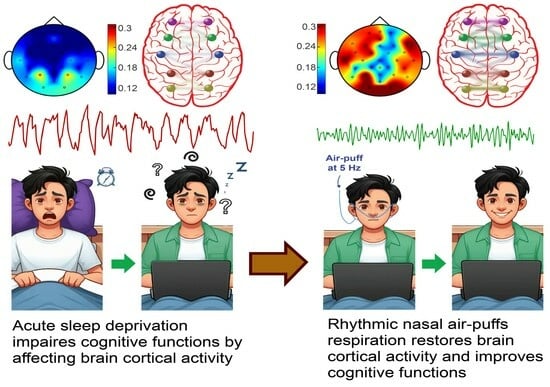
Olfactory Epithelium Stimulation Using Rhythmic Nasal Air-Puffs Improves the Cognitive Performance of Individuals with Acute Sleep Deprivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
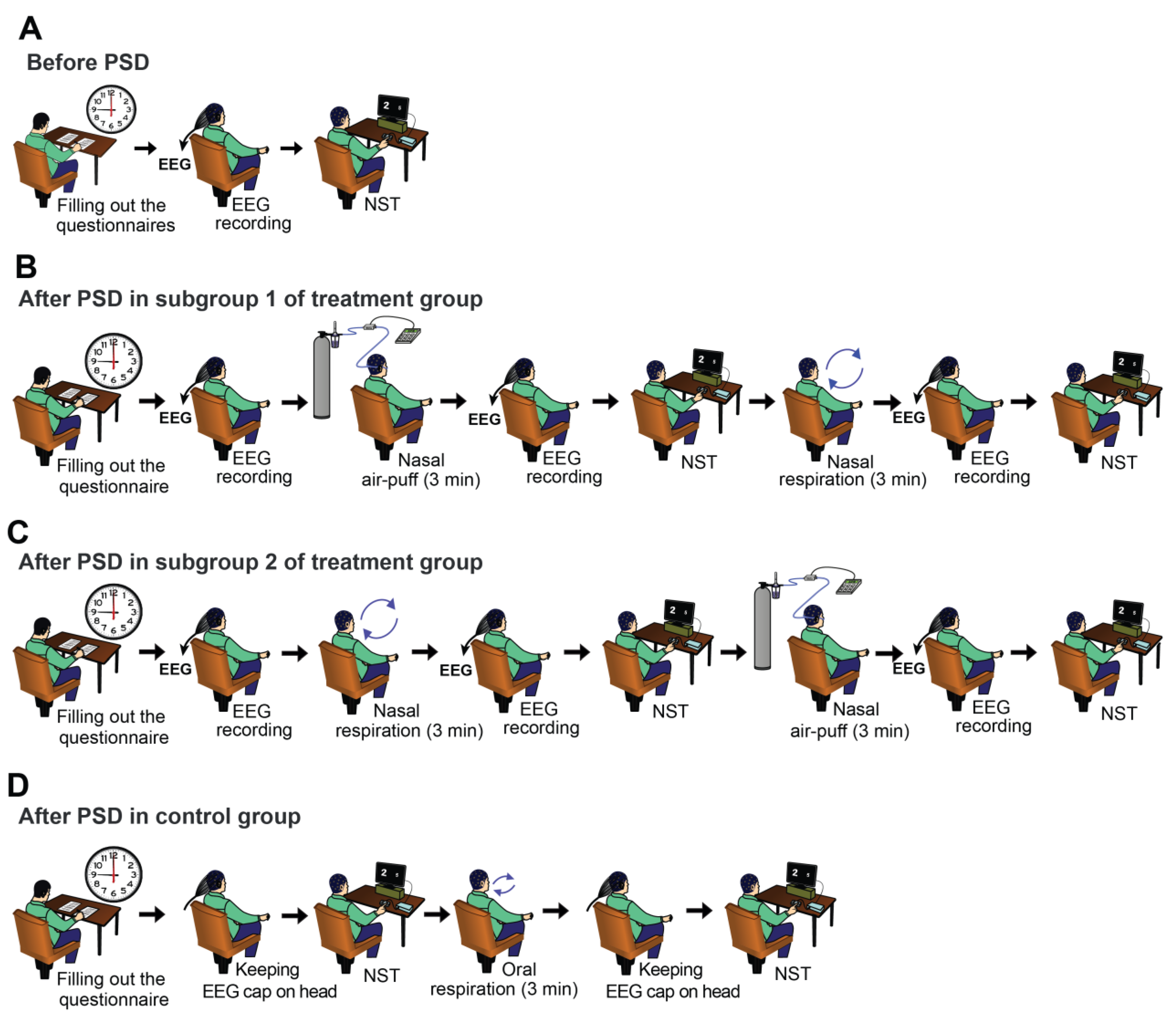
2.2. Experimental Procedure
2.3. Questionnaires
2.3.1. Pittsburgh Sleep Quality Questionnaire
2.3.2. Cognitive Disability Questionnaire
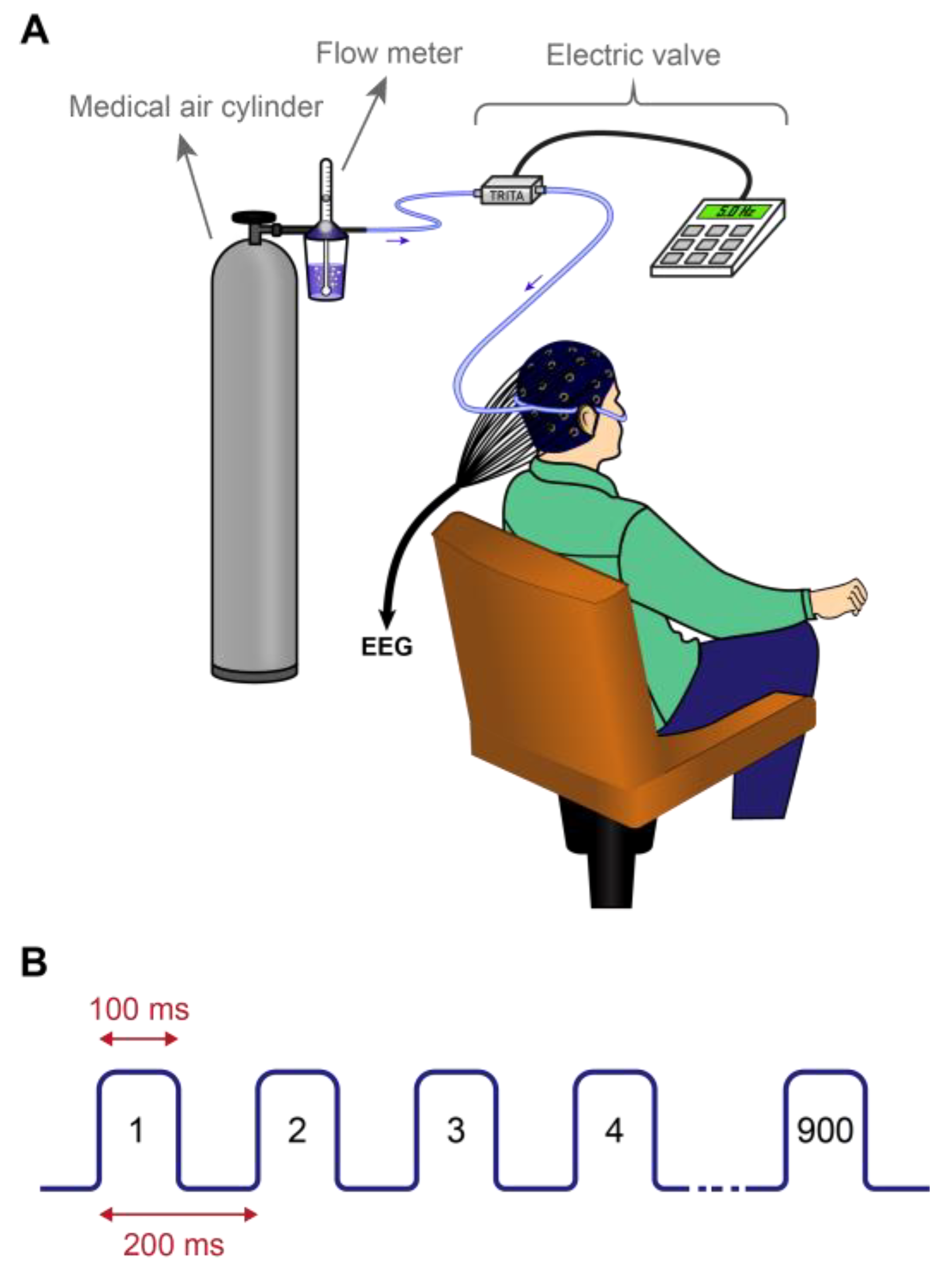
2.4. Applying Nasal Air-Puffs
2.5. Numerical Stroop Test (NST)
2.6. EEG Recording and Pre-Processing
2.7. Signal Processing
2.8. Statistical Analysis
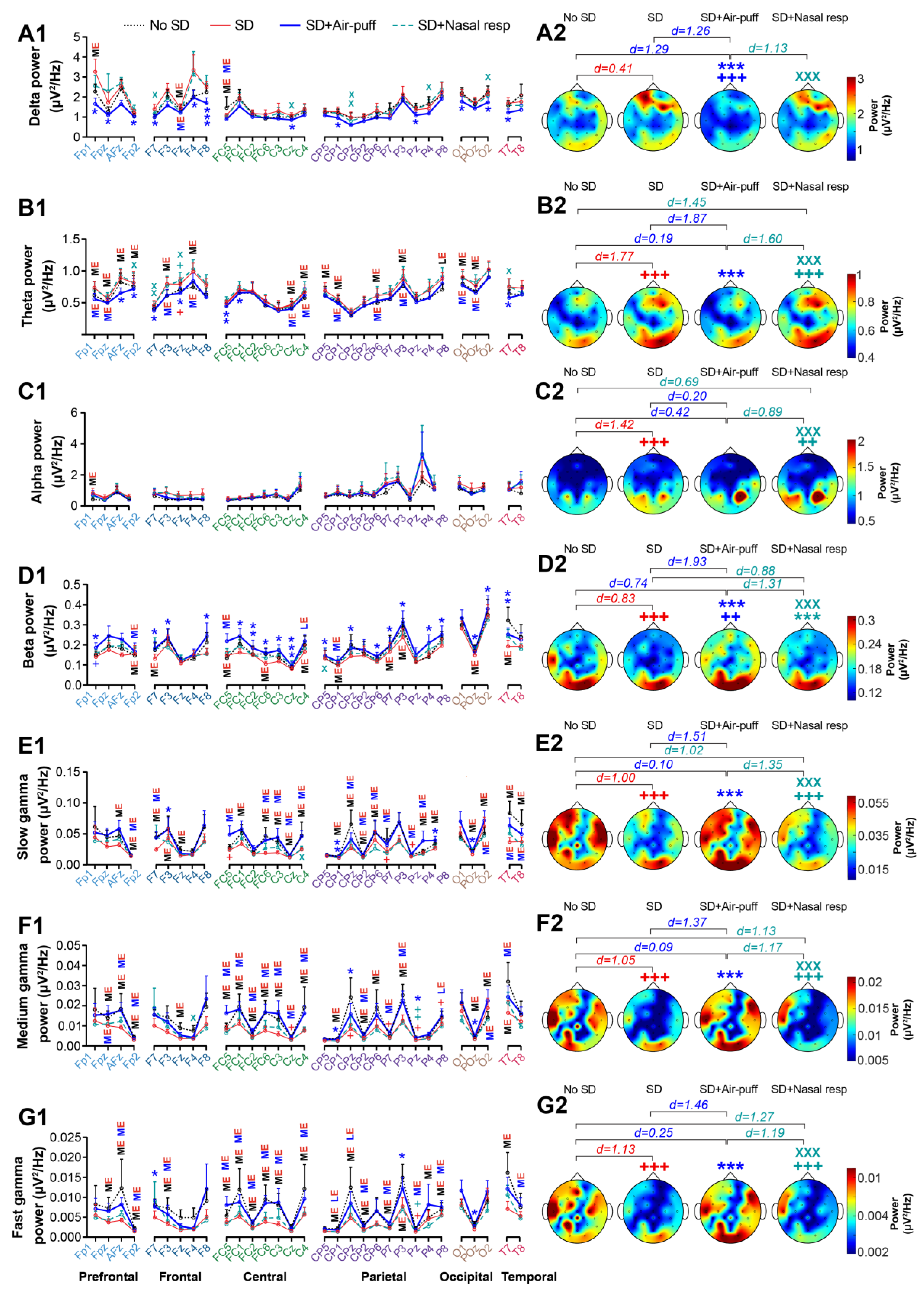
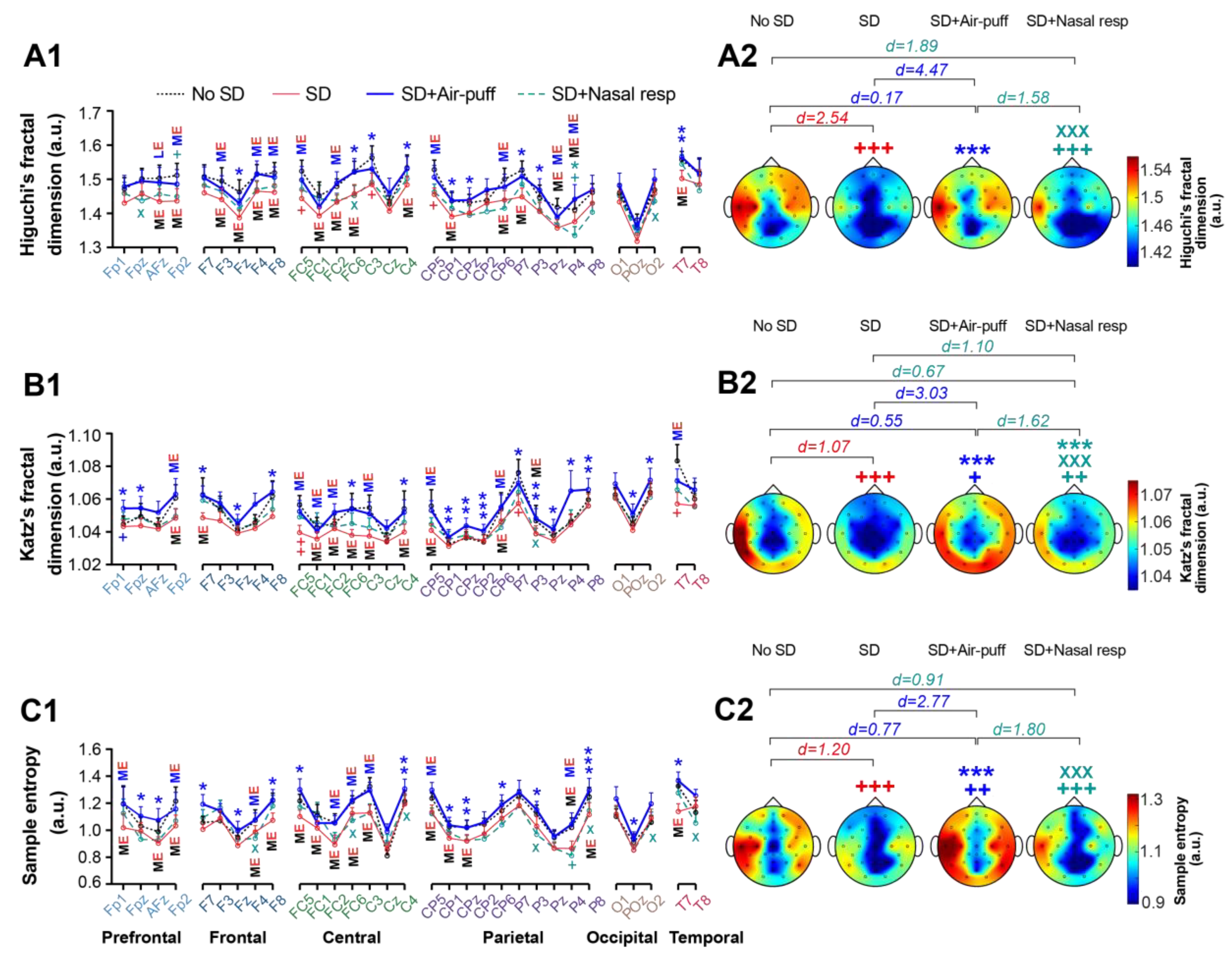
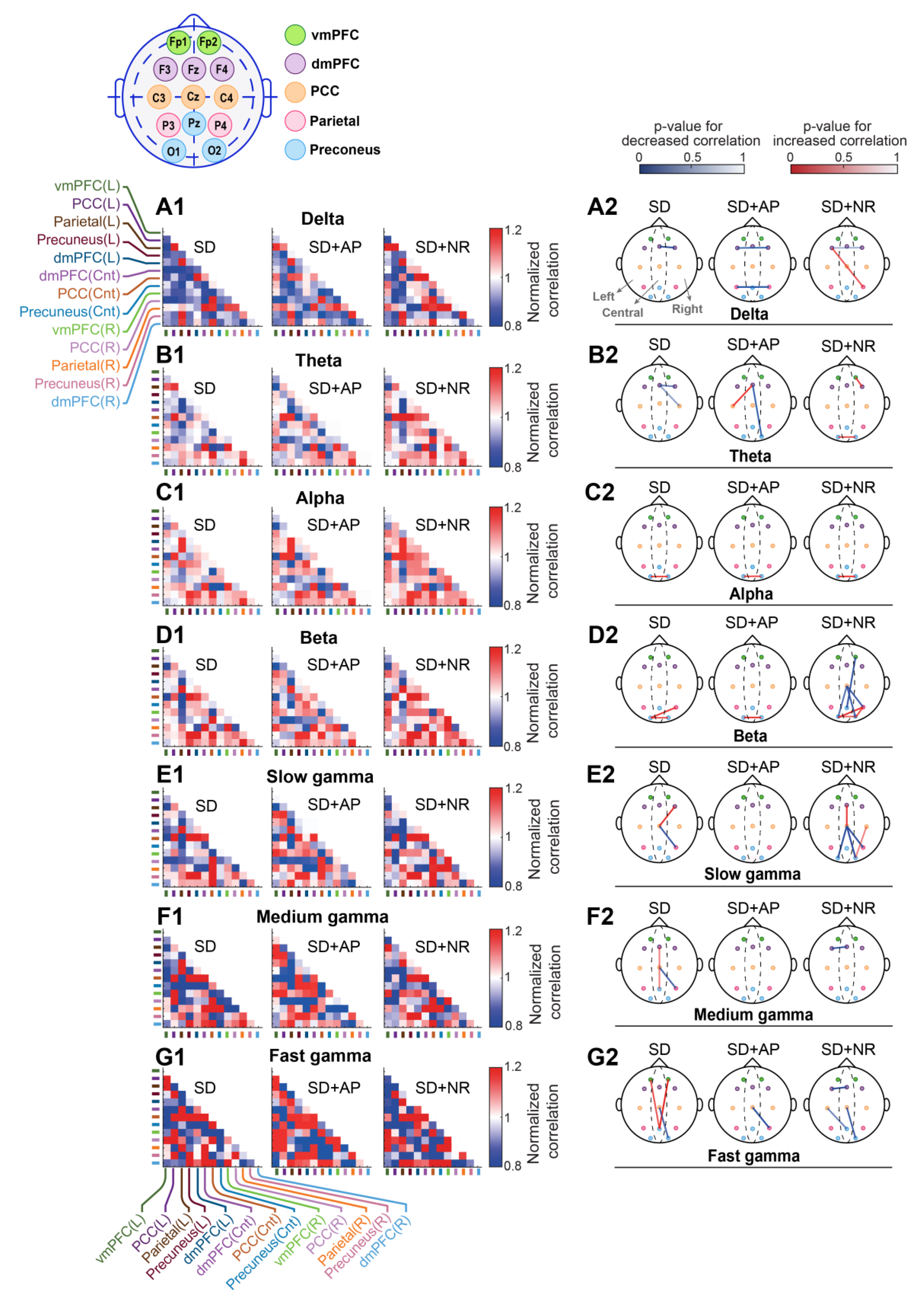
3. Results
3.1. Baseline Characteristics of Participants
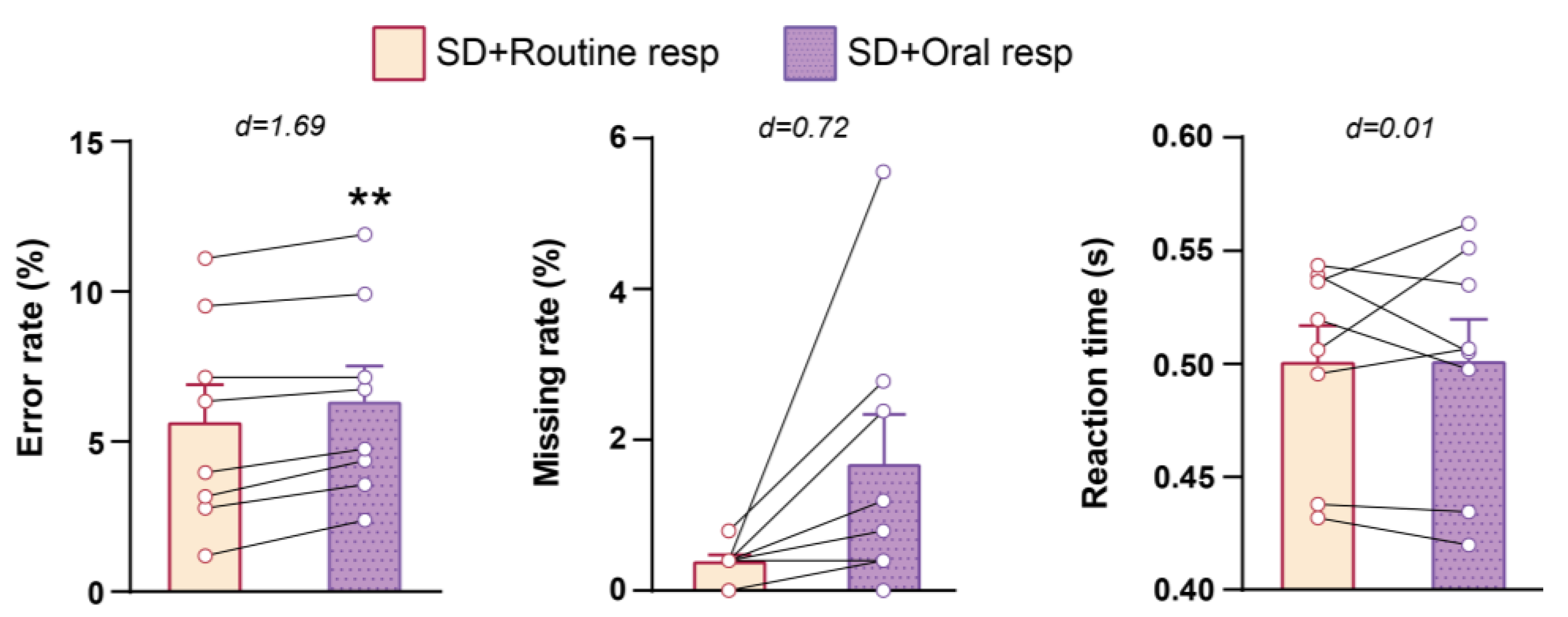
3.2. Cognitive Performance in NST
3.3. EEG Power
3.4. Signals Complexity
3.5. Intra-DMN Functional Connectivity
3.5.1. Cross-Correlation
3.5.2. Coherence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrara, M.; de Gennaro, L. How much sleep do we need? Sleep Med. Rev. 2001, 5, 155–179. [Google Scholar] [CrossRef]
- Sejnowski, T.J.; Destexhe, A. Why do we sleep? Brain Res. 2000, 886, 208–223. [Google Scholar] [CrossRef]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [CrossRef]
- Landolt, H.-P.; Holst, S.C.; Sousek, A.; Bassetti, C.; Dogas, Z.; Peigneux, P. Effects of Acute and Chronic Sleep Deprivation; European Sleep Research Society: Regensburg, Germany, 2014; ISBN 9781119038931. [Google Scholar]
- Sanches, I.; Teixeira, F.; dos Santos, J.M.; Ferreira, A.J. Effects of Acute Sleep Deprivation Resulting from Night Shift Work on Young Doctors. Acta Med. Port. 2015, 28, 457–462. [Google Scholar] [CrossRef]
- Orzeł-Gryglewska, J. Consequences of sleep deprivation. Int. J. Occup. Med. Environ. Health 2010, 23, 95–114. [Google Scholar] [CrossRef]
- Corsi-Cabrera, M.; Ramos, J.; Arce, C.; Guevara, M.A.; Ponce-de León, M.; Lorenzo, I. Changes in the waking EEG as a consequence of sleep and sleep deprivation. Sleep 1992, 15, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Duan, W.; Lei, X. Impaired Coupling of the Brain’s Default Network During Sleep Deprivation: A Resting-State EEG Study. Nat. Sci. Sleep 2020, 12, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-T.; Hsiao, Y.-T.; Yi, P.-L.; Chang, F.-C. Deep Brain Stimulation Increases Seizure Threshold by Altering REM Sleep and Delta Powers During NREM Sleep. Front. Neurol. 2020, 11, 752. [Google Scholar] [CrossRef] [PubMed]
