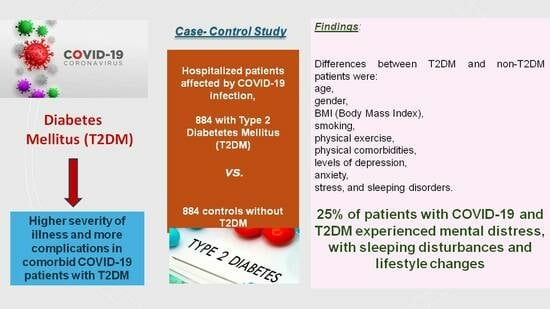
Physical and Mental Health Characteristics of Hospitalized COVID-19 Patients with and without Type 2 Diabetes Mellitus in Turkey
Abstract
1. Introduction
2. Subjects and Methods
2.1. Study Population and Design
2.2. Sample Size Calculation
2.3. Methods and Measurements
2.3.1. The Pittsburgh Sleep Quality Index (PSQI)
2.3.2. The Depression Anxiety Stress Scale (DASS-21)
2.4. Ethical Approvals
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Study Strengths and Limitations
4.2. Highlights
- -
- The COVID-19 pandemic has significantly disrupted the daily life of patients with diabetes and impacted their clinical outcomes.
- -
- Significant changes in HbA1c levels were confirmed among T2DM patients affected by COVID-19 infection.
- -
- Some 25% of COVID-19 patients with T2DM have experienced pervasive psychological and mental burdens during the COVID-19 pandemic and COVID-19 infection in Turkey.
- -
- The majority of T2DM patients reported a lack of sleep and lifestyle changes during COVID-19 infection. Low quality of sleeping, less physical exercise, and more smoking were, alongside other metabolic and serum parameters, factors specifically associated with the clinical presentation of these comorbid patients.
- -
- Vitamin D and ferritin have been identified as useful parameters of reduction in glycated hemoglobin and COVID-19 infection among T2DM patients.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, S.D.; Kim, S.W.; Moon, J.S.; Lee, Y.Y.; Cho, N.H.; Lee, J.H.; Jeon, J.; Choi, Y.K.; Kim, M.K.; Park, K.G. Impact of Social Distancing Due to Coronavirus Disease 2019 on the Changes in Glycosylated Hemoglobin Level in People with Type 2 Diabetes Mellitus. Diabetes Metab. J. 2021, 45, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Lee, S.W.; Lee, W.; Jeon, J.; Kim, J.; Lee, I.; Choi, Y.Y.; Park, K.G. Year-Long Trend in Glycated Hemoglobin Levels in Patients with Type 2 Diabetes during the COVID-19 Pandemic. Endocrinol. Metab. 2021, 36, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, S.; Sinha, B.; Majumder, M.; Misra, A. Estimation of effects of nationwide lockdown for containing coronavirus infection on worsening of glycosylated haemoglobin and increase in diabetes-related complications: A simulation model using multivariate regression analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Gao, L.; Liu, Q.; Dong, K.; Huang, J.; Peng, X.; Yang, Y.; Wang, H.; Yu, X. Factors contributing to glycemic control in diabetes mellitus patients complying with home quarantine during the coronavirus disease 2019 (COVID-19) epidemic. Diabetes Res. Clin. Pract. 2020, 170, 108514. [Google Scholar] [CrossRef] [PubMed]
- Tanji, Y.; Sawada, S.; Watanabe, T.; Morita, T.; Kobayashi, Y.; Murakami, T.; Metoki, H.; Akai, H. Impact of COVID-19 pandemic on glycemic control among outpatients with type 2 diabetes in Japan: A hospital-based survey from a country without lockdown. Diabetes Res. Clin. Pract. 2021, 176, 108840. [Google Scholar] [CrossRef] [PubMed]
- Karataș, S.; Yeşim, T.; Beysel, S. Impact of lockdown COVID-19 on metabolic control in type 2 diabetes mellitus and healthy people. Prim. Care Diabetes 2021, 15, 424–427. [Google Scholar] [CrossRef]
- D’Onofrio, L.; Pieralice, S.; Maddaloni, E.; Mignogna, C.; Sterpetti, S.; Coraggio, L.; Luordi, C.; Guarisco, G.; Leto, G.; Leonetti, F.; et al. Effects of the COVID-19 lockdown on glycaemic control in subjects with type 2 diabetes: The glycalock study. Diabetes Obes. Metab. 2021, 23, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Gentile, A.; Torales, J.; O’Higgins, M.; Figueredo, P.; Castaldelli-Maia, J.M.; De Berardis, D.; Petito, A.; Bellomo, A.; Ventriglio, A. Phone-based outpatients’ follow-up in mental health centers during the COVID-19 quarantine. Int. J. Soc. Psychiatry 2022, 68, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef]
