Effects of Hydrocodone Overdose and Ceftriaxone on Astrocytic Glutamate Transporters and Glutamate Receptors, and Associated Signaling in Nucleus Accumbens as well as Locomotor Activity in C57/BL Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Use Approval
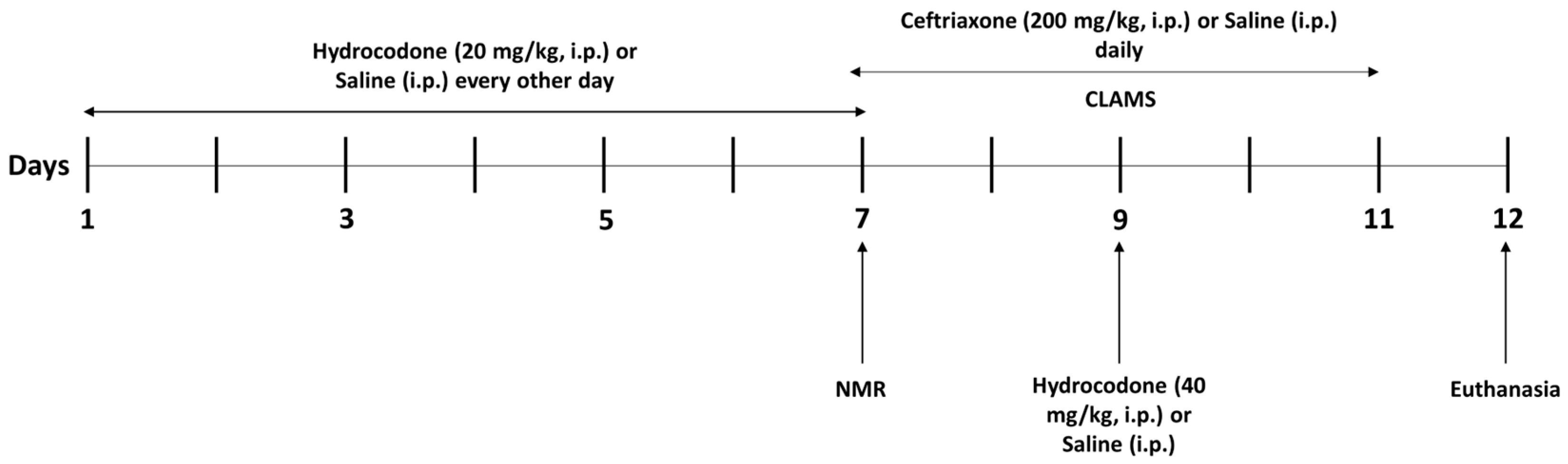
2.2. Animal and Study Design
2.3. Comprehensive Laboratory Animal Monitoring System (CLAMS)
2.4. Brain Tissue Extraction
2.5. Western Blot Analyses
2.6. Statistical Analyses
3. Results
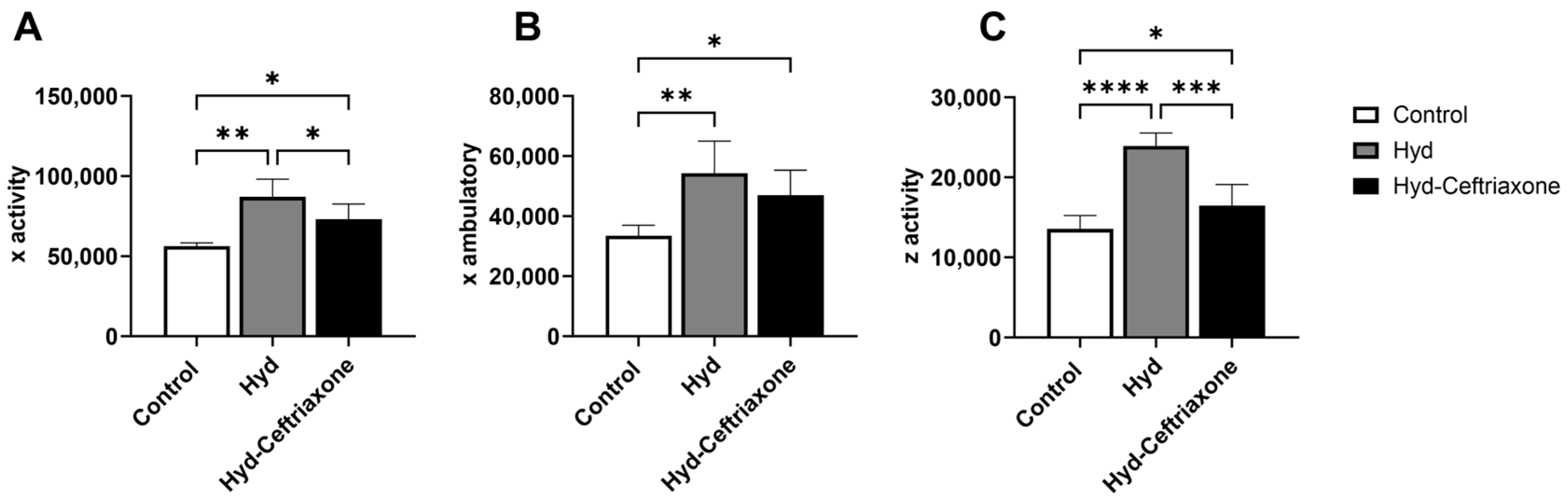
3.1. Effects of Exposure to Hydrocodone Overdose and Ceftriaxone Treatment on Locomotion Activity
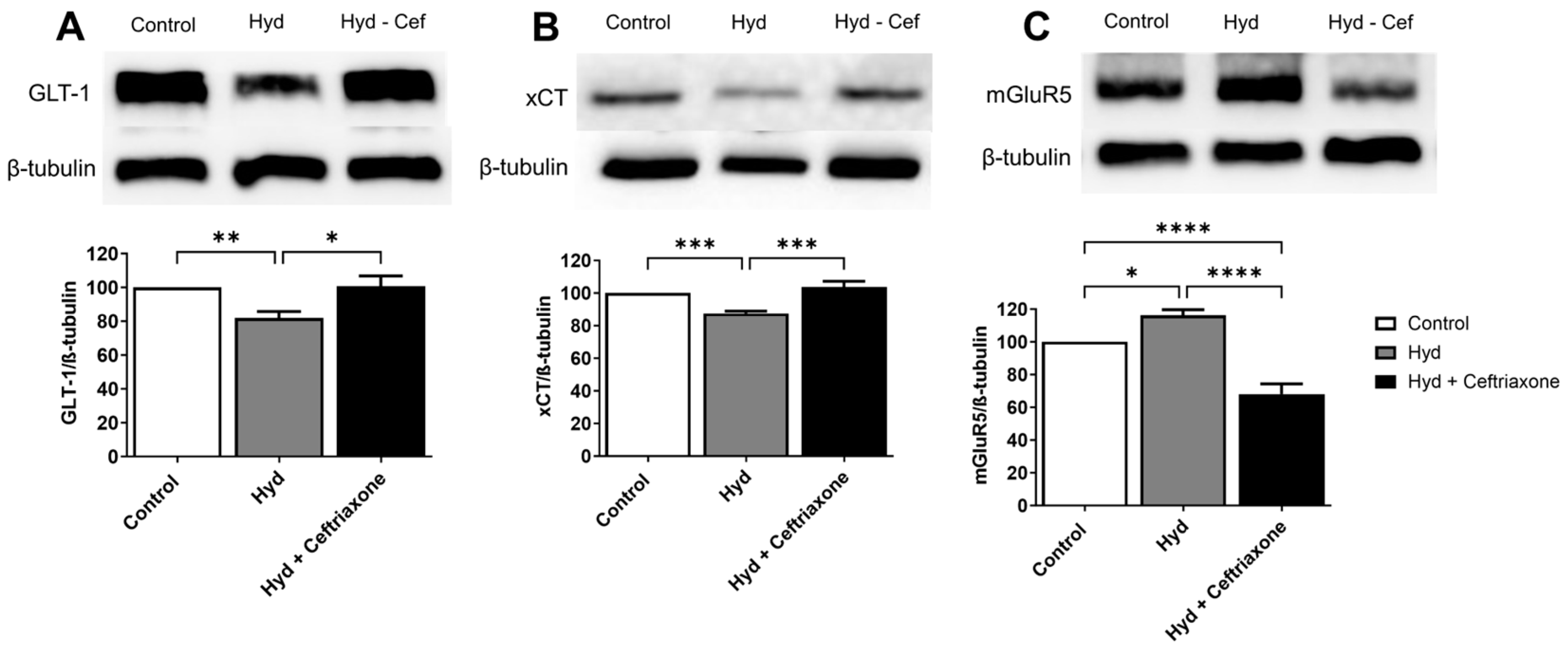
3.2. Effects of Exposure to Hydrocodone Overdose and Ceftriaxone on GLT-1, xCT, and mGluR5 Protein Expressions in the NAc
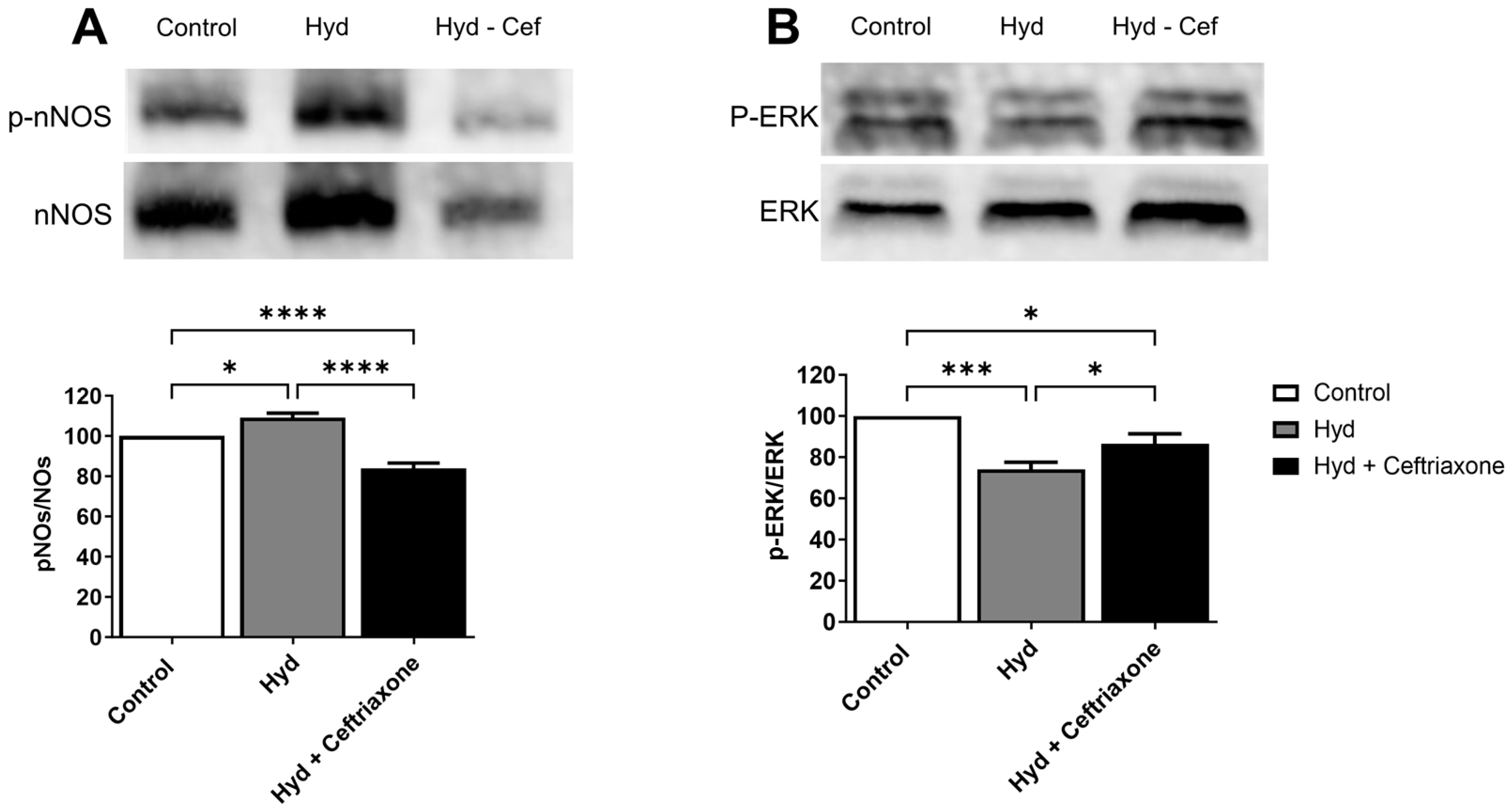
3.3. Effects of Exposure to Hydrocodone Overdose and Ceftriaxone on nNOS and ERK Protein Expression in the NAc
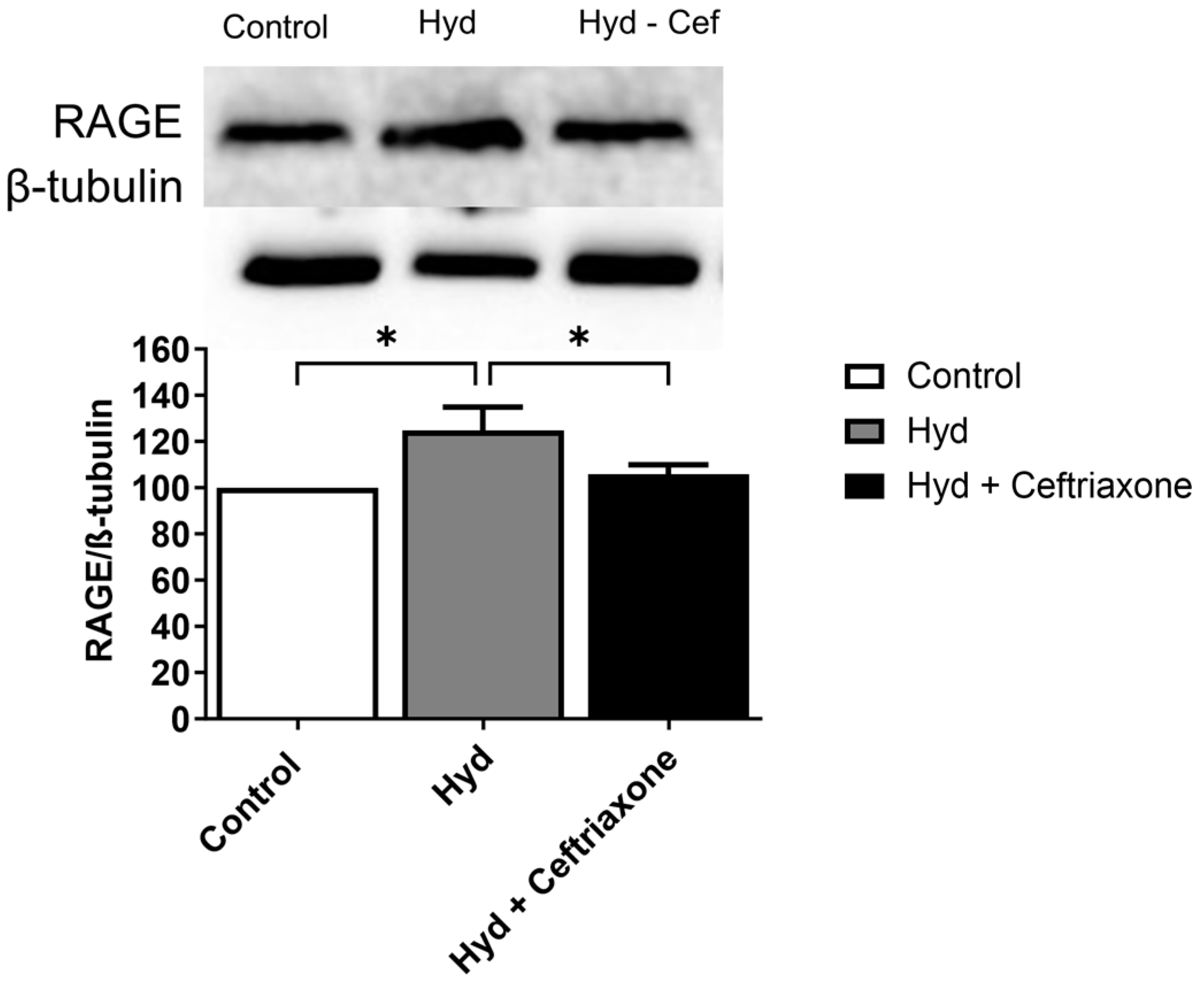
3.4. Effects of Exposure to Hydrocodone Overdose and Ceftriaxone on RAGE Protein Expression in the NAc
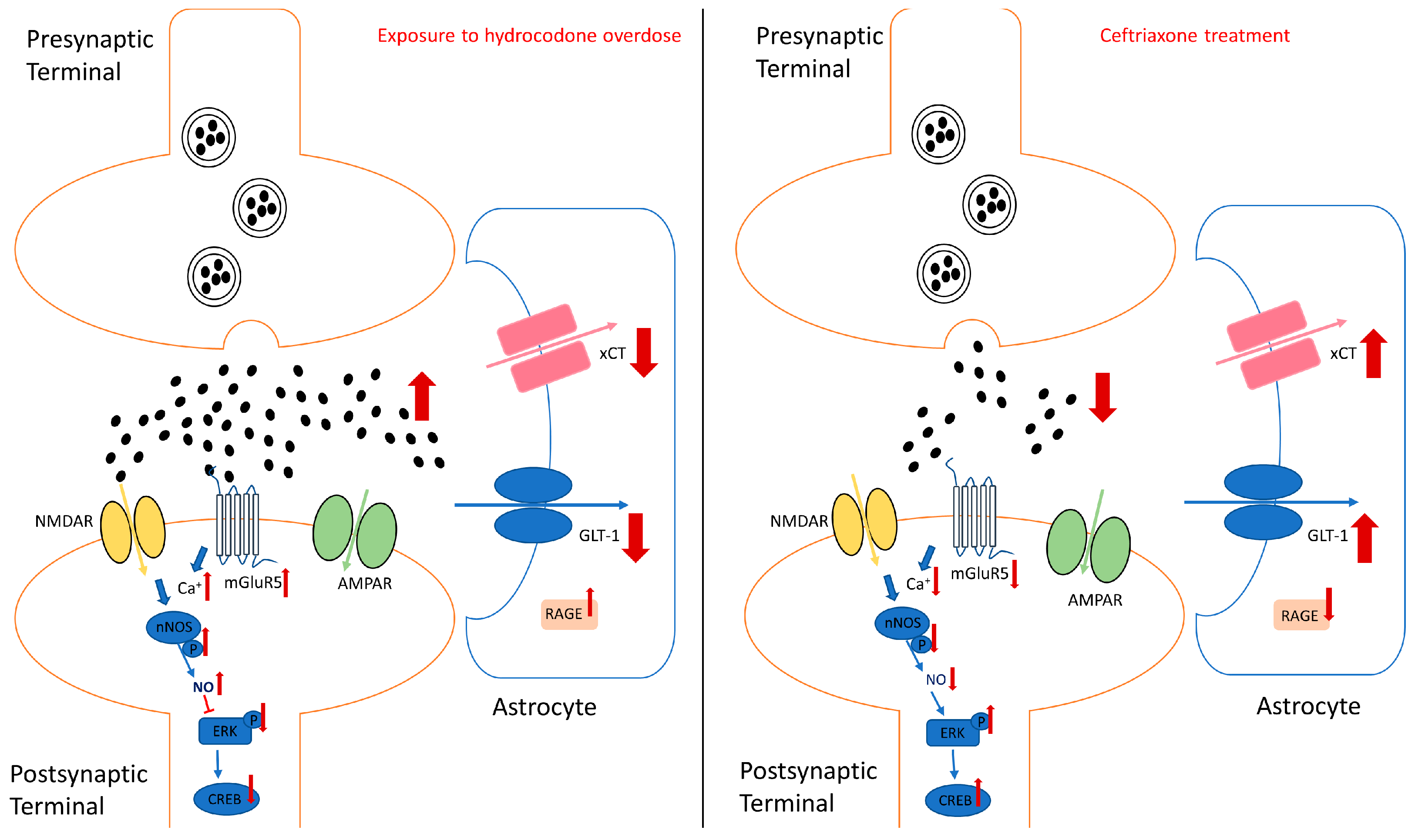
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cicero, T.J.; Ellis, M.S.; Surratt, H.L.; Kurtz, S.P. Factors influencing the selection of hydrocodone and oxycodone as primary opioids in substance abusers seeking treatment in the United States. PAIN® 2013, 154, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Fish, E.W.; DeBold, J.F.; Miczek, K.A. Repeated alcohol: Behavioral sensitization and alcohol-heightened aggression in mice. Psychopharmacology 2002, 160, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, M.; Alam, M.R. Enhancing effect of methamphetamine on ambulatory activity produced by repeated administration in mice. Pharmacol. Biochem. Behav. 1981, 15, 925–932. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Stewart, J. Dopamine transmission in the initiation and expression of drug-and stress-induced sensitization of motor activity. Brain Res. Rev. 1991, 16, 223–244. [Google Scholar] [CrossRef]