- Kurtis, M.M.; Rajah, T.; Delgado, L.F.; Dafsari, H.S. The effect of deep brain stimulation on the non-motor symptoms of Parkinson's disease: A critical review of the current evidence. NPJ Parkinsons. Dis. 2017, 3, 16024. [Google Scholar] [CrossRef]
- Diekelmann, S. Sleep for cognitive enhancement. Front. Syst. Neurosci. 2014, 8, 46. [Google Scholar] [CrossRef]
- Luber, B.; Steffener, J.; Tucker, A.; Habeck, C.; Peterchev, A.V.; Deng, Z.-D.; Basner, R.C.; Stern, Y.; Lisanby, S.H. Extended remediation of sleep deprived-induced working memory deficits using fMRI-guided transcranial magnetic stimulation. Sleep 2013, 36, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Dalong, G.; Jiyuan, L.; Ying, Z.; Lei, Z.; Yanhong, H.; Yongcong, S. Transcranial direct current stimulation reconstructs diminished thalamocortical connectivity during prolonged resting wakefulness: A resting-state fMRI pilot study. Brain Imaging Behav. 2020, 14, 278–288. [Google Scholar] [CrossRef] [PubMed]
- McIntire, L.K.; McKinley, R.A.; Goodyear, C.; McIntire, J.P. The Effects of Anodal Transcranial Direct Current Stimulation on Sleep Time and Efficiency. Front. Hum. Neurosci. 2020, 14, 357. [Google Scholar] [CrossRef]
- McIntire, L.K.; McKinley, R.A.; Goodyear, C.; Nelson, J. A comparison of the effects of transcranial direct current stimulation and caffeine on vigilance and cognitive performance during extended wakefulness. Brain Stimul. 2014, 7, 499–507. [Google Scholar] [CrossRef] [PubMed]
- McIntire, L.K.; McKinley, R.A.; Nelson, J.M.; Goodyear, C. Transcranial direct current stimulation versus caffeine as a fatigue countermeasure. Brain Stimul. 2017, 10, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- San-Juan, D.; Mas, R.N.M.; Gutiérrez, C.; Morales, J.; Díaz, A.; Quiñones, G.; Galindo, A.K.; Baigts, L.A.; Ximenez-Camilli, C.; Anschel, D. Effect of the anodal transcranial direct current electrical stimulation on cognition of medical residents with acute sleep deprivation. Sleep Sci. 2022, 15, 89–96. [Google Scholar] [CrossRef] [PubMed]
