatsumi, K.; Isonishi, A.; Yamasaki, M.; Kawabe, Y.; Morita-Takemura, S.; Nakahara, K.; Terada, Y.; Shinjo, T.; Okuda, H.; Tanaka, T.; et al. Olig2-Lineage Astrocytes: A Distinct Subtype of Astrocytes That Differs from GFAP Astrocytes. Front. Neuroanat. 2018, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Eyo, U.B.; Wu, L.J. Microglia: Lifelong patrolling immune cells of the brain. Prog. Neurobiol. 2019, 179, 101614. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; Tang, Y.; Illes, P.; Verkhratsky, A. The Safeguarding Microglia: Central Role for P2Y(12) Receptors. Front. Pharmacol. 2020, 11, 627760. [Google Scholar] [CrossRef] [PubMed]
- Mildner, A.; Huang, H.; Radke, J.; Stenzel, W.; Priller, J. P2Y(12) receptor is expressed on human microglia under physiological conditions throughout development and is sensitive to neuroinflammatory diseases. Glia 2017, 65, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.G.; Hong, J.J.; Lee, Y.; Yi, K.S.; Jeon, C.Y.; Park, J.; Won, J.; Seo, J.; Ahn, Y.J.; Kim, K.; et al. Increased CD68/TGFβ Co-expressing Microglia/ Macrophages after Transient Middle Cerebral Artery Occlusion in Rhesus Monkeys. Exp. Neurobiol. 2019, 28, 458–473. [Google Scholar] [CrossRef]
- Kirton, A.; deVeber, G. Paediatric stroke: Pressing issues and promising directions. Lancet Neurol. 2015, 14, 92–102. [Google Scholar] [CrossRef]
- Markey, K.M.; Saunders, J.C.; Smuts, J.; von Reyn, C.R.; Garcia, A.D.R. Astrocyte development-More questions than answers. Front. Cell Dev. Biol. 2023, 11, 1063843. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. New insights into glial scar formation after spinal cord injury. Cell Tissue Res. 2022, 387, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, Y.; Cai, W.H.; Hurlock, E.C.; Wu, H.; Kernie, S.G.; Parada, L.F.; Lu, Q.R. A crucial role for Olig2 in white matter astrocyte development. Development 2007, 134, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.L.; Martínez-Cerdeño, V.; Noctor, S.C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 4216–4233. [Google Scholar] [CrossRef] [PubMed]
- Park, D.S.; Kozaki, T.; Tiwari, S.K.; Moreira, M.; Khalilnezhad, A.; Torta, F.; Olivié, N.; Thiam, C.H.; Liani, O.; Silvin, A.; et al. iPS-cell-derived microglia promote brain organoid maturation via cholesterol transfer. Nature 2023, 623, 397–405. [Google Scholar] [CrossRef]
- Pelvig, D.P.; Pakkenberg, H.; Stark, A.K.; Pakkenberg, B. Neocortical glial cell numbers in human brains. Neurobiol. Aging 2008, 29, 1754–1762. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Gong, P.; Qi, G.; Tang, H.; Gui, R.; Qi, C.; Qin, S. Dynamic Changes in Neuroglial Reaction and Tissue Repair after Photothrombotic Stroke in Neonatal Mouse. Brain Sci. 2024, 14, 152. https://doi.org/10.3390/brainsci14020152
Liu Y, Gong P, Qi G, Tang H, Gui R, Qi C, Qin S. Dynamic Changes in Neuroglial Reaction and Tissue Repair after Photothrombotic Stroke in Neonatal Mouse. Brain Sciences. 2024; 14(2):152. https://doi.org/10.3390/brainsci14020152
Chicago/Turabian StyleLiu, Yitong, Pifang Gong, Guibo Qi, Han Tang, Runshan Gui, Congcong Qi, and Song Qin. 2024. "Dynamic Changes in Neuroglial Reaction and Tissue Repair after Photothrombotic Stroke in Neonatal Mouse" Brain Sciences 14, no. 2: 152. https://doi.org/10.3390/brainsci14020152
APA StyleLiu, Y., Gong, P., Qi, G., Tang, H., Gui, R., Qi, C., & Qin, S. (2024). Dynamic Changes in Neuroglial Reaction and Tissue Repair after Photothrombotic Stroke in Neonatal Mouse. Brain Sciences, 14(2), 152. https://doi.org/10.3390/brainsci14020152