- Ruiz-Roso, M.B.; Knott-Torcal, C.; Matilla-Escalante, D.C.; Garcimartín, A.; Sampedro-Núñez, M.; Dávalos, A.; Marazuela, M. COVID-19 lockdown and changes of the dietary pattern and physical activity habits in a cohort of patients with type 2 diabetes mellitus. Nutrients 2020, 12, 2327. [Google Scholar] [CrossRef]
- Ghosh, A.; Arora, B.; Gupta, R.; Anoop, S.; Misra, A. Effects of nationwide lockdown during COVID-19 epidemic on lifestyle and other medical issues of patients with type 2 diabetes in north India. Diabetes Metab. Syndr. 2020, 14, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Takahara, M.; Watanabe, H.; Shiraiwa, T.; Maeno, Y.; Yamamoto, K.; Shiraiwa, Y.; Yoshida, Y.; Nishioka, N.; Katakami, N.; Shimomura, I. Lifestyle changes and their impact on glycemic control and weight control in patients with diabetes during the coronavirus disease 2019 pandemic in Japan. J. Diabetes Investig. 2022, 13, 375–385. [Google Scholar] [CrossRef]
- Sankar, P.; Ahmed, W.; Koshy, V.M.; Jacob, R.; Sasidharan, S. Effects of COVID-19 lockdown on type 2 diabetes, lifestyle and psychosocial health: A hospital-based cross-sectional survey from South India. Diabetes Metab. Syndr. 2020, 14, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Shikuma, J.; Nagai, Y.; Sakurai, M.; Udagawa, K.; Ito, R.; Miwa, T.; Suzuki, R. Impact of gender differences on lifestyle and glycemic control in Japanese patients with diabetes during COVID-19 lockdowns. Prim. Care Diabetes 2022, 16, 350–354. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 1. Improving Care and Promoting Health in Populations: Standards of Medical Care in Diabetes—2020. Diabetes Care 2019, 43 (Suppl. S1), S7–S13. [Google Scholar] [CrossRef]
- Morgül, E.; Bener, A.; Atak, M.; Akyel, S.; Aktaş, S.; Bhugra, D.; Ventriglio, A.; Jordan, T.R. COVID-19 pandemic and psychological fatigue in Turkey. Int. J. Soc. Psychiatry 2021, 67, 128–135. [Google Scholar] [CrossRef]
- Bener, A.; Griffiths, M.D.; Barışık, C.C.; İnan, F.Ç.; Morgül, E. Impacts of Covid-19 and Lockdown on Mental Health: Depression, Anxiety, Stress and Fear among Adult Population in Turkey. Arch. Clin. Biomed. Res. 2022, 6, 34815. [Google Scholar] [CrossRef]
- Bener, A.; Morgül, E.; Atak, M.; Barışık, C.C. The Impact of COVID-19 Pandemic Disease Exposed with Mental Health in Turkey. Int. J. Clin. Psych. Mental Health 2020, 8, 16–19. [Google Scholar] [CrossRef]
- Sultana, M.S.; Islam, M.S.; Sayeed, A.; Potenza, M.N.; Sikder, M.T.; Rahman, M.A.; Koly, K.N. Prevalence and correlates of diabetes distress and depressive symptoms among individuals with type-2 diabetes mellitus during Ramadan fasting: A cross-sectional study in Bangladesh amid the COVID-19. Diabetes Res. Clin. Pract. 2022, 185, 109210. [Google Scholar] [CrossRef]
- Kishimoto, M.; Ishikawa, T.; Odawara, M. Behavioral changes in patients with diabetes during the COVID-19 pandemic. Diabetol. Int. 2020, 12, 241–245. [Google Scholar] [CrossRef]
- Bener, A.; Yıldırım, E.; Barışık, C.C.; Demirtakan, T. The Global COVID-19 Pandemic Quarantining Effect on Mental Health, Sleeping Quality and Life-Style. Int. J. Clin. Psychiatry Mental Health 2021, 9, 31–39. [Google Scholar] [CrossRef]