- D’Atri, A.; Scarpelli, S.; Gorgoni, M.; Alfonsi, V.; Annarumma, L.; Giannini, A.M.; Ferrara, M.; Ferlazzo, F.; Rossini, P.M.; de Gennaro, L. Bilateral Theta Transcranial Alternating Current Stimulation (tACS) Modulates EEG Activity: When tACS Works Awake It Also Works Asleep. Nat. Sci. Sleep 2019, 11, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.D.; Sengupta, S.; Chitnis, S.; Amara, A.W. Deep Brain Stimulation and Sleep-Wake Disturbances in Parkinson Disease: A Review. Front. Neurol. 2018, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.W.; Watts, R.L.; Walker, H.C. The effects of deep brain stimulation on sleep in Parkinson's disease. Ther. Adv. Neurol. Disord. 2011, 4, 15–24. [Google Scholar] [CrossRef]
- Herrero Babiloni, A.; Bellemare, A.; Beetz, G.; Vinet, S.-A.; Martel, M.O.; Lavigne, G.J.; de Beaumont, L. The effects of non-invasive brain stimulation on sleep disturbances among different neurological and neuropsychiatric conditions: A systematic review. Sleep Med. Rev. 2021, 55, 101381. [Google Scholar] [CrossRef]
- Grosmaitre, X.; Santarelli, L.C.; Tan, J.; Luo, M.; Ma, M. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat. Neurosci. 2007, 10, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Ito, A.; LaFever, B.J. Subpopulations of Projection Neurons in the Olfactory Bulb. Front. Neural Circuits 2020, 14, 561822. [Google Scholar] [CrossRef]
- Yanovsky, Y.; Ciatipis, M.; Draguhn, A.; Tort, A.B.L.; Brankačk, J. Slow oscillations in the mouse hippocampus entrained by nasal respiration. J. Neurosci. 2014, 34, 5949–5964. [Google Scholar] [CrossRef]
- Zelano, C.; Jiang, H.; Zhou, G.; Arora, N.; Schuele, S.; Rosenow, J.; Gottfried, J.A. Nasal Respiration Entrains Human Limbic Oscillations and Modulates Cognitive Function. J. Neurosci. 2016, 36, 12448–12467. [Google Scholar] [CrossRef] [PubMed]
- Juventin, M.; Ghibaudo, V.; Granget, J.; Amat, C.; Courtiol, E.; Buonviso, N. Respiratory influence on brain dynamics: The preponderant role of the nasal pathway and deep slow regime. Pflugers Arch. 2023, 475, 23–35. [Google Scholar] [CrossRef]
- Karalis, N.; Sirota, A. Breathing coordinates cortico-hippocampal dynamics in mice during offline states. Nat. Commun. 2022, 13, 467. [Google Scholar] [CrossRef]
- Herrero, J.L.; Khuvis, S.; Yeagle, E.; Cerf, M.; Mehta, A.D. Breathing above the brain stem: Volitional control and attentional modulation in humans. J. Neurophysiol. 2018, 119, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Piarulli, A.; Zaccaro, A.; Laurino, M.; Menicucci, D.; de Vito, A.; Bruschini, L.; Berrettini, S.; Bergamasco, M.; Laureys, S.; Gemignani, A. Ultra-slow mechanical stimulation of olfactory epithelium modulates consciousness by slowing cerebral rhythms in humans. Sci. Rep. 2018, 8, 6581. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Liu, Y.; Wang, L.; Li, B.; Xu, F. Activity Patterns Elicited by Airflow in the Olfactory Bulb and Their Possible Functions. J. Neurosci. 2017, 37, 10700–10711. [Google Scholar] [CrossRef]