- Kita, T.; Okamoto, M.; Nakashima, T. Nicotine-induced ambulatory stimulant effect and its reverse tolerance. Yakubutsu Seishin Kodo = Jpn. J. Psychopharmacol. 1992, 12, 17–25. [Google Scholar]
- Steketee, J.D.; Kalivas, P.W. Drug wanting: Behavioral sensitization and relapse to drug-seeking behavior. Pharmacol. Rev. 2011, 63, 348–365. [Google Scholar] [CrossRef] [PubMed]
- Emery, M.A.; Bates, M.L.; Wellman, P.J.; Eitan, S. Differential effects of oxycodone, hydrocodone, and morphine on the responses of D2/D3 dopamine receptors. Behav. Brain Res. 2015, 284, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Nazarian, A.; Are, D.; Tenayuca, J.M. Acetaminophen modulation of hydrocodone reward in rats. Pharmacol. Biochem. Behav. 2011, 99, 307–310. [Google Scholar] [CrossRef]
- Alshehri, F.S.; Hakami, A.Y.; Althobaiti, Y.S.; Sari, Y. Effects of ceftriaxone on hydrocodone seeking behavior and glial glutamate transporters in P rats. Behav. Brain Res. 2018, 347, 368–376. [Google Scholar] [CrossRef]
- Bridges, R.J.; Natale, N.R.; Patel, S.A. System xc(-) cystine/glutamate antiporter: An update on molecular pharmacology and roles within the CNS. Br. J. Pharmacol. 2012, 165, 20–34. [Google Scholar] [CrossRef]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef] [PubMed]
- Tallaksen-Greene, S.J.; Kaatz, K.W.; Romano, C.; Albin, R.L. Localization of mGluR1a-like immunoreactivity and mGluR5-like immunoreactivity in identified populations of striatal neurons. Brain Res. 1998, 780, 210–217. [Google Scholar] [CrossRef] [PubMed]
- She, W.C.; Quairiaux, C.; Albright, M.J.; Wang, Y.C.; Sanchez, D.E.; Chang, P.S.; Welker, E.; Lu, H.C. Roles of mGluR5 in synaptic function and plasticity of the mouse thalamocortical pathway. Eur. J. Neurosci. 2009, 29, 1379–1396. [Google Scholar] [CrossRef] [PubMed]