- Rowlands, A.V.; Henson, J.J.; Coull, N.A.; Edwardson, C.L.; Brady, E.; Hall, A.; Khunti, K.; Davies, M.; Yates, T. The impact of COVID-19 restrictions on accelerometer-assessed physical activity and sleep in individuals with type 2 diabetes. Diabet. Med. 2021, 38, e14549. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new in-strument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Lovibond, S.H.; Lovibond, P.F. Manual for the Depression Anxiety Stress Scales, 2nd ed.; Psychology Foundation: Sydney, Australia, 1995. [Google Scholar]
- Alessi, J.; de Oliveira, G.B.; Franco, D.W.; Brino do Amaral, B.; Becker, A.S.; Knijnik, C.P.; Kobe, G.L.; de Carvalho, T.R.; Telo, G.H.; Schaan, B.D.; et al. Mental health in the era of COVID-19: Prevalence of psychiatric disorders in a cohort of patients with type 1 and type 2 diabetes during the social distancing. Diabetol. Metab. Syndr. 2020, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Bruine de Bruin, W. Age Differences in COVID-19 Risk Perceptions and Mental Health: Evidence From a National U.S. Survey Conducted in March 2020. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2021, 76, e24–e29. [Google Scholar] [CrossRef] [PubMed]
- Shamshirgaran, S.M.; Mamaghanian, A.; Aliasgarzadeh, A.; Aiminisani, N.; Iranparvar-Alamdari, M.; Ataie, J. Age differences in diabetes-related complications and glycemic control. BMC Endocr. Disord. 2017, 17, 25. [Google Scholar] [CrossRef]
- Falcetta, P.; Aragona, M.; Ciccarone, A.; Bertolotto, A.; Campi, F.; Coppelli, A.; Dardano, A.; Giannarelli, R.; Bianchi, C.; Del Prato, S. Impact of COVID-19 lockdown on glucose control of elderly people with type 2 diabetes in Italy. Diabetes Res. Clin. Pract. 2021, 174, 108750. [Google Scholar] [CrossRef]
- Bener, A.; Morgul, E.; Tokaç, M.; Ventriglio, A.; Jordan, T.R. Sleep quality, quality of life, fatigue, and mental health in COVID-19 post-pandemic Türkiye: A cross-sectional study. Front. Public Health 2024, 12, 1250085. [Google Scholar] [CrossRef]
- Schäfer, A.A.; Santos, L.P.; Manosso, L.M.; Quadra, M.R.; Meller, F.O. Relationship between sleep duration and quality and mental health before and during COVID-19 pandemic: Results of population-based studies in Brazil. J. Psychosom. Res. 2022, 158, 110910. [Google Scholar] [CrossRef]
- Sacre, J.W.; Holmes-Truscott, E.; Salim, A.; Anstey, K.J.; Drummond, G.R.; Huxley, R.R.; Magliano, D.J.; Van Wijngaarden, P.; Zimmet, P.; Speight, J.; et al. Impact of the COVID-19 pandemic and lockdown restrictions on psychosocial and behavioural outcomes among Australian adults with type 2 diabetes: Findings from the PREDICT cohort study. Diabet. Med. 2021, 38, e14611. [Google Scholar] [CrossRef]
- Felix, H.C.; Andersen, J.A.; Willis, D.; Malhis, J.R.; Selig, J.P.; McElfish, P.A. Control of type 2 diabetes mellitus during the COVID-19 pandemic. Prim. Care Diabetes 2021, 15, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Ren, B.; Chen, Y.; Jiang, M.; Ning-Sheng, W.; Niu, R.; Feng, B. Predictors of Glycemic Control among Patients with Type 2 Diabetes in Western China: A Multi-center Cross Sectional Study. Biol. Pharm. Bull. 2021, 44, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Raghavani, P.H.; Sirajwala, H.B. Serum ferritin level in patients with type 2 diabetes mellitus. IJBAR 2014, 5, 272–274. [Google Scholar] [CrossRef]
- Khondker, F.; Roy, M.N.; Saha, P.R.; Huq, R.; Ahmed, R.; Biswas, S. Relationship between serum ferritin level and HBA1C in Bangladeshi type 2 diabetic patients. Anwer Khan Mod. Med. Coll. J. 2018, 9, 29–33. [Google Scholar] [CrossRef][Green Version]
- Diabetes. Available online: http://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 15 March 2023).