- Ito, J.; Roy, S.; Liu, Y.; Cao, Y.; Fletcher, M.; Lu, L.; Boughter, J.D.; Grün, S.; Heck, D.H. Whisker barrel cortex delta oscillations and gamma power in the awake mouse are linked to respiration. Nat. Commun. 2014, 5, 3572. [Google Scholar] [CrossRef]
- Baghdadi, G.; Kamarajan, C.; Hadaeghi, F. Editorial: Role of brain oscillations in neurocognitive control systems. Front. Syst. Neurosci. 2023, 17, 1182496. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Kann, M.; Wang, Q. Neuromodulation of Neural Oscillations in Health and Disease. Biology 2023, 12, 371. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Farrahi Moghaddam, J.; Nakhaee, N.; Sheibani, V.; Garrusi, B.; Amirkafi, A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Sleep Breath. 2012, 16, 79–82. [Google Scholar] [CrossRef]
- Nejati, V. Cognitive Abilities Questionnaire: Development and Evaluation of Psychometric Properties. ICSS 2013, 15, 11–19. [Google Scholar]
- Fakhari, A.; Azizi, H.; Mostofi, M.; Sadeghpour, S.; Farahbakhsh, M. Validity and Reliability of the Iranian Rapid Assessment for Psychiatric Illness Screening Instrument (IRA-PISI) in Primary Health Care. Iran. J. Psychiatry Behav. Sci. 2022, 16, e112790. [Google Scholar] [CrossRef]
- Zielinski, M.C.; Tang, W.; Jadhav, S.P. The role of replay and theta sequences in mediating hippocampal-prefrontal interactions for memory and cognition. Hippocampus 2020, 30, 60–72. [Google Scholar] [CrossRef]
- Beldzik, E.; Domagalik, A.; Froncisz, W.; Marek, T. Dissociating EEG sources linked to stimulus and response evaluation in numerical Stroop task using Independent Component Analysis. Clin. Neurophysiol. 2015, 126, 914–926. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Huang, Z. Brief history and development of electrophysiological recording techniques in neuroscience. In Signal Processing in Neuroscience; Springer: Singapore, 2016; pp. 1–10. [Google Scholar]
- Zappasodi, F.; Olejarczyk, E.; Marzetti, L.; Assenza, G.; Pizzella, V.; Tecchio, F. Fractal dimension of EEG activity senses neuronal impairment in acute stroke. PLoS ONE 2014, 9, e100199. [Google Scholar] [CrossRef]