- Drouin-Ouellet, J.; Brownell, A.L.; Saint-Pierre, M.; Fasano, C.; Emond, V.; Trudeau, L.E.; Lévesque, D.; Cicchetti, F. Neuroinflammation is associated with changes in glial mGluR5 expression and the development of neonatal excitotoxic lesions. Glia 2011, 59, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Abulseoud, O.A.; Alasmari, F.; Hussein, A.M.; Sari, Y. Ceftriaxone as a Novel Therapeutic Agent for Hyperglutamatergic States: Bridging the Gap Between Preclinical Results and Clinical Translation. Front. Neurosci. 2022, 16, 841036. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.; Sari, Y. Effects of Chronic Hydrocodone Exposure and Ceftriaxone on the Expression of Astrocytic Glutamate Transporters in Mesocorticolimbic Brain Regions of C57/BL Mice. Toxics 2023, 11, 870. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.A.; Baron, D.A.; Kim, J.K.; Unterwald, E.M.; Rawls, S.M. β-Lactam antibiotic produces a sustained reduction in extracellular glutamate in the nucleus accumbens of rats. Amino Acids 2011, 40, 761–764. [Google Scholar] [CrossRef][Green Version]
- Orsini, C.; Bonito-Oliva, A.; Conversi, D.; Cabib, S. Susceptibility to conditioned place preference induced by addictive drugs in mice of the C57BL/6 and DBA/2 inbred strains. Psychopharmacology 2005, 181, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, S.L.; Bryant, C.D. Behavioral architecture of opioid reward and aversion in C57BL/6 substrains. Front. Behav. Neurosci. 2015, 8, 450. [Google Scholar] [CrossRef]
- Paxinos, G.; Halliday, G.; Watson, C.; Kassem, M.S. Atlas of the Developing Mouse Brain; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Alasmari, F.; Alhaddad, H.; Wong, W.; Bell, R.L.; Sari, Y. Ampicillin/Sulbactam Treatment Modulates NMDA Receptor NR2B Subunit and Attenuates Neuroinflammation and Alcohol Intake in Male High Alcohol Drinking Rats. Biomolecules 2020, 10, 1030. [Google Scholar] [CrossRef]