- Chellappan, D.K.; Bhandare, R.R.; Shaik, A.B.; Prasad, K.a.R.; Suhaimi, N.a.A.; Yap, W.S.; Das, A.; Banerjee, P.; Ghosh, N.; Guith, T.; et al. Vaccine for Diabetes-Where Do We Stand? Int. J. Mol. Sci. 2022, 23, 9470. [Google Scholar] [CrossRef]
| Variables | T2DM, n = 884 | Controls, n = 884 | p-Value Significance | |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Age groups in Years | ||||
| <45 | 286 (32.4) | 141 (16.0) | ||
| 45–54 | 229 (25.9) | 184 (20.8) | 0.001 | |
| 55–64 | 192(21.7) | 231 (26.1) | ||
| ≥65 | 177 (20.0) | 328 (37.1) | ||
| Gender | ||||
| Males | 360 (40.7) | 434 (49.1) | 0.001 | |
| Females | 524 (59.3) | 450 (50.9) | ||
| BMI | ||||
| Normal (<25 kg/m2) | 231 (26.1) | 243 (27.5) | ||
| Overweight (29–30 kg/m2) | 426 (48.2) | 367 (41.5) | 0.011 | |
| Obese (>30 kg/m2) | 227 (25.7) | 274 (31.0) | ||
| Smoking cigarette | ||||
| Yes | 168 (19.0) | 127 (14.4) | 0.009 | |
| No | 716 (81.0) | 757 (85.6) | ||
| Nargile smoking | ||||
| Yes | 161 (18.9) | 120 (13.2) | 0.008 | |
| No | 723 (81.1) | 764 (86.8) | ||
| Physical activity 30 min/day | ||||
| Yes | 221 (25.0) | 273 (30.9) | 0.006 | |
| No | 663 (75.0) | 611(69.1) | ||
| Co-Morbidities Variables | T2DM, n = 884 | Controls, n = 884 | p-Value Significance | |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Metabolic Syndrome (ATP III) | ||||
| Yes | 195 (22.1) | 157 (17.8) | 0.024 | |
| No | 689(77.9) | 727 (82.2) | ||
| Metabolic Syndrome (IDF) | ||||
| Yes | 220 (24.9) | 167 (18.9) | 0.002 | |
| No | 664 (75.1) | 717 (81.1) | ||
| Thyroid Issues | ||||
| Yes | 254 (28.7) | 112 (12.7) | 0.001 | |
| No | 630 (71.3) | 772 (87.3) | ||
| Chronic Obstructive Pulmonary Disease (COPD) | ||||
| Yes | 257 (29.1) | 140 (15.8) | 0.001 | |
| No | 627 (70.9) | 744 (84.2) | ||
| Infection | ||||
| Yes | 250 (28.3) | 207 (23.4) | 0.019 | |
| No | 634 (71.7) | 677 (76.6) | ||
| Stroke | ||||
| Yes | 170 (19.2) | 87 (9.80) | 0.001 | |
| No | 714 (80.8) | 797 (90.2) | ||