- Susmáková, K.; Krakovská, A. Discrimination ability of individual measures used in sleep stages classification. Artif. Intell. Med. 2008, 44, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Approach to an irregular time series on the basis of the fractal theory. Phys. D Nonlinear Phenom. 1988, 31, 277–283. [Google Scholar] [CrossRef]
- Salimi, M.; Javadi, A.-H.; Nazari, M.; Bamdad, S.; Tabasi, F.; Parsazadegan, T.; Ayene, F.; Karimian, M.; Gholami-Mahtaj, L.; Shadnia, S.; et al. Nasal Air Puff Promotes Default Mode Network Activity in Mechanically Ventilated Comatose Patients: A Noninvasive Brain Stimulation Approach. Neuromodulation 2022, 25, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Zeydabadinezhad, M.; Horn, C.C.; Mahmoudi, B. Quantifying the effects of vagus nerve stimulation on gastric myoelectric activity in ferrets using an interpretable machine learning approach. PLoS ONE 2023, 18, e0295297. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.J. Fractals and the analysis of waveforms. Comput. Biol. Med. 1988, 18, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar]
- Krause, A.J.; Simon, E.B.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef]
- Khan, M.A.; Al-Jahdali, H. The consequences of sleep deprivation on cognitive performance. Neurosciences 2023, 28, 91–99. [Google Scholar] [CrossRef]
- Yoo, S.-S.; Hu, P.T.; Gujar, N.; Jolesz, F.A.; Walker, M.P. A deficit in the ability to form new human memories without sleep. Nat. Neurosci. 2007, 10, 385–392. [Google Scholar] [CrossRef]
- Sagaspe, P.; Sanchez-Ortuno, M.; Charles, A.; Taillard, J.; Valtat, C.; Bioulac, B.; Philip, P. Effects of sleep deprivation on Color-Word, Emotional, and Specific Stroop interference and on self-reported anxiety. Brain Cogn. 2006, 60, 76–87. [Google Scholar] [CrossRef]
- Hudson, A.N.; van Dongen, H.P.A.; Honn, K.A. Sleep deprivation, vigilant attention, and brain function: A review. Neuropsychopharmacology 2020, 45, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Alhola, P.; Polo-Kantola, P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr. Dis. Treat. 2007, 3, 553–567. [Google Scholar] [PubMed]