- Alhaddad, H.; Wong, W.; Abou-Gharbia, M.; Childers, W.; Melenski, E.; Bell, R.L.; Sari, Y. Effects of a Novel Beta Lactam Compound, MC-100093, on the Expression of Glutamate Transporters/Receptors and Ethanol Drinking Behavior of Alcohol-Preferring Rats. J. Pharmacol. Exp. Ther. 2022, 383, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Berríos-Cárcamo, P.; Quezada, M.; Santapau, D.; Morales, P.; Olivares, B.; Ponce, C.; Ávila, A.; De Gregorio, C.; Ezquer, M.; Quintanilla, M.E. A novel morphine drinking model of opioid dependence in rats. Int. J. Mol. Sci. 2022, 23, 3874. [Google Scholar] [CrossRef] [PubMed]
- Gaulden, A.D.; Burson, N.; Sadik, N.; Ghosh, I.; Khan, S.J.; Brummelte, S.; Kallakuri, S.; Perrine, S.A. Effects of fentanyl on acute locomotor activity, behavioral sensitization, and contextual reward in female and male rats. Drug Alcohol Depend. 2021, 229, 109101. [Google Scholar] [CrossRef] [PubMed]
- Allouche, S.; Le Marec, T.; Noble, F.; Marie, N. Different patterns of administration modulate propensity of methadone and buprenorphine to promote locomotor sensitization in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 40, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Delage, C.; Morel, A.; de Witt, P.; Jauffret-Roustide, M.; Bloch, V.; Noble, F.; Vorspan, F.; Marie, N. Behavioral sensitization to psychostimulants and opioids: What is known in rodents and what still needs to be explored in humans? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 127, 110824. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.; Unterwald, E.M.; Rawls, S.M. Glutamate transporter subtype 1 (GLT-1) activator ceftriaxone attenuates amphetamine-induced hyperactivity and behavioral sensitization in rats. Drug Alcohol Depend. 