| Coronary Heart Failure | ||||
| Yes | 222 (25.1) | 163 (18.4) | 0.001 | |
| No | 662 (74.9) | 721 (81.6) | ||
| Malignancy | ||||
| Yes | 108 (12.2) | 63 (7.10) | 0.001 | |
| No | 776 (87.8) | 821 (92.9) | ||
| Hypertension | ||||
| Yes | 210 (23.8) | 125 (14.1) | 0.001 | |
| No | 674 (76.2) | 759 (85.9) | ||
| Cardiovascular disease | ||||
| Yes | 187 (21.2) | 93 (10.5) | 0.001 | |
| No | 697 (78.8) | 791 (89.5) | ||
| Variables and Scores | T2DM, n= 884 Yes n (%) | Controls, n = 884 Yes n (%) | p-Value Significance |
|---|---|---|---|
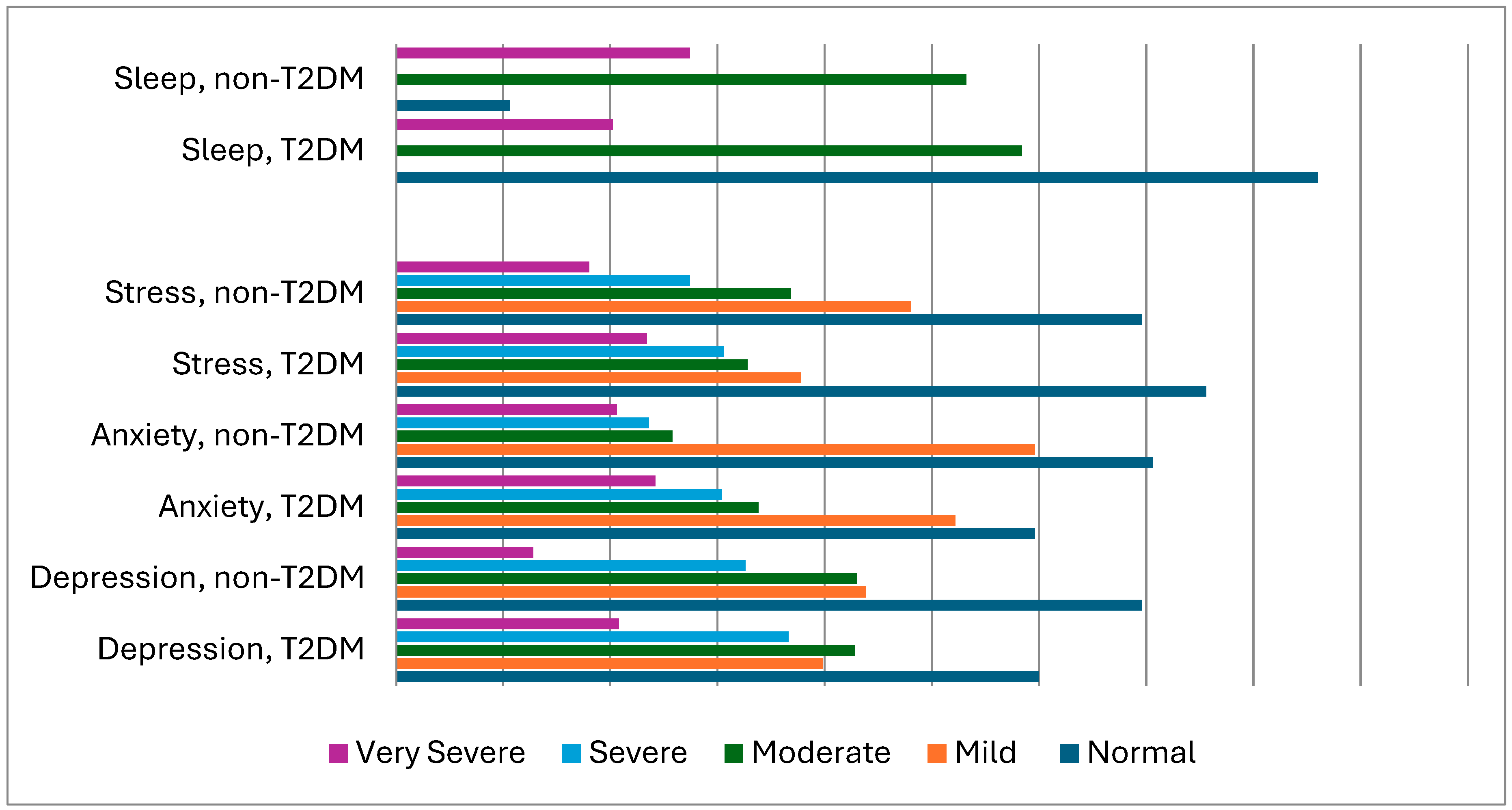
| Depression | |||
| Normal (0–9) | 265 (30.0) | 308 (34.3) | |
| Mild (10–13) | 176 (19.9) | 194 (21.9) | |
| Moderate (14–20) | 189 (21.4) | 181 (21.5) | 0.009 |
| Severe (21–27) | 162 (18.3) | 144 (16.3) | |
| Very severe > 28 | 92 (10.4) | 57 (6.44) | |
| Anxiety | |||
| Normal (0–7) | 263 (29.8) | 312 (35.3) | |
| Mild (8–9) | 231 (26.1) | 263 (29.8) | |
| Moderate (10–14) | 149 (16.9) | 114 (12.9) | 0.003 |
| Severe (15–19) | 134 (15.2) | 104 (11.8) | |
| Very severe > 20 | 107 (12.1) | 91 (10.3) | |
| Stress | |||
| Normal (0–14) | 334 (37.8) | 308 (34.8) | |
| Mild (15–18) | 167 (18.9) | 212 (24.0) | |
| Moderate (19–25) | 145 (16.4) | 163 (18.4) | 0.025 |
| Severe (26–33) | 135 (15.3) | 121 (13.7) | |
| Very severe > 34 | 103 (11.7) | 80 (9.00) | |
| Pittsburgh Sleep Quality Index: | |||
| Good (PSQI ≤ 5) | 380 (43.0) | 445 (50.3) | |
| Average (6 ≤ PSQI ≤ 8) | 258 (29.2) | 235 (26.6) | 0.006 |
| Poor (PSQI > 8) | 246 (27.8) | 204 (29.1) | |
| Vitamin D Levels | |||
| Deficiency < 20 ng/mL | 552 (62.4) | 466 (52.7) | |
| Insufficiency 20–29 ng/mL | 243 (27.5) | 297 (33.6) | 0.001 |
| Sufficiency ≥ 30 ng/mL | 89 (10.1) | 121 (13.7) |
| Variables | T2DM, n = 884 Mean ± SD | Controls, n = 884 Mean ± SD | p-Value Significance |
|---|---|---|---|
| Hemoglobin (g/dL) | 13.20 ± 0.49 | 13.59 ± 1.06 | 0.006 |
| HbA1c | 7.46 ± 0.81 | 5.65 ± 0.035 | 0.001 |