- Wu, J.; Zhou, Q.; Li, J.; Chen, Y.; Shao, S.; Xiao, Y. Decreased resting-state alpha-band activation and functional connectivity after sleep deprivation. Sci. Rep. 2021, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Chee, M.W.L.; Zhou, J. Functional connectivity and the sleep-deprived brain. Prog. Brain Res. 2019, 246, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Y.; Yao, Y.; Pan, Y.; Sun, Y. Sleep deprivation disturbed regional brain activity in healthy subjects: Evidence from a functional magnetic resonance-imaging study. Neuropsychiatr. Dis. Treat. 2016, 12, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Durbin, R.P. Letter: Acid secretion by gastric mucous membrane. Am. J. Physiol. 1975, 229, 1726. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, V.; Chuah, Y.M.L.; Huettel, S.A.; Chee, M.W.L. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep 2007, 30, 603–609. [Google Scholar] [CrossRef]
- Mullin, B.C.; Phillips, M.L.; Siegle, G.J.; Buysse, D.J.; Forbes, E.E.; Franzen, P.L. Sleep deprivation amplifies striatal activation to monetary reward. Psychol. Med. 2013, 43, 2215–2225. [Google Scholar] [CrossRef]
- Killgore, W.D.S.; Balkin, T.J.; Wesensten, N.J. Impaired decision making following 49 h of sleep deprivation. J. Sleep Res. 2006, 15, 7–13. [Google Scholar] [CrossRef]
- Olson, E.A.; Weber, M.; Rauch, S.L.; Killgore, W.D.S. Daytime Sleepiness Is Associated with Reduced Integration of Temporally Distant Outcomes on the Iowa Gambling Task. Behav. Sleep Med. 2016, 14, 200–211. [Google Scholar] [CrossRef]
- Biskamp, J.; Bartos, M.; Sauer, J.-F. Organization of prefrontal network activity by respiration-related oscillations. Sci. Rep. 2017, 7, 45508. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.T.; Peace, S.T.; Cleland, T.A. Properties and mechanisms of olfactory learning and memory. Front. Behav. Neurosci. 2014, 8, 238. [Google Scholar] [CrossRef]
- Zald, D.H.; Pardo, J.V. Emotion, olfaction, and the human amygdala: Amygdala activation during aversive olfactory stimulation. Proc. Natl. Acad. Sci. USA 1997, 94, 4119–4124. [Google Scholar] [CrossRef]
- Bhattarai, J.P.; Etyemez, S.; Jaaro-Peled, H.; Janke, E.; Leon Tolosa, U.D.; Kamiya, A.; Gottfried, J.A.; Sawa, A.; Ma, M. Olfactory modulation of the medial prefrontal cortex circuitry: Implications for social cognition. Semin. Cell Dev. Biol. 2022, 129, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Feurer, C.; Jimmy, J.; Bhaumik, R.; Duffecy, J.; Medrano, G.R.; Ajilore, O.; Shankman, S.A.; Langenecker, S.A.; Craske, M.G.; Phan, K.L.; et al. Anterior cingulate cortex activation during attentional control as a transdiagnostic marker of psychotherapy response: A randomized clinical trial. Neuropsychopharmacology 2022, 47, 1350–1357. [Google Scholar] [CrossRef]