2011, 118, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, C.S.; Corley, G.; Kovalevich, J.; Yen, W.; Langford, D.; Rawls, S.M. Ceftriaxone attenuates locomotor activity induced by acute and repeated cocaine exposure in mice. Neurosci. Lett. 2013, 556, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.D.; Houston, A.C.; Desai, R.I.; Doyle, M.R.; Bergman, J.; Mian, M.; Mannix, R.; Sulzer, D.L.; Choi, S.J.; Mosharov, E.V. Behavioral phenotyping and dopamine dynamics in mice with conditional deletion of the glutamate transporter GLT-1 in neurons: Resistance to the acute locomotor effects of amphetamine. Psychopharmacology 2018, 235, 1371–1387. [Google Scholar] [CrossRef]
- Iovino, L.; Tremblay, M.; Civiero, L. Glutamate-induced excitotoxicity in Parkinson’s disease: The role of glial cells. J. Pharmacol. Sci. 2020, 144, 151–164. [Google Scholar] [CrossRef]
- Pitt, D.; Werner, P.; Raine, C.S. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med. 2000, 6, 67–70. [Google Scholar] [CrossRef]
- Arundine, M.; Tymianski, M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 2003, 34, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Sari, Y.; Smith, K.D.; Ali, P.K.; Rebec, G.V. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J. Neurosci. 2009, 29, 9239–9243. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-w.; Scofield, M.D.; Boger, H.; Hensley, M.; Kalivas, P.W. Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J. Neurosci. 2014, 34, 5649–5657. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Nakagawa, T.; Shige, K.; Minami, M.; Satoh, M. Changes in the expression of glial glutamate transporters in the rat brain accompanied with morphine dependence and naloxone-precipitated withdrawal. Brain Res. 2001, 905, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Hubert, G.W.; Paquet, M.; Smith, Y. Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey substantia nigra. J. Neurosci. 2001, 21, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Sesma, M.A.; McDonald, C.T.; O’malley, K.; van den Pol, A.N.; Olney, J.W. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J. Comp. Neurol. 