| Fasting blood glucose (mmol/L) | 135.18 ± 76.19 | 120.05 ± 56.23 | 0.009 |
| Vitamin D (mmol/L) | 18.02 ± 6.60 | 20.87 ± 7.28 | 0.001 |
| Vitamin B12 (mmol/L) | 252.68 ± 128.0 | 271.97 ± 11.96 | 0.001 |
| Calcium (mmol/L) | 1.72 ± 0.44 | 1.90 ± 0.27 | 0.001 |
| Urea (mg/dL) | 26.14 ± 3.37 | 32.003 ± 4.31 | 0.001 |
| Phosphor (mmol/L) | 1.74 ± 0.41 | 3.51 ± 1.22 | 0.001 |
| Creatinine (mmol/L) | 77.51 ± 19.14 | 72.10 ± 18.98 | 0.001 |
| Total cholesterol (mmol/L) | 166.52 ± 47.26 | 167.49 ± 44.10 | 0.665 |
| HDL (mmol/L) | 1.23 ± 0.27 | 1.29 ± 0.31 | 0.001 |
| LDL (mmol/L) | 180.49 ± 76.10 | 188.43 ± 89.91 | 0.343 |
| Triglyceride (mmol/L) | 164.49 ± 87.42 | 146.73 ± 97.58 | 0.001 |
| Uric acid (mmol/L) | 5.87 ± 2.19 | 5.42 ± 1.65 | 0.003 |
| Ferritin (ug/L) | 78.06 ± 18.984 | 72.10 ± 18.98 | 0.001 |
| Fe (ug/L) | 57.83 ± 28.97 | 59.13 ± 30.99 | 0.362 |
| TSH | 2.54 ± 1.18 | 1.71 ± 1.03 | 0.001 |
| Creatine kinase (ug/L) | 37.15 ± 18.47 | 36.97 ± 17.16 | 0.844 |
| Creatine kinase–myocardial band (ug/L) | 13.37 ± 6.24 | 13.21 ± 6.06 | 0.601 |
| Hematocrit (ug/L) | 36.191 ± 5.93 | 36.06 ± 5.76 | 0.631 |
| White blood cells (×103/µL) | 7591.1 ± 1511.8 | 7649.2 ± 1507.5 | 0.415 |
| Red blood cells (×103/µL) | 4.37 ± 0.63 | 4.19 ± 0.48 | 0.001 |
| Neutrophils (×103/µL) | 5.76 ± 3.04 | 5.66 ± 3.00 | 0.487 |
| Lymphocytes (×103/µL) | 1.63 ± 0.87 | 1.50 ± 0.90 | 0.230 |
| Platelets (×103/µL) | 239.35 ± 94.88 | 227.52 ± 829.7 | 0.012 |
| Aspartate transaminase (U/L) | 27.25 ± 15.29 | 24.67 ± 11.370 | 0.001 |
| Alanine transaminase (U/L) | 24.86 ± 11.96 | 20.10 ± 7.56 | 0.001 |
| C-reactive protein (mg/L) | 8.95 ± 3.318 | 7.23 ± 3.32 | 0.001 |
| Procalcitonin (ug/L) | 0.24 ± 0.10 | 0.24 ± 010 | 0.873 |
| Gamma-glutamyltransferase (GGT) | 25.81 ± 15.58 | 23.97 ± 10.96 | 0.004 |
| Systolic blood pressure (mmHg) | 132.26 ± 13.59 | 130.34 ± 9.75 | 0.001 |
| Diastolic blood pressure (mmHg) | 79.31 ± 9.12 | 78.29 ± 6.98 | 0.008 |