- Alvites, R.; Caine, A.; Cherubini, G.B.; Prada, J.; Varejão, A.S.P.; Maurício, A.C. The Olfactory Bulb in Companion Animals-Anatomy, Physiology, and Clinical Importance. Brain Sci. 2023, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Ghazvineh, S.; Salimi, M.; Nazari, M.; Garousi, M.; Tabasi, F.; Dehdar, K.; Salimi, A.; Jamaati, H.; Mirnajafi-Zadeh, J.; Arabzadeh, E.; et al. Rhythmic air-puff into nasal cavity modulates activity across multiple brain areas: A non-invasive brain stimulation method to reduce ventilator-induced memory impairment. Respir. Physiol. Neurobiol. 2021, 287, 103627. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Li, C.-T.; Juan, C.-H. A review of critical brain oscillations in depression and the efficacy of transcranial magnetic stimulation treatment. Front. Psychiatry 2023, 14, 1073984. [Google Scholar] [CrossRef]
- Jiang, Y.; Jessee, W.; Hoyng, S.; Borhani, S.; Liu, Z.; Zhao, X.; Price, L.K.; High, W.; Suhl, J.; Cerel-Suhl, S. Sharpening Working Memory with Real-Time Electrophysiological Brain Signals: Which Neurofeedback Paradigms Work? Front. Aging Neurosci. 2022, 14, 780817. [Google Scholar] [CrossRef]
- Guan, A.; Wang, S.; Huang, A.; Qiu, C.; Li, Y.; Li, X.; Wang, J.; Wang, Q.; Deng, B. The role of gamma oscillations in central nervous system diseases: Mechanism and treatment. Front. Cell. Neurosci. 2022, 16, 962957. [Google Scholar] [CrossRef]
- Rojas-Líbano, D.; Del Wimmer Solar, J.; Aguilar-Rivera, M.; Montefusco-Siegmund, R.; Maldonado, P.E. Local cortical activity of distant brain areas can phase-lock to the olfactory bulb's respiratory rhythm in the freely behaving rat. J. Neurophysiol. 2018, 120, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mai, X.; Liu, C. The default mode network and social understanding of others: What do brain connectivity studies tell us. Front. Hum. Neurosci. 2014, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Garcés, P.; Angel Pineda-Pardo, J.; Canuet, L.; Aurtenetxe, S.; López, M.E.; Marcos, A.; Yus, M.; Llanero-Luque, M.; Del-Pozo, F.; Sancho, M.; et al. The Default Mode Network is functionally and structurally disrupted in amnestic mild cognitive impairment—A bimodal MEG-DTI study. Neuroimage Clin. 2014, 6, 214–221. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Sun, F.; Xu, Y.; Xu, F.; Wang, S.; Wang, X. Altered neuromagnetic activity in default mode network in childhood absence epilepsy. Front. Neurosci. 2023, 17, 1133064. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef]