1995, 355, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Sugihara, H.; Nawa, H.; Shigemoto, R.; Mizuno, N.; Nakanishi, S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J. Biol. Chem. 1992, 267, 13361–13368. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Wang, X.; Ge, F.; Li, Y.; Shen, F.; Wang, J.; Cui, C. mGluR 5 in the nucleus accumbens shell regulates morphine-associated contextual memory through reactive oxygen species signaling. Addict. Biol. 2015, 20, 927–940. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef]
- Ribeiro, F.M.; Vieira, L.B.; Pires, R.G.; Olmo, R.P.; Ferguson, S.S. Metabotropic glutamate receptors and neurodegenerative diseases. Pharmacol. Res. 2017, 115, 179–191. [Google Scholar] [CrossRef]
- Biber, K.; Laurie, D.J.; Berthele, A.; Sommer, B.; Tölle, T.R.; Gebicke-Härter, P.J.; Van Calker, D.; Boddeke, H.W. Expression and signaling of group I metabotropic glutamate receptors in astrocytes and microglia. J. Neurochem. 1999, 72, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Pasti, L.; Volterra, A.; Pozzan, T.; Carmignoto, G. Intracellular calcium oscillations in astrocytes: A highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J. Neurosci. 1997, 17, 7817–7830. [Google Scholar] [CrossRef]
- Bruno, V.; Copani, A.; Knöpfel, T.; Kuhn, R.; Casabona, G.; Dell’Albani, P.; Condorelli, D.; Nicoletti, F. Activation of metabotropic glutamate receptors coupled to inositol phospholipid hydrolysis amplifies NMDA-induced neuronal degeneration in cultured cortical cells. Neuropharmacology 1995, 34, 1089–1098. [Google Scholar] [CrossRef]
- Smith, A.C.; Scofield, M.D.; Heinsbroek, J.A.; Gipson, C.D.; Neuhofer, D.; Roberts-Wolfe, D.J.; Spencer, S.; Garcia-Keller, C.; Stankeviciute, N.M.; Smith, R.J. Accumbens nNOS interneurons regulate cocaine relapse. J. Neurosci. 