| Independent Variables | Regression Coefficient | Standard Error | Beta | t-Test | p-Value Significance |
|---|---|---|---|---|---|
| Red blood cells (×103/µL) | −0.144 | 0.021 | −0.165 | −7.016 | 0.001 |
| Vitamin D deficiency (mmol/L) | 0.093 | 0.017 | 0.132 | 5.581 | 0.001 |
| HbA1c | −0.355 | 0.062 | −0.787 | −5.725 | 0.001 |
| Creatinine (µg/L) | 0.187 | 0.039 | 0.387 | 4.769 | 0.001 |
| Uric acid (mmol/L) | −0.160 | 0.037 | −1.057 | −4.299 | 0.001 |
| Smoking (Yes) | 0.075 | 0.018 | 0.056 | 4.232 | 0.001 |
| Vitamin B12 deficiency (mmol/L) | 0.098 | 0.031 | 0.094 | 3.118 | 0.002 |
| Physical vigorous activity | −0.039 | 0.015 | −0.035 | −2.554 | 0.011 |
| PSQI sleep quality | −0.092 | 0.037 | −0.059 | −2.474 | 0.013 |
| Metabolic syndrome (IDF) | 0.075 | 0.031 | 0.062 | 2.402 | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bener, A.; Atmaca, M.; Al-Hamaq, A.O.A.A.; Ventriglio, A. Physical and Mental Health Characteristics of Hospitalized COVID-19 Patients with and without Type 2 Diabetes Mellitus in Turkey. Brain Sci. 2024, 14, 377. https://doi.org/10.3390/brainsci14040377
Bener A, Atmaca M, Al-Hamaq AOAA, Ventriglio A. Physical and Mental Health Characteristics of Hospitalized COVID-19 Patients with and without Type 2 Diabetes Mellitus in Turkey. Brain Sciences. 2024; 14(4):377. https://doi.org/10.3390/brainsci14040377
Chicago/Turabian StyleBener, Abdulbari, Murat Atmaca, Abdulla O. A. A. Al-Hamaq, and Antonio Ventriglio. 2024. "Physical and Mental Health Characteristics of Hospitalized COVID-19 Patients with and without Type 2 Diabetes Mellitus in Turkey" Brain Sciences 14, no. 4: 377. https://doi.org/10.3390/brainsci14040377
APA StyleBener, A., Atmaca, M., Al-Hamaq, A. O. A. A., & Ventriglio, A. (2024). Physical and Mental Health Characteristics of Hospitalized COVID-19 Patients with and without Type 2 Diabetes Mellitus in Turkey. Brain Sciences, 14(4), 377. https://doi.org/10.3390/brainsci14040377