- Rojas-Líbano, D.; Kay, L.M. Olfactory system gamma oscillations: The physiological dissection of a cognitive neural system. Cogn. Neurodyn. 2008, 2, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Lepousez, G.; Nissant, A.; Bryant, A.K.; Gheusi, G.; Greer, C.A.; Lledo, P.-M. Olfactory learning promotes input-specific synaptic plasticity in adult-born neurons. Proc. Natl. Acad. Sci. USA 2014, 111, 13984–13989. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Takeuchi, Y.; Wang, J.; Gellért, L.; Barcsai, L.; Pedraza, L.K.; Nagy, A.J.; Kozák, G.; Nakai, S.; Kato, S.; et al. Reinstating olfactory bulb-derived limbic gamma oscillations alleviates depression-like behavioral deficits in rodents. Neuron 2023, 111, 2065–2075.e5. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 1998, 55, 257–332. [Google Scholar] [CrossRef]
- Carmichael, S.T.; Price, J.L. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 1995, 363, 615–641. [Google Scholar] [CrossRef]


| Groups | n | Gender | Age (Years Old) | Weight (kg) | ||
|---|---|---|---|---|---|---|
| Male (n) | Female (n) | |||||
| Control | 8 | 3 | 5 | 27.50 ± 1.33 | 72 ± 9.34 | |
| Treatment | Subgroup 1 | 7 | 4 | 3 | 27.86 ± 1.908 | 75.57 ± 11.62 |
| Subgroup 2 | 6 | 3 | 3 | 26.67 ± 1.726 | 74.33 ± 8.441 | |
| Subgroup 1 + Subgroup 2 | 13 | 7 | 6 | 27.31 ± 1.26 | 75.00 ± 7.08 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riazi, H.; Nazari, M.; Raoufy, M.R.; Mirnajafi-Zadeh, J.; Shojaei, A. Olfactory Epithelium Stimulation Using Rhythmic Nasal Air-Puffs Improves the Cognitive Performance of Individuals with Acute Sleep Deprivation. Brain Sci. 2024, 14, 378. https://doi.org/10.3390/brainsci14040378
Riazi H, Nazari M, Raoufy MR, Mirnajafi-Zadeh J, Shojaei A. Olfactory Epithelium Stimulation Using Rhythmic Nasal Air-Puffs Improves the Cognitive Performance of Individuals with Acute Sleep Deprivation. Brain Sciences. 2024; 14(4):378. https://doi.org/10.3390/brainsci14040378
Chicago/Turabian StyleRiazi, Hanieh, Milad Nazari, Mohammad Reza Raoufy, Javad Mirnajafi-Zadeh, and Amir Shojaei. 2024. "Olfactory Epithelium Stimulation Using Rhythmic Nasal Air-Puffs Improves the Cognitive Performance of Individuals with Acute Sleep Deprivation" Brain Sciences 14, no. 4: 378. https://doi.org/10.3390/brainsci14040378