2017, 37, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Suzuki, M.; Narita, M.; Niikura, K.; Nakamura, A.; Miyatake, M.; Aoki, T.; Yajima, Y.; Suzuki, T. Involvement of spinal metabotropic glutamate receptor 5 in the development of tolerance to morphine-induced antinociception. J. Neurochem. 2005, 94, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Alhaddad, H.; Alasmari, F.; Alhamadani, B.; Wong, W.; Bell, R.L.; Sari, Y. Effects of chronic ethanol consumption on the expression of GLT-1 and neuroplasticity-related proteins in the nucleus accumbens of alcohol-preferring rats. Brain Res. Bull. 2020, 165, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Z.; Liang, H.Y.; Zhang, L.; Zhou, Q.G.; Ni, H.Y.; Luo, C.X.; Zhu, D.Y. nNOS–CAPON interaction mediates amyloid-β-induced neurotoxicity, especially in the early stages. Aging Cell 2018, 17, e12754. [Google Scholar] [CrossRef] [PubMed]
- Raines, K.W.; Cao, G.-L.; Porsuphatana, S.; Tsai, P.; Rosen, G.M.; Shapiro, P. Nitric oxide inhibition of ERK1/2 activity in cells expressing neuronal nitric-oxide synthase. J. Biol. Chem. 2004, 279, 3933–3940. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Park, S.; Lakatta, E.G. RAGE signaling in inflammation and arterial aging. Front. Biosci. A J. Virtual Libr. 2009, 14, 1403. [Google Scholar] [CrossRef]
- Hudson, B.I.; Lippman, M.E. Targeting RAGE signaling in inflammatory disease. Annu. Rev. Med. 2018, 69, 349–364. [Google Scholar] [CrossRef]
- Gasparotto, J.; Girardi, C.S.; Somensi, N.; Ribeiro, C.T.; Moreira, J.C.; Michels, M.; Sonai, B.; Rocha, M.; Steckert, A.V.; Barichello, T. Receptor for advanced glycation end products mediates sepsis-triggered amyloid-β accumulation, Tau phosphorylation, and cognitive impairment. J. Biol. Chem. 2018, 293, 226–244. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, J.-S.; Zhang, D.-D.; Yang, Y.-Q.; Huang, L.-T.; Yu, Z.; Chen, R.-D.; Yang, H.-K.; Hang, C.-H. Inhibition of the receptor for advanced glycation end-products (RAGE) attenuates neuroinflammation while sensitizing cortical neurons towards death in experimental subarachnoid hemorrhage. Mol. Neurobiol. 2017, 54, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Ma, Y.; Zeng, Z.; Yin, Q.; Hong, Y.; Hou, X.; Liu, X. RAGE-specific inhibitor FPS-ZM1 attenuates AGEs-induced neuroinflammation and oxidative stress in rat primary microglia. Neurochem. Res. 2017, 42, 2902–2911. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Niu, M.; Zhang, X.; Wang, J.; Zhou, C.; Xie, A. RAGE silencing ameliorates neuroinflammation by inhibition of p38-NF-κB signaling pathway in mouse model of Parkinson’s disease. Front. Neurosci. 2020, 14, 353. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, W.; Sari, Y. Effects of Hydrocodone Overdose and Ceftriaxone on Astrocytic Glutamate Transporters and Glutamate Receptors, and Associated Signaling in Nucleus Accumbens as well as Locomotor Activity in C57/BL Mice. Brain Sci. 2024, 14, 361. https://doi.org/10.3390/brainsci14040361
Wong W, Sari Y. Effects of Hydrocodone Overdose and Ceftriaxone on Astrocytic Glutamate Transporters and Glutamate Receptors, and Associated Signaling in Nucleus Accumbens as well as Locomotor Activity in C57/BL Mice. Brain Sciences. 2024; 14(4):361. https://doi.org/10.3390/brainsci14040361
Chicago/Turabian StyleWong, Woonyen, and Youssef Sari. 2024. "Effects of Hydrocodone Overdose and Ceftriaxone on Astrocytic Glutamate Transporters and Glutamate Receptors, and Associated Signaling in Nucleus Accumbens as well as Locomotor Activity in C57/BL Mice" Brain Sciences 14, no. 4: 361. https://doi.org/10.3390/brainsci14040361
APA StyleWong, W., & Sari, Y. (2024). Effects of Hydrocodone Overdose and Ceftriaxone on Astrocytic Glutamate Transporters and Glutamate Receptors, and Associated Signaling in Nucleus Accumbens as well as Locomotor Activity in C57/BL Mice. Brain Sciences, 14(4), 361. https://doi.org/10.3390/brainsci14040361





